It is well known that inflammatory response can cause perturbations in Fe homeostasis( Reference Weiss and Goodnough 1 ). This response often results in the decreased absorption of Fe in the small intestine and the increased sequestration of Fe in the macrophages of the reticulo-endothelial system, resulting in a state of relative Fe deficiency( Reference Wessling-Resnick 2 ). These events contribute to the development of the clinical entity known as anaemia of inflammation or chronic disease (ACD)( Reference Weiss and Goodnough 1 ). ACD is commonly seen in conditions such as infections, trauma, malignancies or autoimmune diseases. Deranged Fe homeostasis, decreased proliferation of erythroid progenitor cells, reduced lifespan of erythrocytes and the decreased production and biological activity of erythropoietin have been shown to contribute to the development of anaemia seen in such conditions( Reference Weiss and Goodnough 1 ).
Cytokines and acute-phase proteins play pivotal roles in altering body Fe distribution. Levels of hepcidin, an Fe regulatory hormone, are increased during inflammatory stress, restricting the efflux of Fe from macrophages and enterocytes( Reference Nicolas, Chauvet and Viatte 3 ). Pro-inflammatory conditions have been shown to cause the induction of hepcidin in the liver, resulting in hypoferraemia and subsequent anaemia( Reference Nicolas, Chauvet and Viatte 3 ). Cytokines, such as interferon-γ and TNF-α, have been shown to induce Fe retention within the cells of the mononuclear phagocyte system by down-regulating the expression of ferroportin and increasing the expression of divalent metal transporter 1 (DMT1)( Reference Ludwiczek, Aigner and Theurl 4 ). Cytokines have also been shown to induce ferritin expression in monocytes and macrophages, leading to an increased sequestration of Fe( Reference Rogers 5 , Reference Torti, Kwak and Miller 6 ). In addition, inflammatory stress has been shown to induce the formation of hepcidin by the mononuclear phagocyte system (monocytes and macrophages), which acts in an autocrine manner, inducing the internalisation and degradation of ferroportin( Reference Theurl, Theurl and Seifert 7 ). Studies have shown alterations in the regulation of Fe homeostasis in patients with ACD. Levels of serum hepcidin and pro-hepcidin have been shown to be elevated in such patients( Reference Theurl, Aigner and Theurl 8 , Reference Theurl, Mattle and Seifert 9 ). The expression of ferroportin was found to be down-regulated in the duodenum and monocytes of these patients( Reference Theurl, Aigner and Theurl 8 , Reference Theurl, Mattle and Seifert 9 ). Hepcidin levels have been shown to be lower in patients with ACD who had concurrent Fe deficiency than in those with ACD without Fe deficiency( Reference Theurl, Aigner and Theurl 8 ).
Patients with inflammatory bowel disease (IBD, comprising ulcerative colitis (UC) and Crohn's disease (CD)) are often anaemic( Reference Gasche, Lomer and Cavill 10 ). There are limited reports available on alterations in Fe homeostasis in such patients. Theurl et al. ( Reference Theurl, Aigner and Theurl 8 ) showed decreases in the expression of DMT1 and ferroportin in the duodenum of patients with ACD. There is little information on how inflammation affects other proteins, such as duodenal cytochrome b (dcytb), hephaestin, ferritin and transferrin receptor 1 (TfR1), involved in duodenal Fe absorption in such patients. Similarly, there is little information on Fe-related proteins in the monocytes of such patients. The effects of inflammation on these proteins are likely to affect Fe homeostasis in these patients. Therefore, the aim of the present study was to determine the expression levels of Fe-related proteins in the duodenal mucosa and monocytes in patients with chronic inflammatory conditions.
Materials and methods
Chemicals and reagents
Absolute alcohol was obtained from Hayman. Agarose was obtained from Genei. Antibodies against DMT1 and ferroportin were obtained from Santa Cruz. An antibody against β-actin was purchased from Sigma. Secondary antibodies and ECL West Dura substrate for the development of Western blots were purchased from Thermo Scientific. Disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride, sodium acetate and NaCl were obtained from Sisco Research Laboratories. Glass vacutainer blood collection tubes, with EDTA as the anticoagulant, were obtained from BD Biosciences. 3-Morpholinopropane sulphonic acid was obtained from Fluka Biochemika/Sigma, and Histopaque 1077, TRI reagent, chloroform, isopropanol, formamide, formaldehyde, EDTA, bovine serum albumin, ethidium bromide and diethyl pyrocarbonate were obtained from Sigma. Magnetic-activated cell sorter (MACS) CD14 magnetic antibodies, MACS mini-column and a mini MACS unit were obtained from Miltenyi Biotec. Reverse transcription core kit, SYBR green PCR master mix kit and gene-specific primers were obtained from Eurogentec. Roswell Park Memorial Institute-1640 medium was obtained from GIBCO, Invitrogen Corporation. All reagents used were of analytical grade.
