INTRODUCTION
Historically, dose escalation in prostate cancer has been limited by normal tissue toxicity, primarily in the rectum and to a lesser degree in the bladder. The introduction of computed tomography (CT)–based 3D conformal radiation therapy (3D-CRT) with blocks or multi-leaf collimators (MLCs) provided for a significant improvement in dose conformality.Reference Perez, Michalski, Purdy and Lockett1 The increased dose sparing for the rectum and bladder encouraged several dose escalation studies.Reference Michalski, Purdy, Winter, Roach, Vijayakumar, Sandler, Markoe, Ritter, Russell, Sailer, Harms, Perez, Wilder, Hanks and Cox2–Reference Schneider, Lomax, Besserer, Pemler, Lombriser and Kaser-Hotz4 With the subsequent development and introduction of intensity-modulated radiation therapy (IMRT), it is now possible to further conform the delivered dose to the outlined planning target volume (PTV), while at the same time maximise the sparing of the critical structures.Reference Cahlon, Hunt and Zelefsky5–Reference Zelefsky, Fuks and Leibel10 This has enabled the attempt to escalate the prescribed dose even further with the goal of not increasing toxicity.Reference Zelefsky, Fuks, Hunt, Yamada, Marion, Ling, Amols, Venkatraman and Leibel11–Reference Martinez, Yan, Lockman, Brabbins, Kota, Sharpe, Jaffray, Vicini and Wong13 With the combination of IMRT and the inverse treatment planning algorithms currently available, dose constraints to critical structures such as the rectum and bladder can be directly included in the optimisation calculation of the optimal solution.Reference O'Donnell, Finnegan, Eliades, Oliveros and Plowman14,Reference Teh, Mai, Grant, Chiu, Lu, Carpenter, Woo and Butler15
As of today, there are no standard criteria for absolute dose limits to critical structures, nor is there a customary system to describe these criteria in the treatment of prostate cancer. The Radiation Therapy Oncology Group (RTOG), which is the premier study group in radiation oncology, has used data from past published works in an effort to define some of the prescription parameters for 3D-CRT and IMRT treatments in prostate cancer. Early investigations, based on results from a randomised study at MD Anderson Hospital, showed the benefits of higher radiation doses to the prostate.Reference Storey, Pollack, Zagars, Smith, Antolak and Rosen16 Evaluating toxicity, they found that if no more than 25% of the rectum received 70 Gy, the risk of rectal bleeding decreased from 46% to 16%. A review of the RTOG 9406 3D-CRT protocol (level 3) study confirmed the same dose constraints.Reference Ryu, Winter, Michalski, Purdy, Markoe, Earle, Perez, Roach, Sandler, Pollack and Cox17 As a result, those have been the parameters for subsequent studies, including the current high priority study RTOG 0415, which is a phase III randomised study of hypofractionated 3D-CRT/IMRT treatment versus conventionally fractionated 3D-CRT/IMRT treatment in patients with favourable risk prostate cancer. The goal of the study is to evaluate daily dose fraction size in the treatment of prostate cancer, and it allows for either IMRT or 3D-CRT delivery, using those previously established constraints.
Given the significant difference in complexity between the planning and delivery of a 3D-CRT plan as compared to an IMRT treatment, the scope of this present study is to investigate whether there are dosimetric advantages of employing IMRT instead of 3D-CRT, utilising the current constraints as described in RTOG 0415.Reference Ludlum and Xia18–Reference Verhey20
MATERIALS AND METHODS
Patient selection and planning methods
Ten consecutive patients (see Table 1 for patient characteristics) who were undergoing, or had recently completed, radiation treatments were loaded into the Pinnacle (Philips Medical Systems, Cleveland, OH) treatment planning system (TPS). With respect to the patients treated at our clinic, the planning CT scans are taken ‘as-is’. There is no effort to keep an empty bladder (e.g., catheter insertion) nor to have a completely empty rectum. However, if after the initial scan is performed it is noted that the patient has a distended rectum, an enema is given and the patient is scanned again, in attempts to minimise the risk of biochemical failure from a distended rectum on the planning CT.Reference de Crevoisier, Tucker, Dong, Mohan, Cheung, Cox and Kuban21
Table 1. Patient characteristics

A single physician outlined the prostate (GTV), seminal vesicles, rectum, bladder, femoral heads, small bowel, and penile bulb. As per convention, the rectum was delineated 2 cm superior and inferior to the last CT slice where the prostate gland was contoured.
