Currently approximately 600 000 people in the UK have dementia but it is estimated that by 2026 there will be 840 000, rising to 1.2 million by 2050. 1 Thus increasing numbers of people will die while suffering from dementia. Reference Brayne, Gao, Dewey and Matthews2 It is likely that many will end their lives in an acute hospital where the quality of care may be suboptimal. Reference Sampson, Gould, Lee and Blanchard3 Cognitive impairment and dementia are thought to be common in older people admitted to the acute general hospital, Reference Joray, Wietlisbach and Bula4 but are often not detected or thought to be clinically relevant. Reference Joray, Wietlisbach and Bula4,Reference Harwood, Hope and Jacoby5 Nationally, there are concerns about the lack of provision of general hospital liaison psychiatry services for older people. Reference Bourne6,7 We do not have adequate UK data regarding either the prevalence of cognitive impairment or dementia in older people admitted to acute hospitals, or the reasons for their admission and how these influence short-term mortality. There is evidence that the admission of a person with dementia and acute medical illness to hospital is a critical event associated with high 6-month mortality rates. Reference Morrison and Siu8 Our aim was to investigate the prevalence and associations of cognitive impairment and dementia in this population and test the hypothesis that people with dementia and cognitive impairment who are admitted to the acute hospital have high short-term mortality, even after controlling for other key influences, including the severity of physical illness.
Method
Study population
Recruitment took place at a large north London general hospital that serves an area of socioeconomic and ethnic diversity. All individuals aged over 70 with unplanned acute admission to the medical acute admissions unit were eligible for inclusion. The majority of acute medical admissions at this National Health Service (NHS) Trust pass from accident and emergency through this unit before being distributed to the ‘home ward’ of the most appropriate medical specialty (health services for elderly people, rheumatology, neurology, hepatology, nephrology, cardiology, diabetes/endocrinology and gastroenterology). Thus we did not recruit those admitted to surgical specialties, gynaecology, ear nose and throat or ophthalmology. The cohort was recruited from 4 June 2007 to 4 December 2007. Participants were excluded if they were admitted for less than 48 h (this was to exclude people undergoing brief admission to the ward) or did not speak sufficient English for basic cognitive assessment.
Study procedures
All participants were assessed within 72 h of admission by an old age psychiatrist and all clinical assessments were conducted before consulting the medical notes. All participants were first screened with the Confusion Assessment Method (CAM). Reference Inouye, van Dyck, Alessi, Balkin, Siegal and Horwitz9 This has a sensitivity of over 94% and a specificity over 90% for the detection of delirium and also distinguishes accurately between delirium and dementia/cognitive impairment. Reference Inouye, van Dyck, Alessi, Balkin, Siegal and Horwitz9 We used the version that maximises sensitivity. This would exclude participants whose cognitive impairment may be caused by delirium and thus minimises misclassification of delirium as dementia. Participants who screened positive for delirium were reassessed 4 days later. If they remained positive for delirium, they were excluded from further analysis.
Definition of cognitive impairment
The Mini-Mental State Examination (MMSE) is the most widely used screening test for cognitive impairment. Reference Folstein, Folstein and McHugh10 It has a maximum score of 30. Normal cognition was defined as MMSE >24, moderate cognitive impairment as 16–23 and severe cognitive impairment as 0–15. Reference Hofman, Rocca, Brayne, Breteler, Clarke and Cooper11 A hearing amplifier and large print versions were used for participants with hearing or visual impairments. If people were unable to complete particular items, scores were adjusted taking into account the change in denominator.
Diagnosis of dementia
A diagnosis of dementia was generated after a structured clinical assessment based on operationalised DSM–IV criteria. 12 Information on premorbid social function and activities of daily living was gathered from relatives (if available) or carers and review of hospital notes and occupational therapy reports. Severity of functional impairment was measured using the Functional Assessment Staging (FAST) scale. Reference Reisberg, Sclan, Franssen, Kluger and Ferris13 This is an observational scale that describes a continuum of seven successive stages and substages of dementia, from normality to the most severe dementia.