Subjects
The present study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki. All procedures involving the patients in the study were approved by the Institutional Review Board of the Christian Medical College, Vellore, India. Written informed consent was obtained from all the subjects. Patients, aged 19–65 years, with chronic inflammatory disorders (chronic IBD (comprising UC and CD) or RA), who were required to have a medically indicated upper gastrointestinal endoscopy as part of their clinical management, were recruited into the study. Such indications included the need for a gastric biopsy to rule out focally enhanced gastritis in CD to distinguish it from UC or to investigate upper gastrointestinal symptoms. Patients with active gastrointestinal bleeding and those on Fe supplements were excluded from the study.
Diagnoses of UC, CD and RA were based on standard consensus criteria( Reference Arnett, Edworthy and Bloch 11 – Reference Truelove and Witts 13 ). Control subjects comprised patients who underwent upper gastrointestinal endoscopy for investigation of dyspepsia and in whom no abnormalities were found on endoscopy. The subjects underwent an upper gastrointestinal endoscopy after an overnight fast. The samples were collected in the mornings of the days of recruitment. Mucosal tissue samples were immediately snap-frozen using liquid N2. Venous blood was drawn from the antecubital area of the arm for the isolation of peripheral blood mononuclear cells and the estimation of hepcidin, C-reactive protein and ferritin. Data on sociodemographic and relevant clinical characteristics and other parameters of interest were obtained from the medical records of the patients.
Isolation of monocytes from whole blood
Peripheral blood mononuclear cells were isolated from blood samples using Histopaque 1077, according to the manufacturer's instructions (Sigma). Monocytes were isolated from purified peripheral blood mononuclear cells, using a MACS (Miltenyi Biotec, GmbH). The purity of the isolated monocytes was assessed by fluorescent-activated cell sorting analysis and was consistently found to be above 95 %.
Isolation of total RNA, reverse transcription and real-time PCR assays
Total RNA was isolated from the duodenal mucosal biopsy specimens and monocytes of all the subjects. RNA was isolated using TRI reagent according to the manufacturer's instructions. Integrity of the isolated RNA was confirmed and DNA contamination was ruled out by subjecting the samples to agarose gel electrophoresis. The RNA isolated from monocytes was found to be contaminated with DNA and was subjected to treatment with DNase. The RNA obtained was reverse transcribed and used for real-time PCR assays. Fe-related proteins studied were DMT1, dcytb, ferroportin, hephaestin, ferritin and TfR1. Gene-specific primers and reaction conditions used for PCR assays for the genes of interest are shown in Table S1 (available online). Primer sequences used for the genes of interest were obtained from previously published work, as indicated in Table S1 (available online)( Reference Theurl, Theurl and Seifert 7 , Reference Theurl, Mattle and Seifert 9 , Reference Gilberts, Greenstein and Katsel 14 , Reference Brookes, Hughes and Turner 15 ). All the reactions were carried out in triplicate. Melting curve analyses carried out for each gene of interest showed the presence of a single peak, indicating the amplification of a single product. Standardisation of the reaction conditions was established using a standard curve generated with serial dilutions of the complementary DNA template for each gene. Negative control tubes were included in the assays to rule out genomic DNA contamination. The relative expression of the various genes of interest was determined using the comparative C t method by normalisation with β-actin, which was used as a housekeeping gene( Reference Schmittgen and Livak 16 ).
Preparation of protein extracts and Western blot assays
Protein extracts were prepared from duodenal biopsies from patients with UC( Reference Barisani, Parafioriti and Bardella 17 ). Snap-frozen tissues were homogenised in radioimmunoprecipitation assay buffer (50 mm-Tris, 0·1 % SDS, 1 % NP-40 and 0·5 % sodium deoxycholate, pH 7·5) containing protease inhibitors (Complete Mini; Roche) with 0·5 mm-phenylmethanesulphonyl fluoride. The homogenates were centrifuged at 16 000 g for 5 min at room temperature. Protein concentrations in all the extracts were determined by the bicinchoninic acid protein assay kit, according to the manufacturer's instructions. Duodenal protein extracts (100 μg) were subjected to SDS–PAGE, using 10 % gels. Isolated proteins were transferred to polyvinylidene fluoride membranes at 25 V over 16 h. The membranes were incubated with blocking buffer containing Tween-20 (0·02 %), skimmed milk (5 %) and PBS (pH 7·4) for 2 h at room temperature. This was followed by overnight incubation with primary antibodies against DMT1 (1:300) and ferroportin (1:200). After quantification of the bands, the membranes were stripped using stripping buffer (100 mm-β-mercaptoethanol, 62·5 mm-Tris–HCl and 2 % SDS) and incubated with an antibody against β-actin (1:2500). Incubations with primary antibodies were followed by those with secondary antibodies, which were labelled with peroxidase (1:1000). The protein bands obtained were visualised using the ECL West Dura substrate and quantified using a chemiluminescent imaging system (FluorChem TM SP; Alpha Innotech). The band intensities obtained for the proteins of interest were normalised to that of β-actin, which was used as a loading control for each sample.
