Diseases of the circulatory system and cancer are among the leading causes of death in Brazilian men according to the last Brazilian mortality database (2012). It is estimated that nearly one quarter of deaths caused by these diseases affect adult men aged between 40 and 59 years( 1 ). Eating habits can have a significant influence in the development of most chronic diseases, affecting peoples' health throughout their lifetime( Reference Bressan, Hermsdorff and Zulet 2 ). Thus, the adoption of a healthy diet, including fruits and vegetables, can play an important role in preventing these diseases in middle-aged individuals, since the consumption of these foods have been inversely associated with chronic diseases( Reference Hermsdorff, Zulet and Puchau 3 , Reference Jung, Kim and Tae 4 ) and oxidative stress( Reference Cocate, Natali and Oliveira 5 , Reference Hermsdorff, Barbosa and Volp 6 ).
Fruits and vegetables are sources of nutritional components with high antioxidant capacity such as carotenoids( Reference Hughes 7 ), which have the power of singlet oxygen quenchers, and are scavengers of a variety of reactive oxygen species that can be involved in modern chronic disease manifestation( Reference Fiedor and Burda 8 ). Among over 600 carotenoids naturally identified( Reference Jomova and Valko 9 ), the β-carotene, β-cryptoxanthin, α-carotene, lycopene and lutein plus zeaxanthin are the major ones found in human plasma or serum( Reference Parker 10 ). Regarding fat-soluble compounds, the lycopene was considered as having the strongest singlet oxygen-quenching ability, followed by α-carotene, β-carotene, zeaxanthin, lutein and β-cryptoxanthin( Reference Di Mascio, Murphy and Sies 11 ). Furthermore, carotenoids can affect transcription factors (NF-κB or nuclear erythroid-2-related factor 2) and their downstream targets, promoting health benefits by reducing oxidative stress and inflammation( Reference Kaulmann and Bohn 12 ).
Thus, the antioxidant and anti-inflammatory properties of carotenoids may prevent lipid oxidation and minimise cardiometabolic risks( Reference Ciccone, Cortese and Gesualdo 13 ). In fact, carotenoids have also been associated with lower concentrations of inflammatory markers( Reference Calder, Ahluwalia and Brouns 14 ), and lower chronic disease risks such as the metabolic syndrome (MetS)( Reference Coyne, Ibiebele and Baade 15 , Reference Sluijs, Beulens and Grobbee 16 ), CVD( Reference Jacques, Lyass and Massaro 17 , Reference Osganian, Stampfer and Rimm 18 ) and cancer( Reference Michaud, Feskanich and Rimm 19 ). However, to our knowledge, the association of carotenoid intake with lipid and oxidative stress markers in apparently healthy subjects has not been clarified yet.
Therefore, we investigated the relationship of carotenoid consumption with lipid and oxidative stress markers in middle-aged men. We hypothesised that there is an inverse association of carotenoids consumption with lipid and oxidative stress biomarkers.
Subjects and methods
Participants
The present cross-sectional study was carried out between March and December 2011. The sample size was calculated( Reference Dean, Dean and Colombier 20 ) in February 2011, considering the total number of male staff (1744 individuals), aged between 40 and 59 years, at the Universidade Federal de Viçosa, Viçosa, Brazil, 95 % confidence level, 24·4 % expected prevalence of metabolic condition highly related with oxidative stress( Reference Sá and Moura 21 )(MetS) and 4·5 % sampling error.
We excluded from the study participants with self-reported body weight alterations greater than 3 kg in the 3 months preceding the study; altered levels of physical activity and eating habits in the 3 months preceding the study; thyroid diseases, heart failure, cerebrovascular diseases, infectious diseases, inflammatory diseases, diseases of the gastrointestinal tract, liver disease, chronic kidney disease and/or a history of kidney stones and cancer in the previous 10 years; eating disorders (anorexia and bulimia) and food allergies. Individuals using vitamin supplements, those using diuretics or drugs that alter food intake and/or the metabolism of nutrients, those with pacemakers and/or prosthetic limbs and elite athletes were also excluded from the study.