Four plans were generated for each patient using the Pinnacle TPS: two 3D-CRT plans (6MV and 18 MV photon beams) and two IMRT plans (6MV and 18MV photon beams). Each of the 3D-CRT plans utilised the same template of six coplanar beams, with opposed lateral (90° and 270°) and paired oblique (125° with 305° and 65° with 245°) beams. The respective Pinnacle IMRT plans were produced using a template of either five, seven or nine equally spaced coplanar beams, depending on patient geometry.Reference Pugachev, Li, Boyer, Hancock, Le, Donaldson and Xing22 The IMRT beam template differed from the 3D-CRT template because using an opposed beam arrangement with an IMRT optimisation algorithm causes the TPS to stall when trying to optimise what is essentially the same beam segment twice. The intensity optimisation for each of the beam portals (for all plans) was achieved with the direct machine parameter optimisation algorithm used in the Pinnacle TPS.
The same CT data and contours were then used to generate two additional 6 MV photon beam IMRT plans for each patient: one using the HiArt tomotherapy planning system (TomoTherapy Inc, Madison, WI) for delivery via helical tomotherapy, and one with the Corvus TPS (Best NOMOS Radiation Oncology, Pittsburgh, PA), for delivery by means of serial tomotherapy. The optimised intensity was realised by the minimisation of least squares optimisation algorithm in HiArt Tomotherapy TPS, and by the continuous annealing optimisation algorithm in the Corvus TPS.
With the Pinnacle TPS, all dose constraint points can be optimised concurrently. The Tomotherapy TPS can only optimise one dose constraint per organ at risk at a time. A second point may be entered once the first has been satisfied, allowing all of the dose–volume histogram (DVH) constraints specified by the protocol to be included. The Corvus TPS can only optimise one point per organ at risk, and for these plans the strictest (and therefore most difficult to achieve) constraint for each organ was selected for the IMRT plan optimisation.
The Pinnacle plans were planned for a Varian 2100C/D linear accelerator (Varian, Palo Alto, CA) with a 120-leaf millennium MLC. When creating an IMRT plan for a linear accelerator equipped with an MLC, there are two delivery options: step-and-shoot and sliding window. In the step-and-shoot method, the multiple dynamic beam segments are delivered only when there is no motion of the gantry or the MLC leaves. In contrast, the sliding window technique allows the beam to remain on while the MLC leaves are in motion. For this study, the step-and-shoot method was utilised.
The Corvus TPS’s serial tomotherapy plans were planned for the NOMOSTAT (Best NOMOS Radiation Oncology, Pittsburgh, PA) in the 1.0 cm mode on a Varian 600C Clinac. The helical tomotherapy plans were planned for the HiArt Tomotherapy machine (TomoTherapy Inc, Madison, WI). Both tomotherapy treatments make use of arcs, with the beam always on and the MLCs continuously modulating the intensity.
In total, six plans were generated for each patient (Pinnacle 18MV and 6MV IMRT, Pinnacle 18MV and 6MV 3D conformal, NOMOS 6 MV IMRT and Tomotherapy 6MV IMRT).
Prescription and IMRT constraints
The PTV was defined as the GTV with 5 mm margins in the anterior, lateral, superior and inferior directions; and a posterior margin of 3mm. The PTV included only the prostate gland with the above mentioned margin; the seminal vesicles were not included. All plans were evaluated using the following criteria: (i) ability to deliver 100% of the dose to the GTV within –5% and +10%; (ii) maximum and minimum dose to the rectal volume; (iii) maximum and minimum dose to the bladder volume. The RTOG 0415 protocol guidelines for PTV dose compliance state that the prescription isodose surface should cover ≥98% of the PTV. A major variation is defined as an isodose surface coverage <95% of the PTV. The protocol also states that the maximum dose to the PTV volume should not exceed the prescription dose by more than 7%. A minor variation with respect to this criterion is <7% to ≤10%; and a major variation is >10%.
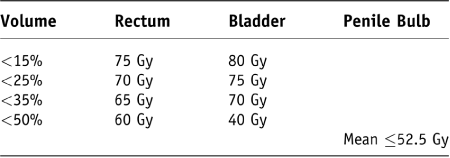
Two different approaches were examined in this study: 3D-CRT and IMRT, using the RTOG 0415 criteria. The prescription used for all plans was 76 Gy to the 95% isodose line of the PTV, in 38 fractions of 2 Gy, independent of the planning/delivery method. For the 3D-CRT modality, no dose constraints for critical structures (e.g., rectum and bladder) were used and conformality was determined strictly by anatomy. The only optimisation enabled was that of monitor units for each beam, in order to deliver the prescribed dose to the PTV. The IMRT plans were optimised based on the RTOG 0415 recommended dose constraints to the rectum, bladder, penile bulb and femoral heads. The RTOG’s bladder, rectum and penile bulb dose constraint parameters are shown in Table 2. For the IMRT plans, the optimisation was carried out only until those constraints were satisfied and for the purposes of this study, no further refinement was attempted.