Other explanatory and outcome variables
Demographic data (age, gender, place of residence) were gathered from the hospital notes. All participants admitted acutely to the hospital received standardised assessment of continence and risk of pressure sores using the Waterlow Scale. Reference Waterlow14 The severity of chronic comorbidity was calculated using the Charlson Co-Morbidity scale, Reference Charlson15 which includes 19 diseases weighted on the basis of their strength of association with mortality. The Acute Physiology and Chronic Health Evaluation (APACHE II) system measures the severity of acute illness using 12 routine physiological parameters, including core temperature, respiratory rate, mean arterial pressure, Glasgow Coma Scale and laboratory values (serum sodium, potassium, creatinine and haematocrit). Reference Knaus, Draper, Wagner and Zimmerman16 We used a modified version as arterial blood gas sampling was not routinely performed on all participants. Reference Adamis, Treloar, Darwiche, Gregson, Macdonald and Martin17 Length of hospital stay and mortality were collected from hospital administrative data.
Diagnosis on admission
Data were collected from Hospital Episode Statistics using the primary ICD–10 18 diagnosis coded for the index admission and categorised according to the Ambulatory Care Sensitive Condition system. Reference Sanderson and Dixon19 We examined the three most common Ambulatory Care Sensitive Condition categories in this cohort; pneumonia (ICD–10 codes J10, 11, 13, 14, 15.3, 15.4, 15.7, 15.9, 16.8, 18.1, 18.8), urinary tract infection (ICD–10 codes N39.0) and acute ischaemic heart disease (I20, 24.0, 24.8, 24.9).
Data analysis
Pearson chi-squared tests were used to examine the relationship between categorical data. Analysis of variance (ANOVA) was used for group differences between continuous variables. Data on length of hospital admission were skewed and thus non-parametric analyses (Mann–Whitney and Kruskall–Wallis tests) were performed. The main exposures were defined as cognitive impairment and dementia and the primary outcome was mortality. Univariate techniques (Mantel–Haenszel) were used to identify potential confounders. Cox regression methods were used to examine the risk of mortality during the index hospital admission. The proportional hazards assumption was checked for each exposure using y-log scale Nelson–Aalen plots. Unadjusted models were used first to examine the effect of cognitive impairment or dementia on mortality risk. Factors identified as potential confounders (significance set at P<0.1 level) were included in the final multivariate models. There was no evidence of any significant interaction terms in the adjusted models. Wald tests were used to examine the significance of the Cox regression models. Data were analysed using STATA version 9 for Windows.
Ethical issues
We sought verbal consent from participants or, if they lacked capacity to consent, verbal assent from their family carers or their key nurse on the ward to ensure that it would not be inappropriate to carry out an assessment. Thus, to minimise selection bias, we were able to collect data on people who may not have had capacity to consent to participate or a family carer to assent for them. The study involved the collection of routine clinical data that have subsequently been fully anonymised. Screening for cognitive impairment, dementia and delirium should be routine on hospital admission. 20 The findings of these assessments were documented on the medical notes so that clinical teams could act on them if they wished. The exclusion of individuals unable to give written informed consent or those without a relative to give assent for their participation may have caused selection bias, excluding the patient population we wished to study. The study was approved by the Royal Free Hospital NHS Trust Ethics Committee.
Results
Study population
A total of 805 people over the age of 70 years had unplanned admissions to the hospital lasting more than 48 h during the 6-month recruitment period. Of these, 45 were discharged before they could be assessed, leaving 760 (94.5%) individuals for further assessment. Of these, 30 people refused to be assessed (3.7%) and 20 did not speak adequate English (2.5%). Therefore 710 (88.2%) were screened to detect and exclude those with delirium (Fig. 1). The remaining 617 people (76.7%) were included in this analysis.