The sample size calculated for this study was of 293 participants. This sample size was increased by 2·5 % of subjects (seven individuals) to allow for possible withdrawals from the study. Then, we selected a total of 300 men based on inclusion criteria using systematic sampling. Four subjects who did not answer the FFQ were excluded later. Therefore, the final sample comprised 296 subjects.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee on Human Research of the Universidade Federal de Viçosa (Reference n° 069/2010). Written informed consent was obtained from all subjects.
Dietary and lifestyle data assessment
An FFQ developed for the Brazilian population( Reference Ribeiro and Cardoso 22 ), which was adapted and published by Queiroz( Reference Queiroz 23 ), was used to assess the usual dietary intake of the participants of the present study, taking into account their habitual intake in the previous 6 months. We evaluated 105 items from the following food-groups: milk and dairy products, fats, breads and bread substitutes, cereals, fruits, legumes, vegetables, meats, eggs, beverages and sweets. The FFQ was administered once to each participant between March and December 2011 (study period). Daily food intake was estimated as frequency × portion × size for each consumed food item. Nutrient intakes including carotenoid intake (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene) were assessed using the United States Department of Agriculture table( 24 ), since these data are not included in Brazilian food tables. The foods in the FFQ which were not listed in the United States Department of Agriculture table were estimated considering appropriate values of foods that had similar nutritional composition and were prepared using similar cooking methods. Intake of each nutrient was evaluated by a standard spreadsheet software (Excel 2003; Microsoft Corporation).
Data about lifestyle factors, including work position and current smoking status, were collected using a questionnaire administered by a trained interviewer. The subjects of the present study occupied technical/administrative positions, classified as levels A, B, C, D, and E, or were professors. They were divided into two groups according to their education level and work positions: the first group ABC was composed of technical and administrative staff, levels A, B and C, indicating an education level up to high school; the second group DEprof was composed of technical and administrative staff of two levels D and E, and the abbreviation ‘prof’ stood for professors; subjects in this second group had all completed graduation or post-graduation. Participants were also categorised as current smokers and non-smokers. Finally, habitual physical activity was estimated by the mean number of daily steps (seven consecutive days) measured by a Digi-Walker SW-200 pedometer (Yamax Corporation), according to instructions previously described( Reference Cocate, de Oliveira and Hermsdorff 25 ).
Anthropometric and biomedical data collection
Anthropometric measurements such as weight, height, and waist circumference as well as systolic and diastolic blood pressures were taken using standard measurement procedures( Reference Cocate, de Oliveira and Hermsdorff 25 ). BMI was calculated as weight (kg) divided by height squared (m2). Total body fat percentage was determined by total body scanning with a dual energy X-ray absorptiometry (enCORE software version 13.31; GE/Lunar) in accordance with the manufacturer's instructions.
A venous blood sample was taken after a 12-h overnight fast for measuring total cholesterol, HDL-cholesterol (HDL-C), TAG, NEFA and oxidised-LDL (ox-LDL). Serum total cholesterol, HDL-C and TAG were determined by the enzymatic colorimetric method (Cobas Mira Plus; Roche Diagnostics GmbH). NEFA concentration was determined by the kinetic spectrophotometry method (EnzyChromFree Fatty Acid Assay; Bioassay Systems), while ox-LDL was assessed by the commercially available ELISA kit (Mercodia) based on the direct sandwich technique.
Castelli index (determined by the ratio between total cholesterol and HDL-C)( Reference Castelli, Abbott and McNamara 26 )and TAG:HDL-C ratio( Reference Gaziano, Hennekens and O'Donnell 27 ), both recognized as atherogenic markes( Reference da Luz, Favarato and Faria-Neto 28 , Reference Malaspina, Bussiere and Le Calve 29 ), were calculated. The MetS was diagnosed by the Alberti et al. ( Reference Alberti, Eckel and Grundy 30 )criteria.
In turn, urine was collected in sterile tubes (after a 12-h overnight fasting) for measuring 8-iso-PGF2α and 8-OHdG, the biomarkers of oxidative stress.
