The National Center for Advancing Translational Sciences (NCATS) has awarded Clinical and Translational Science Awards (CTSA) to more than 50 medical centers in the United States to accelerate the pace of translational research to improve human health. NCATS recently issued a new Funding Opportunity Announcement, PAR-21-293, that will apply to all new and renewal proposals for CTSA hubs beginning in 2022 [1]. PAR-21-293 newly requires each hub to have foundational dissemination and implementation (D&I) capabilities and activities to ensure that translational research results in health impact. In a 2021 article [Reference Mehta, Mahoney and Leppin2], members of the D&I working group of the NCATS consortium shared a vision of how CTSA hubs could integrate D&I sciences into each hub’s methods and processes, workforce development, and evaluation components in order to speed the translation of research into practice. This editorial crosswalks recommendations from the 2021 publication with the new NCATS requirement and describes strategies that CTSA hubs can use to enhance the integration of D&I sciences into the required elements of their application, in line with PAR-21-293.
CTSA Program hubs at 50 medical centers in the United States provide innovative resources, training, and mentorship to investigators to improve the efficiency, quality, and impact of translational research to improve the health of individuals and communities. PAR 21-293 highlights the imperative for CTSA hubs to translate research findings into healthcare practice and community health settings, and specifically requires each hub to develop D&I capabilities and utilize D&I activities to ensure that translational research results in health impact. PAR 21-293 organizes CTSA hub proposals into operational elements. Element B, the Strategic Management Element, includes all activities related to management of the hub to ensure the hub achieves its objectives. PAR 21-293 states that, as part of the Strategic Management Element, “Each CTSA hub is required to engage in D&I activities to support innovative approaches to identifying, understanding, and developing strategies for overcoming barriers to the adoption, adaptation, integration, scale-up and sustainability of evidence-based interventions, tools, policies, and guidelines” with the goal to deliver the benefits of translational science to all. While the PAR provides opportunities for incorporation of D&I sciences within other elements of the hub (e.g., training and outreach, core resources and services), the responsibility for management of D&I resources is centralized within the strategic management element.
PAR-21-293 represents an important and substantive change in CTSA awards. Previously, D&I resources have not been a required component of CTSA hubs. Some CTSA hubs provide D&I scientific resources, most typically as part of community engagement cores [Reference Dolor, Proctor, Stevens, Boone, Meissner and Baldwin3]. PAR 21-293 broadens the scope of D&I beyond its original siloed home in the community engagement core and urges integration of D&I within all components of the CTSA hub. The requirement for D&I activities in PAR 21-293, and the location of these activities within the strategic management element, clearly emphasizes the imperative to bridge the research to practice gap. Centralization of D&I resources in the strategic management element should help ensure that all elements of the CTSA hub, from training and outreach, to core resources and services, leverage D&I sciences. Increased utilization of D&I sciences within all elements of CTSA hubs should speed translational research and increase its relevance for clinical partners, stakeholders, and communities [1,Reference Dolor, Proctor, Stevens, Boone, Meissner and Baldwin3]. PAR 21-293 further requires that hubs disseminate their advancements in clinical and translational science (CTS) to other hubs. The responsibility for dissemination of CTS innovations also rests within the strategic management element and it is expected that the hub will utilize D&I activities to do so.
In this editorial, we highlight two resources produced by members of the NCATS consortium D&I workgroup that can support applicants to address the new requirements in the current PAR. In a 2019 publication [Reference Leppin, Mahoney and Stevens4], members of our NCATS consortium D&I working group proposed a framework that illustrated how D&I sciences can benefit translational endeavors within and between all stages of the translational research spectrum (basic research, preclinical research, clinical research, clinical implementation, public health), ultimately speeding the translation of research towards practice adoption and improved health outcomes and health equity. In a 2021 article [1], members of our D&I working group shared a vision of how CTSA hubs could integrate D&I sciences into their methods and processes, workforce development, and evaluation components. We recommended approaches, and provided examples of promising practices, that our group of experts agreed were useful to help embed D&I sciences into CTSA processes and resources to support translational research. Finally, we emphasized that D&I sciences should be applied to support research across all stages of the translational research spectrum and into practice.
In order to provide initial guidance to CTSA hubs as they respond to the new PAR, Table 1 summarizes our 2021 consensus recommendations for CTSA hubs to integrate D&I sciences into methods and processes, workforce development, and evaluation and crosswalks these consensus recommendations with the NCATS requirements in PAR 21-293. Table 1 additionally shows how our consensus recommendations can be utilized to fulfill the requirement in PAR 21-293 for innovation in CTS “that will be catalytic to translational efficiency and the development and delivery of interventions that improve the health of individuals and communities.” PAR 21-293 recommends areas for potential innovation within each of the elements. In addition, Elements D (Translational Science Pilots) and E (Clinical and Translational Science Research Program) afford opportunities to design, test, and disseminate innovative methodologies, programs and practices that harness D&I sciences to optimize translational research and the adoption and implementation of research into practice.