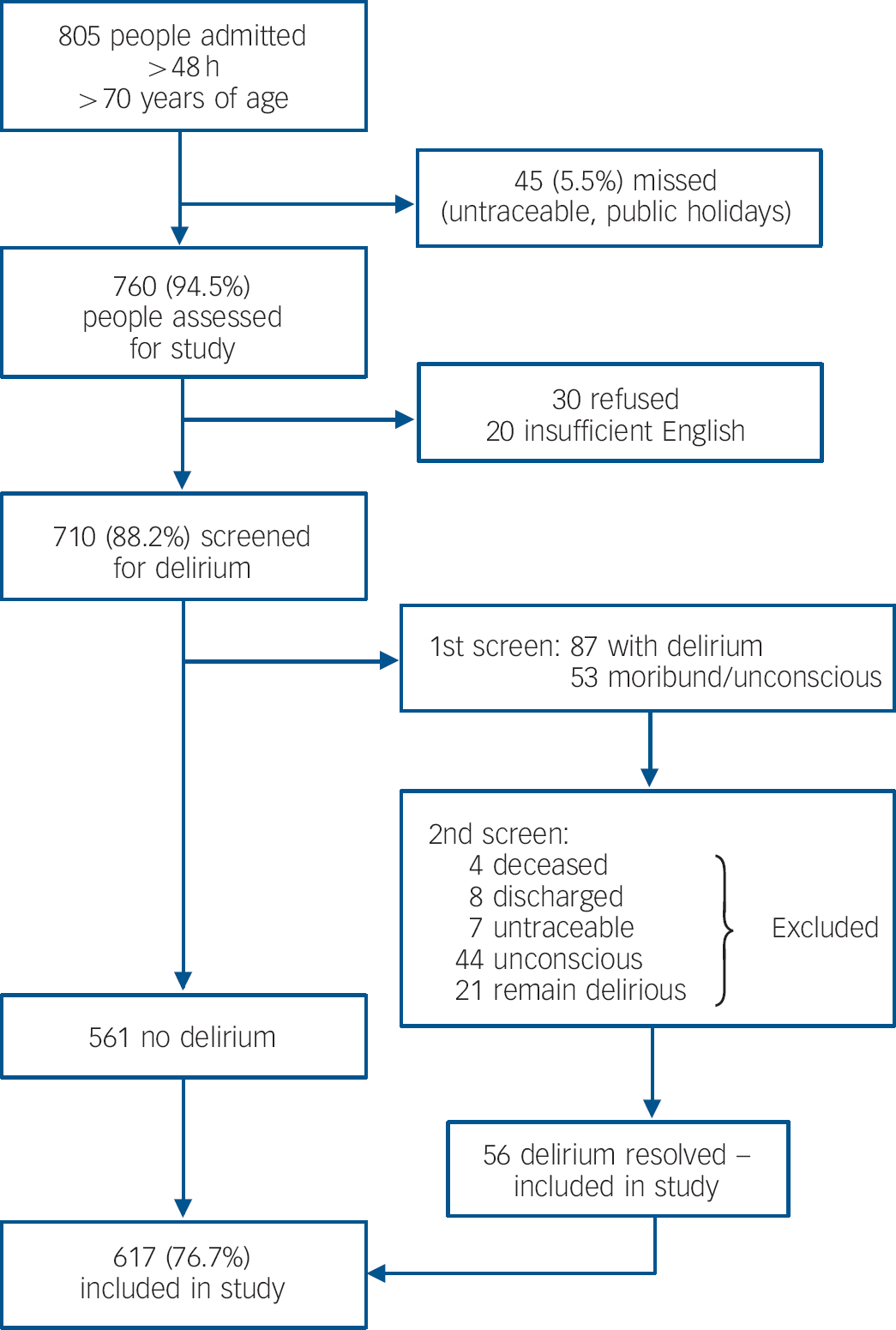
Fig. 1 Study flowchart.
People excluded from this analysis (refused to participate, insufficient English, persistent delirium or moribund) were not significantly different from those who were included with respect to age (83.3 years v. 83.1 years, F = 1.08, χ2(1) = 1.76, P = 0.18), female gender (58.1% v. 59.1%, χ2(1) = 0.04, P = 0.84), Charlson score (2.8 v. 2.7, F = 2.9, χ2(1) = 3.91, P = 0.05) or APACHE II score (13.1 v. 12.0, F = 5.4, χ2(1) = 2.28, P = 0.13). There was, however, significantly increased in-hospital mortality during the index admission in those who were excluded from this analysis (20.4% v. 9.4%, χ2(1) = 10.17, P = 0.001).