Competitive ELISA was used to determine urinary concentrations of 8-iso-PGF2α (Oxford) and 8-OHdG (Cayman). The analyses were performed according to the manufacturers' instructions. Although the Cayman's kit recognises the 8-OHdG from DNA, the ELISA values are always higher than LC/MS inasmuch as this method also detects 8-hydroxyguanosine and 8-hydroxyguanine from either DNA or RNA. The values of urinary 8-iso-PGF2α and 8-OHdG were normalised by mg of urinary creatinine, measured by a kinetic colorimetric method, using a Bioclin commercial kit (Cobas Mira Plus; Roche Diagnostics GmbH).
Statistical analyses
Normal distribution of the data was assessed by the Shapiro–Wilk test. Non-normally distributed variables were log-transformed before statistical analysis. β-Cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene intakes and the sum of these carotenoids consumption was adjusted by daily energy intake using the residual method( Reference Willett 31 ) before statistical analysis. To evaluate the association of these antioxidant compounds with lipid and oxidative stress markers, we used linear multiple regressions, controlled by habitual physical activity (steps/d), work position, daily energy intake (kcal/d), total body fat (percentage) and current smoker status (yes/no).
A stepwise multiple regression analysis was also used to identify the key food-groups and food items from relevant consumptions by the participants that explained carotenoid intake. The participants were then categorised into tertiles based on five investigated carotenoids (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene). Subsequently, as a complementary analysis, we used the χ2 test for linear trend to compare proportions among total carotenoid consumption and MetS.
Energy consumption outliers were defined as previously described( Reference Cocate, Natali and Oliveira 32 ). Data processing and analysis were performed using the software STATA version 9.1 (Stata Corporation), considering P< 0·05.
Results
Anthropometric, clinical and lifestyle characteristics of the study participants are shown in Table 1. Mean total carotenoids consumed by the study subjects was 7500 (sd 5435) μg/d. Regarding the food-groups, we found that the mean fruit and vegetable intake corresponded to 310·9 g/d, and that subjects consumed 54·1 % of their total energy from carbohydrate, 27·5 % from fat and 18·4 % from protein (Table 2).
Table 1 Anthropometric, clinical and lifestyle characteristics of participants (Mean values and standard deviations, n 296)
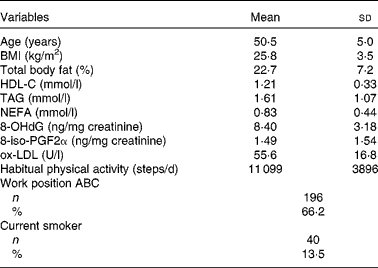
HDL-C, HDL-cholesterol; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; ox-LDL, oxidised LDL; Work position ABC, technical-administrative positions A, B or C.
Table 2 Carotenoids, food-groups and macronutrients consumed by participants (Mean values and standard deviations, n 296)
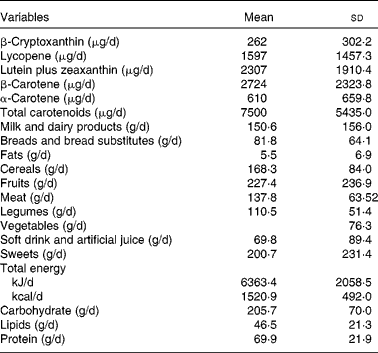
Interestingly, of all food-groups analysed, only fruits, vegetables and eggs explained the variation in carotenoid intake in the present study. Then, we assessed other stepwise multiple regressions with fruit and vegetable items and eggs to verify which items contributed most to the consumption of antioxidants. Thus, the items described in Table 3 accounted for 88·2 % of the total variability in antioxidant carotenoids. When each carotenoid was individually analysed, we also verified that fruit and vegetable food-groups contributed most to the variation in intake than did others. The items from these food-groups accounted for 99·3, 94·2, 55·3, 92·4 and 98·5 % of the total variability in β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene consumption, respectively. We observed that papaya (R 2 0·62) contributed to the highest β-cryptoxanthin intake; watermelon/melon (R 2 0·49) favoured the highest lycopene intake; dark green leaves (R 2 0·40) supplied the highest lutein plus zeaxanthin intake; carrot/pumpkin (R 2 0·76 and 0·98) facilitated the highest β-carotene and α-carotene intake, respectively.