Estimations in serum
Estimations of Hb, serum C-reactive protein (CRP), ferritin and Fe were carried out in routine diagnostic laboratories (Transfusion Medicine, Clinical Microbiology and Clinical Biochemistry) of the institution. These parameters and hepcidin levels were also estimated in a separate group of control subjects (n 19) and those with UC (n 20), from whom only a blood sample was available. Hepcidin levels were estimated in these samples using a commercially available ELISA kit for bioactive hepcidin-25 (DRG Instruments GmbH), according to the manufacturer's instructions. Standards of varying concentrations of synthetic hepcidin provided in the kit were used as positive controls.
Statistical analysis
Statistical analysis of data was carried out using the SPSS software package, version 16. All parameters in the study were tested for a normal distribution using the one-sample Kolmogorov–Smirnov test. All the parameters studied were found to be normally distributed and were analysed by the unpaired t test. A χ2 test was used for the analysis of categorical data. A P value less than 0·05 was considered to be statistically significant in all cases. Bivariate analysis was also carried out on the data using Pearson's correlation coefficient.
Results
Patients
In the present study, 119 patients (control n 44 and chronic inflammatory disorders n 75) were recruited after informed consent. The study was designed to obtain the data on the parameters of interest in all the subjects recruited. However, as the study progressed, it was found that most patients were willing to consent to giving either a duodenal mucosal sample or a sample of blood, but most often not both. Owing to this, it was not possible to obtain complete data for each patient as planned. As a result, there were only forty-three patients (control n 19 and chronic inflammatory disorders n 24) from whom data were available on all the parameters of interest. They consisted of twenty-eight males (65 %) and fifteen females (35 %). The profile of these patients is shown in Table 1. Of the nineteen control patients, thirteen were males and six were females. Of the twenty-four patients with inflammatory disorders, fifteen were males and nine were females. Among the twenty-four patients with disease, eleven had UC (seven males and four females), eight had CD (seven males and one female) and five had RA (one male and four females).
Table 1 Profile of the study patients (Mean values and standard deviations; number of patients and percentages)
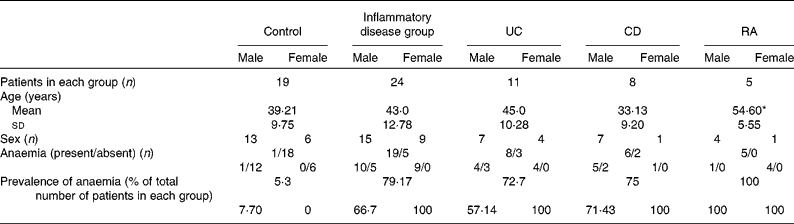
UC, ulcerative colitis; CD, Crohn's disease; RA, rheumatoid arthritis.
* Mean value was significantly different from that of the control group (P< 0·05).
The mean ages of the patients with inflammatory disorders and those who had UC or CD were similar to those of the control subjects; the patients who had RA were found to be significantly older than the control subjects (Table 1). The prevalence of anaemia in each group and in males and females separately is shown in Table 1. It was high among the patients in the disease groups, as assessed using the criteria of the WHO, according to which a Hb level of < 130 g/l in men and < 120 g/l in women is defined as anaemia( Reference Adamson, Longo, Longo, Fauci, Kasper, Hauser, Jameson and Loscalzo 18 ).
Hb levels
Levels of Hb were significantly lower in patients with chronic inflammatory conditions than in control subjects, both when considered as a whole group and as individual conditions (Table 2).
Table 2 Levels of Hb and C-reactive protein (CRP) in the study patients (Mean values and standard deviations)
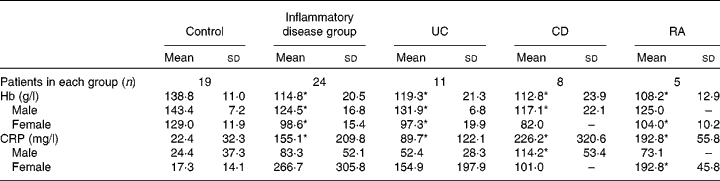
UC, ulcerative colitis; CD, Crohn's disease; RA, rheumatoid arthritis.
* Mean value was significantly different from that of the control group (P< 0·05).