Characteristics of the cohort are presented in Table 1. The mean age of participants was 83.0 years (range 70–101 years) and 59% were female. The majority of participants resided in their own homes but over 20% of participants were from residential or nursing homes. Nearly half of the cohort had signs of cognitive impairment, with 23% having moderate impairment and 25% being severely impaired. DSM–IV dementia was present in 42% of participants. Fewer than half of these (21.1%) had received a diagnosis of dementia prior to the study assessments. Functional Assessment Staging scores demonstrated that over a quarter of the cohort were moderately or severely impaired in activities of daily living.
Table 1 Cohort characteristics (n = 617)

| Variables | |
|---|---|
| Gender, female: % | 59.0 |
| Age, years: % | |
| 70-79 | 37.1 |
| 80-89 | 43.5 |
| 90+ | 19.5 |
| Place of residence, % | |
| Private home | 71.0 |
| Sheltered housing | 7.6 |
| Residential home | 6.8 |
| Nursing home | 14.7 |
| Mini-Mental State Examination score, % | |
| 24-30 | 52.1 |
| 16-23 | 22.8 |
| 0-15 | 25.1 |
| DSM—IV criteria met for dementia, % | |
| No | 57.6 |
| Yes | 42.4 |
| Known diagnosis of dementia prior to index admission, % | 21.1 |
| Functional Assessment Staging score, % | |
| 1. No functional impairment | 42.8 |
| 2-5. Subjective functional deficit, objective functional deficit, difficulties with activities of daily living | 29.2 |
| 6a—c. Help required putting on clothes, toileting or bathing | 11.1 |
| 6d—e. Urinary and faecal incontinence | 10.3 |
| 7a—f. Speaks 5-6 words, speaks 1 word, can no longer walk, sit up, smile, hold up head | 6.5 |
| Charlson score, mean (s.d.) | 2.8 (2.1) |
| Acute Physiology and Chronic Health Evaluation II score, mean (s.d.) | 12.1 (3.7) |
Table 2 demonstrates a significant increase in both cognitive impairment and dementia with age; in participants over the age of 80 years, 49% were cognitively impaired and 48% met the DSM–IV criteria for dementia. Prevalences were markedly higher in women across all age groups.
Table 2 Prevalence of cognitive impairment and dementia by age and gender in 617 participants with acute medical admissions

| % (95% CI) | |||||
|---|---|---|---|---|---|
| Mini-Mental State Examination score | DSM—IV criteria met | ||||
| Age | 24-30 | 16-23 | 0-15 | No | Yes |
| Total cohort (n = 617) | |||||
| 70-79 years (n = 229) | 69.6 (63.5-75.6) | 18.9 (13.8-24.1) | 11.4 (7.2-15.6) | 76.7 (71.2-82.2) | 23.2 (17.7-28.7) |
| 80-89 years (n = 268) | 48.5 (42.4-54.5) | 22.3 (17.3-27.4) | 29.1 (23.6-34.5) | 51.6 (45.6-57.6) | 48.3 (42.3-54.3) |
| 90+ years (n = 120) | 26.8 (18.8-34.9) | 31.1 (22.6-39.5) | 42.0 (33.0-51.1) | 34.4 (25.7-43.1) | 65.5 (56.8-74.2) |
| Men | |||||