Table 3 Main fruit and vegetable items consumed by participants that contribute to carotenoids consumption (β-cryptoxanthin+lycopene+lutein plus zeaxanthin+β-carotene +α-carotene)*
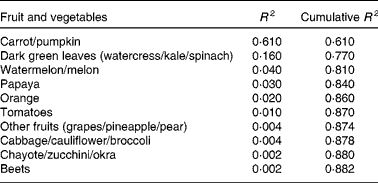
* Banana, apple, lettuce, cucumber and eggs were excluded from stepwise regression model.
In terms of lipid markers, there was a tendency (P= 0·079) and a negative association (P< 0·05) between NEFA concentration and lycopen, lutein plus zeaxanthin, β-carotene and α-carotene, and with the total carotenoid intake, respectively. We observed a significant inverse association of Castelli index values with lycopene, β-carotene and with the sum of all carotenoids consumed. The oxidative stress biomarkers of urinary 8-OHdG and ox-LDL concentrations were also inversely associated with lycopene, lutein plus zeaxanthin, β-carotene, α-carotene and total carotenoid consumption, regardless of confounding variables. Moreover, there was a negative association of urinary 8-iso-PGF2α concentration with lutein plus zeaxanthin, β-carotene and the sum of all carotenoids consumed (Table 4).
Table 4 Multiple linear regression models with the carotenoids consumption as the main independent variable (β Coefficients and 95 % confidence intervals)

8-OHdG, 8-hydroxy-2′-deoxyguanosine; Crn, creatinine; ox-LDL, oxidised LDL.
* Model adjusted for habitual physical activity (steps/d), work position, daily energy intake (kcal/d), total body fat (%) and current smoker (yes/no).
The inclusion of the covariates ‘vitamin C consumption’ and ‘fibre consumption’ in the linear regression model did not lead to a relevant change in statistical significance. Surprisingly, when we included ‘total fat intake’ as a covariate, we found no significant changes either in the statistical outcome (data not shown).
We performed a complementary analysis and observed a significant (P= 0·038) lower presence of MetS (19·0 %) among those subjects who consumed higher quantities of total carotenoids (third tertile = 8250 μg/d), compared to those who consumed lower quantities (21·4 %, second tertile = 4850–8250 μg/d and 31·6 %, first tertile = 4850 μg/d).
Discussion
The hypothesis of the present cross-sectional study was confirmed, as we observed an inverse association of daily carotenoid intake with lipid and oxidative stress markers. In fact, carotenoids are effective antioxidants which can reduce oxidative stress( Reference Fiedor and Burda 8 ). In this sense, fruit and vegetable intakes explained most of the variations in carotenoid consumption in the present study, confirming that these food types are the major sources of carotenoids( Reference Maiani, Caston and Catasta 33 ), and that they lead to lower oxidative stress marker concentrations( Reference Cocate, Natali and Oliveira 5 ).
Another important finding of the present study was the negative association between NEFA concentrations and total carotenoid consumption. NEFA concentrations have been found to be positively associated with insulin resistance and the occurrence of MetS in another study( Reference Athyros, Tziomalos and Karagiannis 34 ). In this context, we observed lower presence of MetS among those subjects who consumed higher quantities of total carotenoids, compared to those who consumed lower quantities. This result is in accordance with a previous cross-sectional study that observed an inverse association between total carotenoid intake (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene consumption) and the presence of MetS in middle-aged and elderly men( Reference Sluijs, Beulens and Grobbee 16 ).
We also showed that lycopene and β-carotene consumption were negatively related with Castelli index. In fact, lycopene appears to have hypocholesterolemic effect probably due to the inhibition of activity and expression of the 3-hydroxy-3-methylglutaryl coenzyme A reductase enzyme. This leads to a decrease in cholesterol synthesis, modulation of LDL receptor activity and inhibition of the acyl-coenzyme A:cholesterol acyltransferase enzyme activity( Reference Palozza, Catalano and Simone 35 ). Furthermore, β-carotene supplementation (0·2 %) for 6 weeks led to a decrease in serum total cholesterol and non-HDL-C, as well as an increase in total lipid and cholesterol contents excreted in the faeces of female Fisher rats fed with a hypercholesterolemic diet, as compared to rats not supplemented with β-carotene( Reference Silva, de Miranda and de Brito Magalhaes 36 ). These mechanisms can partially explain the inverse association between lycopene and β-carotene consumption and lipid markers observed in the present study.