Serum levels of serum C-reactive protein
Serum levels of CRP were significantly higher in the combined and individual groups of patients than in the control group (Table 2).
Serum levels of iron and ferritin
There were no significant differences in serum Fe and ferritin levels between the combined group of patients and control patients. When individual disease groups were considered, the levels of serum Fe were found to be significantly lower in patients with CD when compared with the control subjects and were unaffected in those with UC and RA. There were no significant differences in the serum levels of ferritin between the individual groups of patients and control subjects (Table 3).
Table 3 Serum levels of iron and ferritin in the study patients (Mean values and standard deviations)
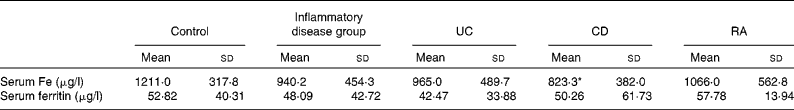
UC, ulcerative colitis; CD, Crohn's disease; RA, rheumatoid arthritis.
* Mean value was significantly different from that of the control group (P< 0·05).
Gene expression of iron-related proteins in the duodenum
In the group of patients with inflammatory diseases, the mRNA levels of DMT1, hephaestin, TfR1 and ferritin were significantly higher than those in the control subjects (Fig. 1(a) and (d)–(f)). The expression levels of ferroportin and dcytb were also higher, with the increases being close to statistical significance (P= 0·08 and P= 0·09, respectively; Fig. 1(b) and (c)). In patients with UC, the mRNA levels of all the proteins of interest were significantly elevated, with the exception of dcytb, where the increase was close to being statistically significant (P= 0·06; Fig. 1(a)–(f)). In patients with CD, the mRNA levels of ferroportin, hephaestin, TfR1 and ferritin were significantly higher than those in the control group; the levels of DMT1 and dcytb were also elevated, but the increases did not reach statistical significance (P= 0·07 for both; Fig. 1(a)–(f)).

Fig. 1 Levels of mRNA of (a) divalent metal transporter 1 (DMT1), (b) ferroportin, (c) duodenal cytochrome b (dcytb), (d) hephaestin, (e) transferrin receptor 1 (TfR1) and (f) ferritin, normalised to that of β-actin, in the duodenal mucosal tissue from the control group, the combined group of subjects with chronic inflammatory conditions, the ulcerative colitis group (UC), the Crohn's disease group (CD) and the rheumatoid arthritis (RA) group. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
In patients with RA, the mRNA levels of DMT1 were found to be significantly higher than those in the control subjects (Fig. 1(a)). The expression levels of mRNA of some of the other proteins of interest also tended to be higher than those in the control subjects, but did not differ significantly (Fig. 1(b)–(f)).
There was no evidence of active disease observed in the duodenal biopsy samples, as assessed by endoscopy and histopathological studies. Therefore, it is unlikely that such factors confounded the findings reported above.
Protein expression of iron-related proteins in the duodenum of patients with ulcerative colitis
The protein levels of DMT1 and ferroportin were found to be significantly higher in patients with UC than in the control subjects (Fig. 2).

Fig. 2 Protein levels of (a) divalent metal transporter 1 (DMT1) and (b) ferroportin, normalised to that of β-actin, in the duodenal mucosal tissue from the control and ulcerative colitis (UC) groups. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). (c) Representative Western blots for each protein studied.
Gene expression of iron-related proteins in monocytes
There were no significant differences found between the patients with UC and the control subjects with respect to the gene expression of Fe-related proteins in monocytes (Fig. S1, available online).
Correlation analysis
Several of the parameters studied showed statistically significant correlations with one another (Table 4). For example, Hb levels negatively correlated with the levels of serum CRP and mRNA for most of the duodenal proteins studied. The gene expression levels of many of the proteins in the duodenum showed significant positive correlations with one another.
Table 4 Correlation analysis of the parameters studied*

CRP, serum C-reactive protein; DMT1, divalent metal transporter 1; TfR1, transferrin receptor 1; dcytb, duodenal cytochrome b.
* Bivariate analysis was carried out on the data obtained, using Pearson's correlation coefficient.
Serum hepcidin levels in a separate group of patients with ulcerative colitis
Serum samples that were available from a separate group of control patients and those with UC were used for the estimation of serum hepcidin. This was the only sample that was available from this group of subjects. The levels of serum hepcidin were found to be significantly lower in the separate group of the patients with UC than in those of the control subjects (Fig. 3). There were no significant differences found between the subjects in this control group and the control subjects from whom both the blood and duodenal samples were obtained in terms of their clinical profile and haematological and inflammatory parameters. Similarly, this group of patients with UC did not show any significant differences from the other group of patients with UC from whom the blood and duodenal samples were obtained, showing that these groups of patients were similar.