| 70-79 years (n = 111) | 75.4 (67.3-83.6) | 15.4 (8.6-22.2) | 9.1 (7.4-19.9) | 83.6 (76.6-90.6) | 16.4 (9.4-23.3) |
| 80-89 years (n = 98) | 56.6 (46.7-66.4) | 24.2 (15.7-32.7) | 19.2 (11.4-27.0) | 59.6 (49.8-69.3) | 40.4 (30.6-50.2) |
| 90+ years (n = 44) | 37.2 (22.4-51.9) | 32.5 (18.2-46.8) | 30.2 (16.2-44.3) | 51.2 (35.9-66.4) | 48.8 (33.6-64.1) |
| Women | |||||
| 70-79 years (n = 118) | 64.1 (55.3-72.8) | 22.2 (14.6-29.8) | 13.7 (7.4-19.9) | 70.3 (62.0-78.6) | 29.6 (21.3-37.9) |
| 80-89 years (n = 170) | 43.8 (36.2-51.3) | 21.3 (15.1-27.5) | 34.9 (27.7-42.1) | 47.1 (39.5-54.6) | 52.9 (45.4-60.5) |
| 90+ years (n = 76) | 21.0 (11.7-30.4) | 30.3 (19.8-40.7) | 48.6 (37.2-60.1) | 25.0 (15.1-34.9) | 75.0 (65.1-84.9) |
Clinical characteristics and outcomes
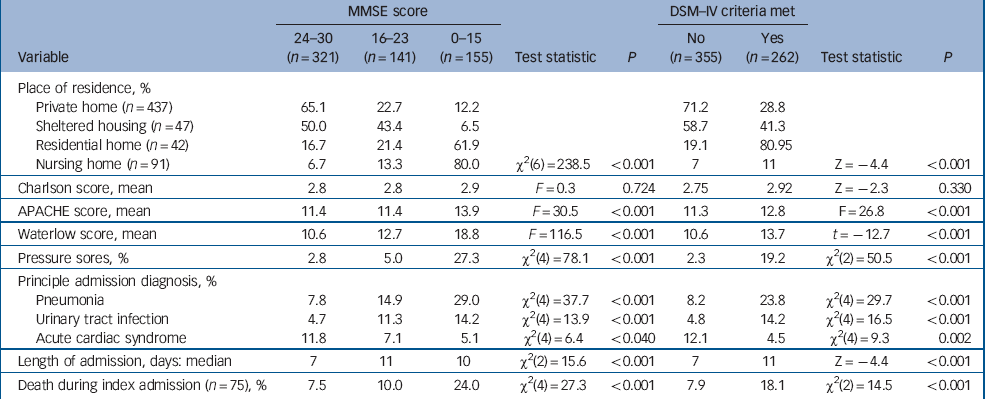
The three most common causes of admission were pneumonia (16.0%), urinary tract infection (9.1%) and acute cardiac ischaemia (9.2%). Admissions for pneumonia or urinary tract infections were associated with increasing severity of cognitive impairment and dementia (Table 3).
Table 3 Clinical characteristics and outcomes of 617 participants with acute medical admissions
| MMSE score | DSM—IV criteria met | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 24-30 (n = 321) | 16-23 (n = 141) | 0-15 (n = 155) | Test statistic | P | No (n = 355) | Yes (n = 262) | Test statistic | P |
| Place of residence, % | |||||||||
| Private home (n = 437) | 65.1 | 22.7 | 12.2 | 71.2 | 28.8 | ||||
| Sheltered housing (n = 47) | 50.0 | 43.4 | 6.5 | 58.7 | 41.3 | ||||
| Residential home (n = 42) | 16.7 | 21.4 | 61.9 | 19.1 | 80.95 | ||||
| Nursing home (n = 91) | 6.7 | 13.3 | 80.0 | χ2(6) = 238.5 | <0.001 | 7 | 11 | Z = -4.4 | <0.001 |
| Charlson score, mean | 2.8 | 2.8 | 2.9 | F = 0.3 | 0.724 | 2.75 | 2.92 | Z = -2.3 | 0.330 |
| APACHE score, mean | 11.4 | 11.4 | 13.9 | F = 30.5 | <0.001 | 11.3 | 12.8 | F = 26.8 | <0.001 |
| Waterlow score, mean | 10.6 | 12.7 | 18.8 | F = 116.5 | <0.001 | 10.6 | 13.7 | t = -12.7 | <0.001 |
| Pressure sores, % | 2.8 | 5.0 | 27.3 | χ2(4) = 78.1 | <0.001 | 2.3 | 19.2 | χ2(2) = 50.5 | <0.001 |
| Principle admission diagnosis, % | |||||||||
| Pneumonia | 7.8 | 14.9 | 29.0 | χ2(4) = 37.7 | <0.001 | 8.2 | 23.8 | χ2(4) = 29.7 | <0.001 |
| Urinary tract infection | 4.7 | 11.3 | 14.2 | χ2(4) = 13.9 | <0.001 | 4.8 | 14.2 | χ2(4) = 16.5 | <0.001 |
| Acute cardiac syndrome | 11.8 | 7.1 | 5.1 | χ2(4) = 6.4 | <0.040 | 12.1 | 4.5 | χ2(4) = 9.3 | 0.002 |
| Length of admission, days: median | 7 | 11 | 10 | χ2(2) = 15.6 | <0.001 | 7 | 11 | Z = -4.4 | <0.001 |
| Death during index admission (n = 75), % | 7.5 | 10.0 | 24.0 | χ2(4) = 27.3 | <0.001 | 7.9 | 18.1 | χ2(2) = 14.5 | <0.001 |