We also found an inverse association between the oxidative stress biomarkers (urinary 8-OHdG, urinary 8-iso-PGF2α and ox-LDL concentration) and the consumption of all carotenoids (sum of β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene) in the present study. In a prospective 15-year follow-up study, the sum of circulating carotenoids (β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin) in men and women in the year zero was inversely associated with F2-isoprostane concentration (a recognised oxidative stress marker), and positively associated with the activity of superoxide dismutase (an antioxidant enzyme) in the year 15( Reference Hozawa, Jacobs and Steffes 37 ). In accordance, an investigation also involving the participants of the Cardiovascular Risk Development in Young Adults (CARDIA) Study found an inverse relationship of the circulating carotenoid concentration (β-carotene, α-carotene, β-cryptoxanthin, lutein and zeaxanthin) with serum γ-glutamyltransferase activity (related to oxidative stress), both cross-sectionally and longitudinally( Reference Lee, Gross and Jacobs 38 ).
Interestingly, when we analysed the consumption of each carotenoid, we obtained an inverse association with at least two of the oxidative stress biomarkers assessed, except for β-cryptoxanthin consumption. In this sense, β-carotene intake was also negatively associated with 8-iso-PGF2α among elderly men in a cohort study( Reference Helmersson, Arnlov and Larsson 39 ). Additionally, plasma β-carotene concentration was the most important determinant of serum LDL conjugated dienes (an indicator of circulating ox-LDL) variation, which was shown to be the most efficient antioxidant among the serum carotenoids in adult men in another cohort study( Reference Karppi, Nurmi and Kurl 40 ). The authors of a study involving patients with coronary artery disease and apparently healthy subjects (control) verified a negative correlation of plasma α- and β-carotene concentration with the susceptibility of DNA to oxidation( Reference Park, Kyoung Park and Kim 41 ). In turn, in a randomised cross-over study, lycopene supplementation in three different treatments (39·2; 50·4 and 75·0 mg/d of lycopene) for a period of a week also led to a significant reduction in LDL oxidation (measured by LDL-thiobarbituric acid-reactive substance and LDL conjugated diene) in healthy adults( Reference Agarwal and Rao 42 ). On the other hand, daily lycopene supplementation (30 mg) for 8 weeks reduced lymphocyte DNA damage and urinary 8-OHdG in healthy adult human subjects in a double-blind, placebo-controlled intervention trial( Reference Devaraj, Mathur and Basu 43 ).
The inverse relationship of carotenoids with oxidative stress biomarkers have been observed in in vitro studies as well. For instance, Kameji et al. ( Reference Kameji, Mochizuki and Miyoshi 44 ) observed higher reactive oxygen species levels (assessed by oxidation of 2′,7′-dichlorodihydrofluorescein diacetate) in 3T3L1 adipocytes pretreated with TNF-α in the absence of β-carotene than in untreated cells, while those cells pretreated with β-carotene had no induction of dichlorodihydrofluorescein diacetate. Thus, they showed that β-carotene accumulation is able to restore reactive oxygen species levels in insulin-resistant adipocytes.Also in another study on human umbilical vein endothelial cells, β-carotene and lycopene affected the inflammation and oxidation induced by TNF-α, through the inhibition of the expression of adhesion molecules, and suppression of the increase in reactive oxygen species generation and of nitrotyrosine (index of ONOO− formation) intracellular levels, which led to augmented NO levels and bioavailability( Reference Di Tomo, Canali and Ciavardelli 45 ). Therefore, in a pro-oxidant condition, physiological concentrations of β-carotene and lycopene can reduce the inflammatory activity, which can act positively on vascular inflammatory states probably by increasing vascular NO bioavailability( Reference Di Tomo, Canali and Ciavardelli 45 ). Moreover, the supplementation of β-carotene and lutein had an inhibitory effect on H2O2 (reative oxygen species)-induced activation of NF-κB in human gastric epithelial cells cultured( Reference Kim, Seo and Kim 46 ). It suggests the antioxidant capacity of carotenoids and their possible protective effect against chronic diseases induced by oxidative stress.