Table 2. RTOG 0415 dose constraints
RESULTS
The plans calculated in this investigation have a noticeable variability with respect to the dose to the bladder and rectum, even within the same modality. Even though all patients, using a particular modality may meet the criteria for an acceptable plan, some are intrinsically better than others—they may have lower hotspots, more homogeneous PTV coverage, or overall lower rectal or bladder doses. A thorough analysis of this variability is beyond the scope of the study, but patient-specific factors such as pelvic anatomy, organ distension and body habitus all appear to have an effect.Reference Sanguineti, Cavey, Endres, Brandon and Bayouth23,Reference Verellen, Linthout, Soete, Van Acker, De Roover and Storme24 In order to generalise the results, an ‘average patient’ was created using averaged data from all 10 patients. The dose–volume results for each organ, for each of the six plans, are shown in Table 3. The doses to the bladder, the rectum and the penile bulb were within the limits, and all 3D-CRT plans were able to easily satisfy the RTOG 0415 critical organ criteria. Figures 1 and 2 show the rectum and bladder DVHs for the average patient, using each modality. Bladder and rectal doses, on average, were greater for the plans using IMRT, and more sparing was observed for the 3D-CRT plans, highlighting the potential benefits of choosing 3D-CRT over IMRT plans created with the RTOG 0415 constraints. No trend was observed with respect to the dose to the critical structures between the IMRT modalities.

Figure 1. The rectum DVHs for the ‘average’ patient, using each modality. Circles indicate the RTOG dose constraint criteria.

Figure 2. The bladder DVHs for the ‘average’ patient, using each modality. Circles indicate the RTOG dose constraint criteria.
Table 3. PTV dose comparison between 3D-CRT and IMRT plans for the ‘average’ patient

Bold indicates a value outside of the allowable range for the RTOG 0415 criteria.
For the evaluation of PTV dose homogeneity, an average PTV DVH was created from six patients, eliminating the two highest and two lowest from each group. Table 4 shows the mean dose, as well as the dose ranges for each modality. The 18 MV 3D-CRT plans in general had maximal doses that exceeded the RTOG allowances but there was only one instance of a major deviation with the 6 MV 3D-CRT plans. Tomotherapy did the best here, with no deviations among its IMRT plans; and Corvus did the worst with five IMRT plans with maximum values outside the allowable range. Corvus also had the largest maximum dose of all: 98.78 Gy.
Table 4. Dose comparison for organs at risk for 3D-CRT and IMRT plans for the ‘average’ patient

The 3D-CRT plans easily met the RTOG 0415 dose constraints.
Figure 3 shows the variation in the high-dose region of the PTV DVHs for each modality. In contrast to the excellent results for the critical structure data, the 3D-CRT plans had poorer homogeneity, with an apparent worsening for plans with higher energies. The Pinnacle and Tomotherapy optimised solutions provided the best PTV homogeneity in almost all cases. Serial tomotherapy (Corvus) was the IMRT technique with the worst plans, in terms of PTV homogeneity. Although the hotspots for the serial tomotherapy plans appeared in a very small volume of the PTV, the doses there were high. Also, for these plans, there were instances where the goals for PTV coverage were not reached during the initial attempt, and several additional trials were required in order to reach the RTOG recommended criteria.

Figure 3. The high-dose region of the PTV DVHs for the ‘average’ patient, for each modality. The vertical dashed line denotes 7600 cGy, the prescription dose for every plan.
DISCUSSION
The 3D-CRT plans calculated with Pinnacle were able to meet the RTOG 0415 criteria, especially when the 6MV photon beam energy was used. Even though the 3D-CRT plans were not optimised utilising critical structure constraints, the rectum, bladder and penile bulb were well within the specified dose limits recommended for their respective DVH points. The average dose to the PTV for the 3D-CRT plans was within the specified criteria for the 6MV photon beam energy but the 18 MV plans showed most of the major deviations as defined by the protocol. The 18MV 3D-CRT plans had hot spots that exceeded the prescribed dose by 110–113%. The PTV homogeneity was improved for all the 3D-CRT plans for both energies when the margins specified by the protocol were used, and with them no major deviations were observed.