There was a much higher prevalence of cognitive impairment and dementia in people admitted from residential and nursing homes than those admitted from other settings (Table 3). There were no differences between the groups in terms of chronic health status (Charlson score) but participants with cognitive impairment and dementia had higher Waterlow scores, greater risk of pressure sores on admission and significantly higher APACHE scores, suggesting a higher burden of acute physiological disturbance (Table 3). Median length of admission was shorter in people who were cognitively intact.
Mortality
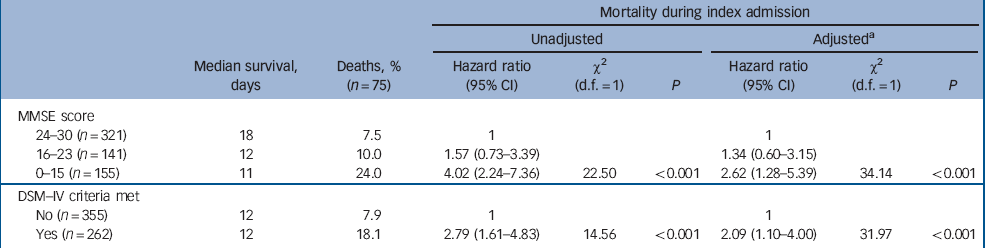
During the index admission 75 people (12.2%) died. Risk of death significantly increased with the level of cognitive impairment (24.0% of those with an MMSE score of 0–15 and 18.1% with dementia had died within 14 days) (Table 4). Of those individuals who died, a significantly higher proportion were admitted from residential (20.8%) or nursing homes (19.3%) compared with those residing in their own homes (9.1%) or in sheltered accommodation (4.0%) (χ2(8) = 17.2, P = 0.002).
Table 4 Cox proportional hazard models for death during index admission associated with cognitive impairment and dementia in people over 70 years of age during acute hospital admission
| Mortality during index admission | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||||
| Median survival, days | Deaths, % (n = 75) | Hazard ratio (95% CI) | χ2 (d.f. = 1) | P | Hazard ratio (95% CI) | χ2 (d.f. = 1) | P | |
| MMSE score | ||||||||
| 24-30 (n = 321) | 18 | 7.5 | 1 | 1 | ||||
| 16-23 (n = 141) | 12 | 10.0 | 1.57 (0.73-3.39) | 1.34 (0.60-3.15) | ||||
| 0-15 (n = 155) | 11 | 24.0 | 4.02 (2.24-7.36) | 22.50 | <0.001 | 2.62 (1.28-5.39) | 34.14 | <0.001 |
| DSM—IV criteria met | ||||||||
| No (n = 355) | 12 | 7.9 | 1 | 1 | ||||
| Yes (n = 262) | 12 | 18.1 | 2.79 (1.61-4.83) | 14.56 | <0.001 | 2.09 (1.10-4.00) | 31.97 | <0.001 |
In the unadjusted Cox regression model, there was a significant increase in mortality risk during the index admission with increasing levels of cognitive impairment. Compared with those scoring 24 and over on the MMSE, those scoring 0–15 on the MMSE had a hazard ratio for mortality of 4.02 (95% CI 2.24–7.36, χ2 = 22.5, P<0.001). In univariate analyses of the explanatory variables only age and APACHE score were identified as potential confounders of the association between cognitive impairment or dementia and mortality at the P<0.1 level. These were added to give the adjusted multivariate model. Other putative confounders such as place of residence (i.e. community dwelling v. nursing home residents), length of hospital stay, chronic comorbidity and function did not have any impact and were not included in the final model. After this adjustment, mortality risk remained higher in those with cognitive impairment and was significantly increased in those with a DSM–IV diagnosis of dementia.