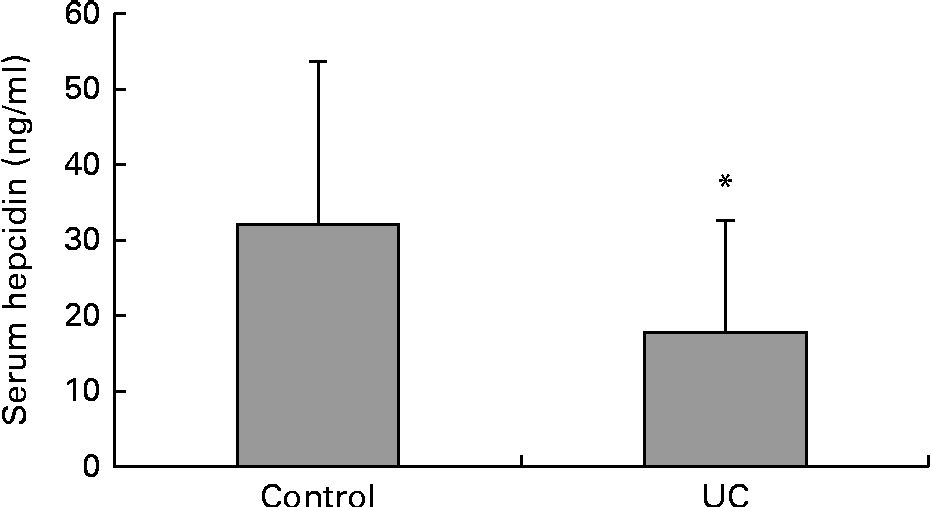
Fig. 3 Serum levels of hepcidin from a separate group of control (n 19) and ulcerative colitis (n 20) subjects. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05).
Discussion
A total of 119 patients (control n 44 and chronic inflammatory disorders n 75) were recruited after informed consent for the present study. However, since most patients were willing to consent to giving either a duodenal mucosal sample or a sample of blood, but most often not both, it was not possible to obtain complete data for each patient as planned. As a result, there were only forty-three patients (control n 19 and chronic inflammatory disorders n 24) from whom data were available on all the parameters of interest. Data were analysed separately for the total number of 119 patients recruited and the subgroup of forty-three patients. Interestingly, the findings were very similar in both the groups (data for the group of 119 patients not shown). This is an indication of the robustness of the findings, which were observed even with a smaller sample size.
The prevalence of anaemia was high in patients with chronic inflammatory disorders in the present study. In those with IBD, which comprises the majority of patients in the study, several mechanisms are known to contribute to anaemia. These include gastrointestinal blood loss, inflammation-induced reduction in erythropoiesis and duodenal Fe absorption, and deficiencies in vitamin B12 and folate( Reference Gasche, Lomer and Cavill 10 , Reference Peeters, Jongen-Lavrencic and Raja 19 , Reference Ganz 20 ). Recruitment criteria for the present study excluded patients with active or recent gastrointestinal bleeding. Serum levels of vitamin B12 and folate in the disease groups did not differ significantly from those in the control group (data not shown). The mean lengths of time since the diagnosis of UC and CD were 8·9 (sd 8·8) months and 8·4 (sd 3·5) months, respectively. These patients had thus been ill for many months. Therefore, it appears likely that inflammation-induced effects contributed to the anaemia observed in patients in the present study.