Major deviations were also found within some IMRT plans. None of the Pinnacle or Tomotherapy plans had such deviations, but some of the cases that were optimised with the Corvus TPS produced hot spots that were not satisfactory according to the RTOG criteria. Under typical clinical circumstances the plans with these excessive inhomogeneities could have been modified and deemed acceptable, but the immediate goal of this study was to reach the normal organ constraints.
In an analogous fashion, the suboptimal 3D-CRT plans could have been improved, through better beam angle selection or by adding more beams. The heaviest patients would realise the greatest benefit if more beams, both coplanar and non-coplanar, were introduced to deliver the prescribed dose to the target. However, because this study required the same arrangement of beams (in both number and angle) to ensure intercomparability, the cases where better results could have been obtained (if the angles of the photon beams had been optimised) were left as initially calculated.
The plans calculated with the IMRT TPSs required more time in order to set the criteria for each organ at risk, despite the use of a library of initial objectives to minimise this problem. These plans also required longer to calculate a solution that satisfied the specified criteria, particularly with respect to the time required for optimisation. In some instances an IMRT plan utilising RTOG criteria took up to 15 times longer than the respective 3D-CRT plan—without major improvement in either the PTV dose coverage or sparing of the critical organs. For patients where IMRT trials with nine beams had to be used for the criteria to be met; starting over after several attempts using five beams and again with seven, made the disparity even greater.
As previously mentioned, there are cases where the RTOG criteria were not satisfied by applying IMRT. These cases occurred with the Corvus TPS, and similar results have been noted by other researchers.Reference Ayyangar, Fung, Li, Pillai, Yoe-Sein, Zhen and Enke25 A likely reason for this is the serial arc delivery of the Corvus TPS using a fixed field width, without the option to use some sort of ‘pitch’ between arcs. That restriction results in hot and cold spots, especially in the most inferior and posterior portions of the prostate gland. It is believed that further investigation would be required in order to devise a more standard solution for Corvus plans. In contrast, the optimisation in the helical tomotherapy plans was fairly easy, and for most cases a solution was achieved within a few (10–30) iterations. These plans were also technically superior to the serial tomotherapy plans in most cases.
These results suggest, in contrast to some of the previous work published in the field, that the optimisation of prostate plans using these three different IMRT modalities was not superior to the six-field 3D-CRT plans calculated with the Pinnacle TPS.Reference Lee, Dong, Ahamad, Choi, Cheung, Lee, Horne DF, Breaux and Kuban26–Reference Zhu, Mizowaki, Nagata, Takayama, Norihisa, Yano and Hiraoka29 Although the IMRT plans in general showed better PTV dose homogeneity, the homogeneity of the 3D-CRT plans was similar to the IMRT plans and could have been further improved with more planning time. This is not a trivial issue, as hot spots in critical structures (such as the urethra) could result in increased toxicity.Reference Gambacorta, Manfrida, D'Agostino, Digesù, D'Onofrio, Rosato and Valentini30,Reference Roach31
It is clear that in using the basic RTOG 0415 criteria for prostate IMRT optimisation, we are not taking advantage of the real power of IMRT. In that regard, we also evaluated a simple four field box plan for each patient (data not shown) and in most cases the RTOG criteria were met. Much stricter dose constraints for the rectum and bladder can be met by IMRT and would likely result in a further decrease in toxicity; with the added possibility of higher doses to the PTV.
CONCLUSIONS
IMRT plans optimised according to the RTOG 0415 criteria did not show any significant dosimetric advantage over the plans that were produced using 3D-CRT. The dose to the bladder, rectum and penile bulb for the 3D-CRT plans satisfied the RTOG 0415 criteria. The benefit of the IMRT optimised plans was the higher uniformity in PTV dose coverage. Also, the IMRT plans had less major variations compared to the 3D-CRT plans, but this could change if effort is put towards improving beam angle selection for the latter plans. Our results showed that following the RTOG 0415 criteria does not take full advantage of the capabilities of IMRT. Dosimetrists and others involved in the treatment planning process should be encouraged to continue optimising plans after the RTOG 0415 protocol constraints have been met. This will preclude a situation where a solution that is ‘good enough’ is accepted for treatment, and allow the considerable benefits IMRT offers to be maximised. Making full use of these technologies is an ethical way to bring the greatest benefit to the patient and justify the added cost associated with the time, effort and technology needed for IMRT planning.
Acknowledgements
The authors thank Fan-Chi Su M.S. for assisting with treatment planning and data collection. Dr. Gregory Swanson is the SWOG co-chair for the RTOG study 0415 currently in progress.