Discussion
Acute medical in-patients with cognitive impairment and dementia have high short-term mortality after acute medical admission. Over three times as many people with dementia (18%) and five times as many with MMSE scores of 0–15 (24%) died during their index admission. This association remained significant after controlling for age and severity of acute physical illness. Other studies have found a similar association between cognitive impairment, dementia and 6-month mortality in community populations and after hospital admission for pneumonia or hip fracture Reference Morrison and Siu8,Reference Neale, Brayne and Johnson21,Reference Nightingale, Holmes, Mason and House22 but the association with short-term mortality has not previously been reported.
Why do these individuals have such high mortality? It is possible that they had been identified as terminally ill and that curative or invasive care was appropriately withdrawn. We believe this is unlikely and have demonstrated how people with dementia receive more active interventions and less palliative care compared with similar individuals without dementia. Reference Sampson, Gould, Lee and Blanchard3 The combination of frailty, acute physical illness and dementia may be damaging, or the interaction between the acute hospital environment and people with dementia particularly ‘malignant’. This finding may reflect how people with dementia receive poorer quality care in the acute hospital and the lack of skill of acute hospital staff in caring for people with dementia has been widely documented. Reference Bourne6 People with dementia receive suboptimal end-of-life care Reference Sampson, Gould, Lee and Blanchard3 and are at greatly increased risk of adverse events, iatrogenic harm and greater functional decline after acute hospital admission. Reference Creditor23
Dementia and cognitive impairment are common in this acute hospital population and, as would be expected, increase markedly with age, reflecting and exaggerating gender differences found in the community. For example, 29.6% of women and 27.5% of men aged 90–94 years residing in the community would be expected to have dementia. Reference Knapp and Prince24 In our acute hospital population 45% of men and 75% of women over the age of 90 years had dementia. The prevalence of dementia (91.9%) and cognitive impairment (80%) was very high in participants admitted from nursing homes. Our estimates may be higher than those of previous studies because our population was older and we did not exclude people admitted from institutional care. Over half of the people with dementia in this cohort had not previously received a diagnosis. Early recognition of dementia in the community is preferable but acute hospital admission may be an opportunity to identify undiagnosed cases. This would help to close the ‘dementia gap’ where currently in the UK two-thirds of sufferers do not receive a formal diagnosis and have difficulty accessing specialist services and support. Reference Bourne6 The UK National Audit Office has highlighted, using a detailed hospital case study and economic modelling, how screening older people admitted to the acute hospital for dementia and taking a ‘whole system approach’ to their further management could accrue significant cost savings and improve outcomes. Reference Bourne6 Of course, this approach relies on appropriately skilled acute hospital medical and nursing staff and adequate provision of liaison psychiatry teams for older people.