Additionally, an inverse relationship of carotenoids (i.e. β-carotene, lycopene and lutein plus zeaxanthin) with the manifestation of some chronic diseases has been verified( Reference Rao and Rao 47 , Reference Ribaya-Mercado and Blumberg 48 ). In the present study, we assessed the concentration of oxidative stress biomarkers highly associated with CVD (F2-isoprostane and ox-LDL)( Reference Chen and Keaney 49 , Reference Schwedhelm, Bartling and Lenzen 50 )and cancer (8-OHdG)( Reference Valavanidis, Vlachogianni and Fiotakis 51 ). However, the beneficial role of β-carotene( Reference Ye, Li and Yuan 52 ) and lycopene( Reference Osganian, Stampfer and Rimm 18 ) consumption in the prevention of cardiovascular events has not been observed in some studies. This discrepancy may be due to the consumption of excessive doses associated with synthetic carotenoids consumption, which could favour the occurrence of pro-oxidant instead of antioxidant effects( Reference Ciccone, Cortese and Gesualdo 13 ) and/or the influence of some dietetic and lifestyle variables (e.g. high fat intake, sedentary life style and smoking). Despite this observation, results of the present study indicate the important role of carotenoid intake, mainly those derived from fruits and vegetables, in reducing oxidative stress biomarker concentrations. It also suggests that the assessment of habitual carotenoid consumption may be a useful tool to assess the oxidative stress status in healthy middle-aged men.
It is noteworthy, that our outcomes were independent of relevant environmental (smoking and habitual physical activity) and dietary confounding factors such as vitamin C and fibre intake, and thus showing the powerful inverse relationship of carotenoid intake with lipid and oxidative stress biomarkers. Thus, these bioactive compounds (carotenoids) may have a promising role in the prevention or control of chronic diseases.
The present study has to acknowledge, however, its limitations. First, given the cross-sectional nature of the investigation, the results must be cautiously considered as they cannot guarantee ‘cause and effect’ relationship, although we controlled the potential confounding variables as much as possible. Second, borrowing nutrient intake data from the USDA table and applying them to the Brazilian food intake situation might not have truly reflected the research scenario of the present study. Third, it was difficult to take other dietary confounding factors of polyphenols and vitamin E into account. And fourth, we did not assess serum carotenoid concentrations. However, the present study draws strength from the fact that analyses of carotenoid consumption based on FFQ have been used in previous epidemiological studies( Reference Sluijs, Beulens and Grobbee 16 , Reference Sahni, Hannan and Blumberg 53 ).
Conclusion
Daily carotenoid intake was cross-sectionally and negatively associated with relevant lipid and oxidative stress markers in the middle-aged men participants of the present study; the results suggest that regular consumption of fruits and vegetables could lead to health benefits, since daily consumption of these bioactive compounds was mostly derived from these food-groups.
Acknowledgements
The authors thank the participants of the present study from the Universidade Federal de Viçosa, Viçosa, Brazil for their contribution, the nursing staff for excellent technical assistance and the students for untiring help in the fieldwork.
This work was supported by the Foundation for Research Support of the State of Minas Gerais (grant number CDS-APQ-02189-10 and CDS-APQ-02535-13). A. J. N., R. C. G. A., and H. H. M. H. are CNPq fellows.
None of the authors has any conflict of interest to declare.
The authors's contributions are as follows: P. G. C. designed the study, collected and analysed the data, wrote the initial draft and organised the final version of the manuscript; A. d. O. and E. C. d. S. collected the data; A. J. N., R. C. G. A. and H. H. M. H. designed the study, analysed the data and helped in the organisation of the manuscript. All authors assisted in editing the manuscript. All authors read and approved the final version of the manuscript.