Inflammation has been shown to be associated with decreased duodenal Fe absorption( Reference Ganz 20 ). Hepcidin, induced by inflammation, is thought to mediate this effect( Reference Frazer, Wilkins and Becker 21 ). In the present study, it was possible to measure serum hepcidin levels only in a separate small group of twenty patients with UC and nineteen control subjects. Serum hepcidin levels were significantly lower in these patients than in the corresponding control subjects. These control and UC subjects were similar to the corresponding subjects among the forty-three patients from whom data were obtained for all the parameters of interest. Arnold et al. ( Reference Arnold, Sangwaiya and Bhatkal 22 ) also reported lower serum levels of hepcidin in patients with IBD. Theurl et al. ( Reference Theurl, Aigner and Theurl 8 ) showed that serum hepcidin levels were significantly lower in patients with Fe-deficiency anaemia (IDA) and in those with ACD and IDA than in the control subjects. The present findings are in agreement with these observations. However, other studies have reported elevations in serum hepcidin levels in patients with IBD( Reference Basseri, Nemeth and Vassilaki 23 – Reference Semrin, Fishman and Bousvaros 25 ); however, these studies differ from the present one in many respects. The most prominent difference is that patients with UC in the present study, in whom hepcidin levels were estimated, had a high prevalence of anaemia (77 %). Serum hepcidin levels have been shown to be low in those with IDA and elevated in the presence of inflammation( Reference Ganz, Olbina and Girelli 26 ). In the aforementioned published studies, patients either did not have anaemia( Reference Ganz, Olbina and Girelli 26 ) or such patients were excluded( Reference Oustamanolakis, Koutroubakis and Messaritakis 24 ). In the study by Oustamanolakis et al. ( Reference Oustamanolakis, Koutroubakis and Messaritakis 24 ), a subgroup of patients who had IDA as well as IBD were found to have lower serum hepcidin levels (but did not differ significantly, with P= 0·09) than those without IDA, which is in agreement with the findings of the present study. We postulate that the presence or absence of anaemia is possibly the crucial factor that accounts for the differences observed in serum hepcidin levels in the studies published earlier and the present one, as anaemia is known to have a profound effect on serum hepcidin levels( Reference Shanmugam, Ellenbogen and Trebicka 27 ). It is likely that the initial phase of inflammation in chronic inflammatory disorders is associated with increased hepcidin levels induced by inflammatory stimuli, which contributes to the development of anaemia of inflammation. Once anaemia sets in, homeostatic mechanisms operate to suppress the induction of hepcidin. We suggest that the latter is the situation in which the present study was performed. The present results indicate that when inflammation and anaemia coexist, the effect of the latter on hepcidin overrides that of the former, as has been suggested by Theurl et al. ( Reference Theurl, Aigner and Theurl 8 ). Shanmugam et al. ( Reference Shanmugam, Ellenbogen and Trebicka 27 ) also reported decreased hepcidin expression in the liver in two models of colitis in mice. However, it is not clear how these correlate with serum levels of this peptide and whether such observations have any bearing upon the situation in patients. Of the patients with UC, in whom hepcidin levels were measured, 40 % had mild disease, 50 % had moderate disease and 10 % had severe disease (based on the Truelove–Witts scoring system( Reference Truelove and Witts 13 )). Some of these patients were on standard anti-IBD medication, while others were not. From the data available, it does not appear likely that any of these factors may be responsible for the low levels of serum hepcidin in these patients.
Several mechanisms have been suggested to account for the suppression of hepcidin secondary to anaemia. These include the negative regulatory effects of matriptase 2, growth differentiation factor 15 (GDF15) and twisted gastrulation factor 1 (TWSG1) on hepcidin expression( Reference Tanno, Bhanu and Oneal 28 – Reference Tanno, Porayette and Sripichai 30 ). The latter two are secreted by erythroid precursor cells, with high levels reported in patients with thalassemic syndromes, resulting in the suppression of hepcidin synthesis( Reference Tanno, Bhanu and Oneal 28 , Reference Tanno, Porayette and Sripichai 30 ). Among the patients in whom hepcidin was estimated, nearly 90 % of them had either ACD and Fe deficiency (as indicated by serum ferritin values between 300 and 1000 μg/l in the presence of inflammation) or Fe deficiency only (as indicated by serum ferritin values below 300 μg/l)( Reference Weiss and Goodnough 1 , Reference Gasche, Berstad and Befrits 31 ). It has been reported that serum levels of GDF15 were significantly elevated in patients who had ACD and ACD with IDA( Reference Theurl, Finkenstedt and Schroll 32 ); these levels did not, however, correlate with hepcidin levels in these patients, suggesting that in the presence of inflammation, factors other than GDF15 may be involved in the regulation of hepcidin. One such factor may be the pathway of bone morphogenetic protein/SMAD signalling, known to be involved in inflammation-mediated hepcidin expression( Reference Steinbicker, Sachidanandan and Vonner 33 , Reference Theurl, Schroll and Nairz 34 ). Steinbicker et al. ( Reference Steinbicker, Sachidanandan and Vonner 33 ) showed that the inhibition of bone morphogenetic protein-6 signalling was found to reduce IL-6-mediated hepcidin expression. In the liver of rats with ACD, the phosphorylation of SMAD1/5/8 and the mRNA expression of hepcidin were found to be increased; in rats with ACD with co-existent Fe deficiency, SMAD1/5/8 signalling was found to be reduced, with subsequent decreased hepcidin expression( Reference Theurl, Aigner and Theurl 8 , Reference Theurl, Schroll and Nairz 34 ). It is possible that such mechanisms may be operational in humans with ACD. High levels of erythropoietin, which are observed in response to anaemia, have also been shown to decrease hepcidin expression, possibly by down-regulating CCAAT/enhancer-binding protein α( Reference Pinto, Ribeiro and Pontes 35 ). It would have been useful to estimate the levels of erythropoietin, GDF15 and TWSG1 in the sera of the subjects in the present study. However, it was not possible to do this, as the amounts of serum available were inadequate to carry out these estimations. These aspects are currently under investigation.