Table 1. Recommendations to enhance integration of D&I sciences within CTSAs [1] and corresponding requirements and opportunities in the new NCATS PAR-21-293 [Reference Mehta, Mahoney and Leppin2]
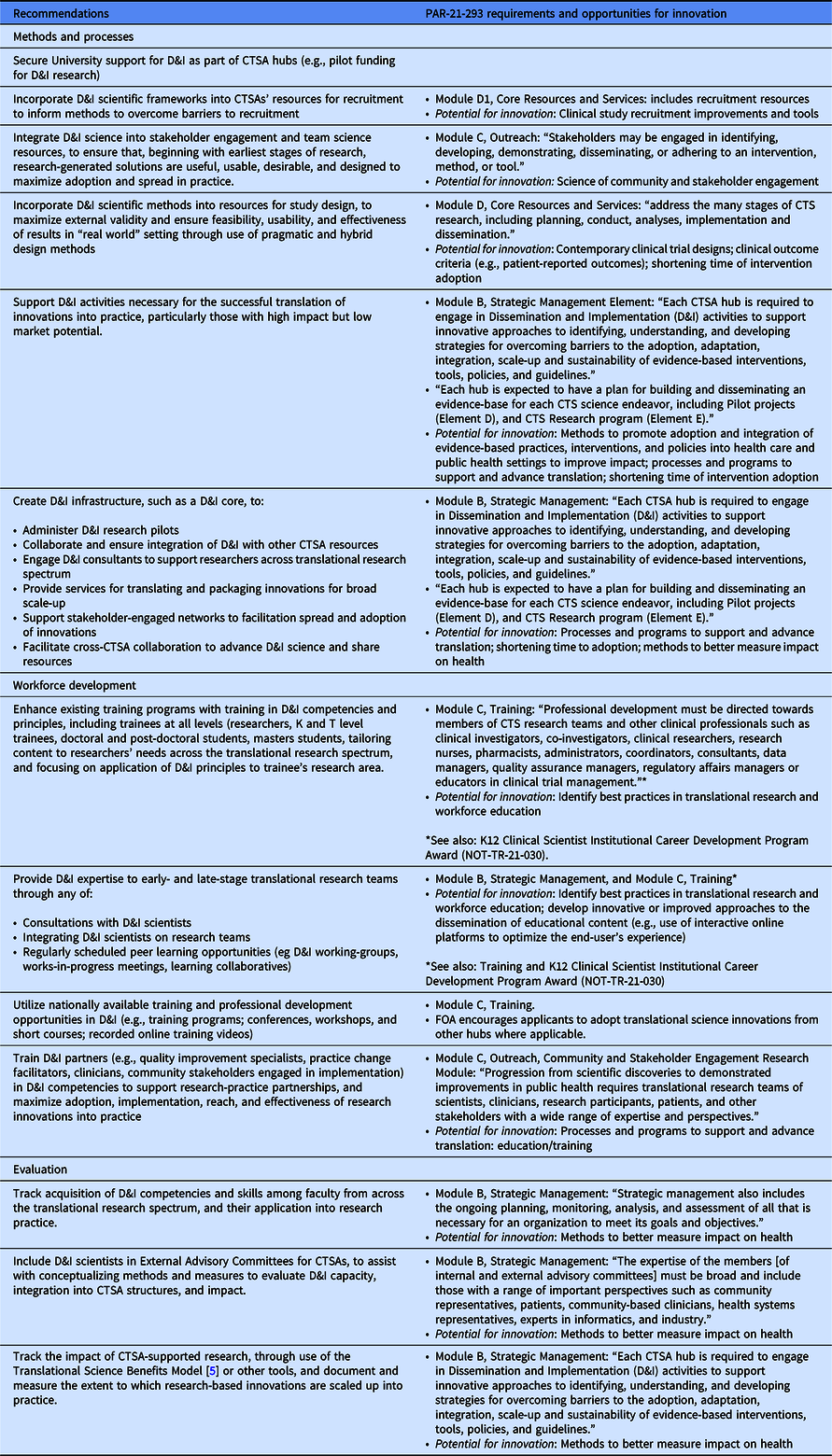
CTSA, Clinical and Translational Science Award; CTS, Clinical and translational science; D&I, Dissemination and Implementation; FOA, Funding Opportunity Announcement.
In addition to the recommended approaches shown in Table 1, our 2021 publication listed 12 areas where further research is needed to understand how to optimize translational research and the adoption and implementation of research into practice. We provided five recommendations for further research on novel ways to incorporate D&I sciences in methods and processes to support translational science; four recommendations for further research to identify core D&I competencies needed by different sectors of the workforce; and three recommendations for research on how best to evaluate the processes and outcomes of integration of D&I sciences into translational research, track the scale-up of innovations into practice, and evaluate the ultimate impact of translational research endeavors on improving health and reducing disparities. Our recommendations are highly congruent with the areas for translational science innovation that are highlighted in PAR 21-293. Elements D and E of PAR 21-293 provide opportunities for further research along the lines of these recommendations.
Ultimately, PAR 21-293 promotes the stronger incorporation of D&I sciences into the work of CTSA hubs to enhance the translation of research into practice to improve health and reduce disparities. The publications from members of the D&I working group of the CTSA consortium [1,Reference Dolor, Proctor, Stevens, Boone, Meissner and Baldwin3] provide a starting point for CTSA hubs as they respond to the new requirements of PAR 21-293 and suggest opportunities to design, study, and disseminate translational science innovations. We hope our recommended approaches and the corresponding examples of promising practices will help CTSA hubs meet the new NCATS requirements.
The D&I working group of the CTSA consortium continues to identify and disseminate best practices and welcomes new CTSA members to join.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) by grants 1UL1TR002373, UL1TR002645, and UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We gratefully acknowledge our coauthors AL Leppin, R Yousefi-Nooraie, BH Pollock, RC Shelton, R Dolor, H Pincus, S Patel, and JB Moore on the publication “Integrating dissemination and implementation sciences within Clinical and Translational Science Award programs to advance translational research: Recommendations to national and local leaders.”
Disclosures
The authors have no conflicts of interest.