Cognitive impairment and dementia were significantly associated with admission for pneumonia and urinary tract infection, which are common in this patient group. Reference Carter and Porell25 In our cohort 43% of admissions of people with dementia were caused by these. These are ‘Ambulatory Care Sensitive Conditions’ for which admissions are thought to be avoidable or manageable with prompt access to medical care, i.e. those that could have been prevented or treated in the community. They are used to evaluate outcomes and quality of health service provision. People with dementia in residential and nursing homes use fewer health services compared with those who are cognitively intact, despite higher levels of physical illness Reference Nelson, Livingston, Knapp, Manela, Kitchen and Katona26 and our findings support calls by the UK National Audit Office to improve intermediate and community care for people with dementia in order to reduce emergency admissions. Reference Bourne6
Strengths and limitations
Although the sample was large and follow-up rates were high over a 6-month period, the study was conducted in a single acute hospital. Thus, it is possible that the increased mortality risk of those with dementia and cognitive impairment is atypical. However, the NHS trust in which this work was conducted receives individuals for acute medical admission from five separate primary care trusts covering a total population of 1.2 million and has one of the lowest standardised mortality ratios in the UK, therefore risk may actually be higher in other settings. 27,28 Residual confounding is possible; in particular we could not measure all possible confounders such as concurrent depressive illness or nutritional status.
Our participants had a comprehensive cognitive and functional assessment, using informant information, where available, in a challenging environment. Cognitive impairment has a wide range of causes (some reversible), whereas ‘dementia’ implies progressive neurodegeneration causing global cognitive and functional decline. Although there are more methodologically rigorous semi-structured techniques for diagnosing dementia (for example the Geriatric Mental State–Automated Geriatric Examination for Computer Assisted Taxonomy (GMS–AGECAT) system), Reference Copeland, Dewey, Henderson, Kay, Neal and Harrison29 and the use of these may have reduced the risk of diagnostic error, we chose our methodology for a number of reasons. We had to be pragmatic about screening the large number of people passing through the medical acute admissions unit, which has a rapid patient turnover rate, in a challenging and busy environment and we wished to minimise selection bias by screening as high a percentage of individuals as possible. We also wished to demonstrate how the use of a widely available cognitive screening tool, such as the MMSE, may be useful in the acute hospital and have clinical utility. For example, in this study it appears to demonstrate important differences in risk of short-term mortality. The DSM–IV dementia criteria have high interrater reliability and agreement with the gold standard NINCDS–ADRDA Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan30 (National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences) criteria but, compared with ICD–10, may identify more cases, particularly of mild dementia, Reference Erkinjuntti, Ostbye, Steenhuis and Hachinski32 mainly because ICD–10 requires duration of at least 6 months. Reference Stevens, Livingston, Kitchen, Manela, Walker and Katona33
We excluded individuals with persistent delirium to avoid misclassification of delirium as dementia. However, given that people with pre-existing dementia are at much higher risk of developing delirium, Reference Fick, Agostini and Inouye34 we may have underestimated the prevalence of dementia and the associated mortality risk.
Clinical implications
Dementia is common in acute medical in-patients over the age of 70 years and is associated with more severe acute physical illness and longer hospital stays. We cannot judge from our data whether these admissions were necessary, but the high short-term mortality risk in people with dementia suggests that this intervention did not prolong life for a meaningful length of time. Individuals may have received better-quality care in a familiar environment if more support was available in the community. Issues surrounding place of care and place of death have been discussed in the UK Government End of Life Care Strategy. 35 It includes consideration of ways to support care of the frail elderly at the end of life in both primary and secondary care, in particular those in residential or nursing homes. The inevitable rise in dementia prevalence and its associated functional disability and behavioural and psychiatric problems will challenge acute hospital staff, require effective implementation of the Mental Capacity Act and improvements at the interface between social, community and mental health services. Numerous recent reports have highlighted how people with dementia in the acute hospital receive poor-quality care Reference Bourne6 and many general hospitals in the UK do not have mental health liaison services for older people. 7 Significant investment will be required to meet future need.
Funding
E.L.S. was funded by a Medical Research Council (UK) Special Training Fellowship in Health Services Research. The study sponsor had no influence on the study design, collection, analysis and interpretation of data, the writing of the report or the decision to submit the paper for publication.
Acknowledgements
We thank Dr Dan Lee (Health Services for Elderly People, Royal Free Hospital NHS Trust), the staff of the Medical Acute Admissions Unit and Ginnette Kitchen, Lucy Watkin, Noel Collins, Jenny Drife and Viv Green for assistance with data collection and processing.








eLetters
No eLetters have been published for this article.