In the present study, serum levels of hepcidin were estimated by ELISA, as has been done in previous studies( Reference Ganz, Olbina and Girelli 26 , Reference Galesloot, Vermeulen and Geurts-Moespot 36 ). Mass spectrometric techniques have also been employed to determine serum hepcidin levels( Reference Ganz, Olbina and Girelli 26 ). Levels of serum hepcidin measured by ELISA have been reported to correlate with those measured by mass spectrometry( Reference Koliaraki, Marinou and Vassilakopoulos 37 ). A recent study on a group of geriatric patients has reported that serum hepcidin levels estimated by ELISA did not distinguish between patients with IDA from those with ACD( Reference Geerts, Vermeersch and Joosten 38 ). However, the results of this study on a small group of geriatric patients (who often have co-morbidities that affect Fe homeostasis) may not be representative of the true picture. The findings of the present study and those of Theurl et al. ( Reference Theurl, Aigner and Theurl 8 ), with regard to serum hepcidin levels, show that trends observed in serum hepcidin levels are similar, even though measured by different techniques.
The findings of the present study on the up-regulation of mRNA for the proteins involved in duodenal Fe absorption are in agreement with those of Theurl et al. ( Reference Theurl, Aigner and Theurl 8 ) who observed that in patients with ACD and concurrent Fe deficiency (as assessed by serum ferritin levels and soluble TfR:log ferritin ratios), the expression of duodenal ferroportin was up-regulated, when compared with those with ACD alone. Similar findings were observed in children with CD, where ferroportin protein expression was significantly higher in anaemic children than in non-anaemic subjects( Reference Burpee, Mitchell and Fishman 39 ). Serum ferritin and Fe levels reflect Fe stores in the body. However, these indices are known to be dysregulated in the states of inflammatory stress( Reference Brugnara 40 ). Soluble TfR (levels of which are elevated in Fe deficiency and normal in the conditions of chronic inflammatory stress) or soluble haemojuvelin (levels of which are increased in patients with ACD) may have been better indicators of the true Fe status of subjects in the present study( Reference Punnonen, Irjala and Rajamaki 41 , Reference Brasse-Lagnel, Poli and Lesueur 42 ). It was not possible to estimate these parameters in the present study due to inadequate serum samples.
Theurl et al. ( Reference Theurl, Mattle and Seifert 9 ) reported significantly decreased expression of ferroportin protein in monocytes from patients with ACD. Lowered expression levels would be in keeping with reports that have shown that Fe tends to be sequestered in the reticulo-endothelial system in response to inflammation( Reference Weiss and Goodnough 1 ). In the present study, no significant changes were observed in the levels of mRNA of Fe-related proteins in monocytes from patients with UC. The small sample size in the present study may be a limitation in this respect. Further work with increased numbers of patients would be necessary before definitive conclusions can be made in this regard.
The correlation analysis of the parameters of interest showed that many of them showed significant correlations with one another (Table 4). The levels of Hb negatively correlated with serum CRP. This is in keeping with the development of anaemia during chronic inflammatory stress( Reference Theurl, Theurl and Seifert 7 , Reference Theurl, Aigner and Theurl 8 ). Inflammation is known to negatively affect the proliferation and differentiation of erythroid cells, the production and biological activity of erythropoietin and the expression of Fe-related proteins, all of which are postulated to decrease the levels of Hb( Reference Weiss and Goodnough 1 ). Hb also negatively correlated with the mRNA levels of most of the proteins of interest, suggesting that the presence of anaemia and the consequent increased erythropoietic drive were likely to be responsible for the up-regulation of the proteins. This influence appeared to override the reported tendency of inflammation to down-regulate these proteins. The levels of mRNA of Fe-related proteins in the duodenum showed significant positive correlations with one another, suggesting that the expression of these proteins in response to anaemia occurs in a coordinated fashion.
The strength of the present study is that it was performed in specific inflammatory disorders, unlike earlier studies( Reference Theurl, Theurl and Seifert 7 – Reference Theurl, Mattle and Seifert 9 ) that have been carried out on those with heterogeneous conditions, making it difficult to elucidate mechanisms that may have been involved in the effects observed. In addition, we have studied the genes of interest in the duodenal mucosa as well as in the monocytes of patients with UC, as both these sites are involved in the regulation of Fe homeostasis. Thus, we believe that the present study contributes to an improved understanding of the events involved in the anaemia of inflammation in such conditions.
Clinical management of ACD is often a challenge. Oral supplementation with Fe is often not effective enough to raise Hb and transferrin levels, both of which are goals to be achieved in this respect( Reference Laftah, Ramesh and Simpson 43 , Reference Andrews 44 ). Intravenous preparations of Fe are often used in Fe-refractory settings and have been found to be partially effective( Reference Finberg 45 ). Targeting hepcidin is another potential therapeutic intervention that is increasingly being considered for tackling ACD( Reference Sun, Vaja and Babitt 46 ). Modalities that are currently under investigation in this respect include the use of anti-hepcidin antibodies, RNA interference and antisense oligonucleotides targeted at hepcidin, hepcidin-binding proteins and spiegelmers, inhibitors of signalling pathways involving bone morphogenic proteins and agonists of haemojuvelin and ferroportin( Reference Sun, Vaja and Babitt 46 ). These modalities, if they prove successful, would be effective in the treatment of ACD. The results of the present study suggest that serum hepcidin levels and the true Fe status of patients with chronic inflammatory disorders may be important factors in deciding whether to use a therapeutic intervention that targets hepcidin. Targeting hepcidin would be useful when its levels are elevated, as such an intervention would be likely to prevent the development of ACD. Once anaemia has set in, hepcidin levels tend to be down-regulated, as was observed in a group of patients with UC in the present study. In such a setting, targeting hepcidin may not be beneficial; in contrast, supplementation with Fe may be effective in this setting, as Fe absorption is likely to be increased with the up-regulation of proteins involved in the process. The results of the present study thus indicate that at different time points in the course of chronic inflammatory diseases, the underlying abnormalities involved in causing the dysregulation of Fe homeostasis may differ. A clear understanding of the events involved would enable us to choose appropriate therapies for anaemia of inflammation. It is possible that estimation of serum hepcidin and markers of Fe status in patients with IBD may help distinguish between those with only ACD and those with ACD and co-existent Fe deficiency. It is conceivable that such information would help clinicians decide whether oral Fe supplements would benefit individual patients with IBD.
The present study has its limitations. Although it was designed to obtain data for all the parameters of interest in all the subjects recruited, we were unable to do so for reasons explained earlier. Therefore, the number of patients studied was small. It was also not possible to make correlations between the levels of serum hepcidin and the expression levels of Fe-related proteins in the duodenum, as these were done in different groups of patients with UC. However, the two groups of patients with UC and the two control groups were similar in their clinical profiles and haematological and inflammatory parameters, suggesting that the events involved in the two groups may be similar. Further studies are warranted to more fully elucidate the relationships among the various parameters of interest.
In conclusion, the present data show that the expression of several Fe-related proteins in the duodenum was up-regulated in patients with chronic inflammatory conditions, especially in those with UC and CD, who had a high prevalence of anaemia. The levels of mRNA of many of the proteins of interest seemed to be coordinately increased, probably in response to anaemia. The levels of mRNA of these proteins in the monocytes of patients with UC were not significantly affected. Serum hepcidin levels were found to be significantly lower in a separate group of patients with UC who also had a high prevalence of anaemia. Collectively, these results suggest that when inflammation and anaemia co-exist, the effect of anaemia appears to override that of inflammation on hepcidin and Fe-related proteins. We suggest that these findings may have implications in the context of choosing appropriate therapeutic modalities for the management of ACD.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513003334
Acknowledgements
The authors acknowledge the following faculty members for facilitating routine blood tests done in the diagnostic laboratories at Christian Medical College, Vellore: Dr Sukesh Chandran, Head of the Department of Transfusion Medicine (Hb), Dr R. Selvakumar, Head of the Department of Clinical Biochemistry (serum Fe and ferritin), and Dr Mary Mathews, Head of the Department of Clinical Microbiology (CRP), and their colleagues.
The present study was supported by the Department of Biotechnology (DBT), New Delhi, India (grant no. BT/PR5925/Med/14/716/2005) and a fluid research grant from Christian Medical College, Vellore (IRB minute no. 7372). Neither the DBT nor the research grant committee had any role in the design, analysis or writing of this article. A. S. was supported by a Senior Research Fellowship from the Council of Scientific and Industrial Research, New Delhi, India (grant no. 08/016(0042)/2010 EMR-I, dated 10 March 2010).
The authors' contributions are as follows: A. S. carried out most of the experimental work, analysed and interpreted the data and wrote the manuscript; J. J. helped in carrying out the Western blot work and was involved in the statistical analysis and the interpretation of the data; H. P. J. performed some of the assays and analysed and interpreted the data; A. A. and L. M. S. helped with some of the assays; B. S. R. helped in recruiting the patients, assessing and interpreting the clinical data and revising the manuscript; D. D. helped in recruiting the subjects and read the manuscript; V. J. was involved in the data analysis and interpretation; M. J. conceptualised and designed the study, supervised the work, analysed and interpreted the data and wrote the manuscript.
The authors declare that there are no conflicts of interest.









