Introduction
The novel photocatalytic wastewater treatment system was developed and applied for dairy cattle wastewater treatment with a simple titanium dioxide (TiO2) sol–gel preparation protocol by the research team of National Taiwan University (NTU) (Su et al., Reference Su, Wang and Su2018). The photocatalytic treatment technique (ultraviolet/titanium dioxide/silicon dioxide (UV/TiO2/SiO2)) had been applied to treat certain sulphur-containing compounds such as hydrogen sulphide (H2S), C2H6S (dimethyl sulphide, DMS) and C2H6S2 (dimethyl disulphide, DMDS) (Canela et al., Reference Canela, Alberici and Jardim1998; Nishikawa and Takahara, Reference Nishikawa and Takahara2001). Based on the study of Canela et al., the catalyst, TiO2, was coated onto the internal glass surface of the photocatalytic reactor (TiO2 film thickness = 5.3 μm) using an aqueous slurry, followed by drying with hot air. The photocatalytic reactor with a 30 W black light lamp (wavelength = 365 nm and the irradiation strength = 4.5 mW/cm2) was applied and the interior reactor was filled with 0.21 (v/v) oxygen (O2) under the humidity ⩾0.23 (v/v) for H2S removal. Results showed that the H2S removal efficiency was higher than 0.99 (v/v) when the inlet H2S concentrations of 46.2–1197 mg/m3 under sufficient O2 conditions. However, there was about 0.95 (v/v) of sulphate adsorbed on the TiO2 surface of the reactor and only about 0.0002 (v/v) of sulphate ion was detected in the reactor effluent when the reactor was working under higher H2S concentrations (e.g. 840 mg/m3) (Canela et al., Reference Canela, Alberici and Jardim1998).
The photocatalytic activity of three semiconductor catalysts (tungsten trioxide (WO3), TiO2 and NiO (nickel oxide)) for the conversion of methane (CH4) into methanol at room temperature with an ultraviolet (UV) laser (355 nm) in the aqueous solution has been investigated. The maximum percentage conversions (v/v) of 0.29, 0.21 and 0.20 were observed for WO3, TiO2 and NiO, respectively (Gondal et al., Reference Gondal, Hameed, Yamani and Arfaj2004). Moreover, Fourier-transform infrared spectroscopy has been employed to investigate the adsorption and photo-oxidation of CH4 over powdered TiO2. The interaction between the CH4 and TiO2 surface is weak. It is found that no CH4 molecules are adsorbed on the surface at 35 °C in a vacuum. Under UV irradiation, CH4 decomposes to form carbon monoxide (CO), carbon dioxide (CO2), water (H2O) and formate (HCOO) in the presence of O2 (Lien et al., Reference Lien, Chen, Lin and Lin2004). Thus, CH4 is tough to be completely oxidized to methanol under ambient conditions with TiO2/UV light and the photocatalytic desulphurization reactor (PDR) of this study may be applied to the livestock farms.
Biogas produced from anaerobic digestion of animal manure wastewater from Taiwan's livestock farms is comprised of CH4 (0.60–0.76, v/v), CO2 (0.18–0.30, v/v), a trace amount of H2S and others (Su et al., Reference Su, Liu and Chang2003, Reference Su, Wang and Su2018). However, some nitrogen was introduced during the biogas desulphurization process (Su et al., Reference Su, Chang, Chen, Chang and Lee2013, Reference Su, Chen and Chang2014; Su and Chen, Reference Su and Chen2015; Su and Hong, Reference Su and Hong2020). To promote CH4 concentration in the desulphurized biogas, a hollow fibre CO2 adsorption system was introduced and applied on a livestock farm for biogas upgrading. Some commercial applications were carried out for desulphurized biogas upgrading including (Chen et al., Reference Chen, Vinh-Thang, Ramirez, Rodrigue and Kaliaguine2015): (1) absorption: water scrubbing, organic physical scrubbing, amine scrubbing and inorganic solvent scrubbing; (2) adsorption: pressure swing adsorption, vacuum swing adsorption, temperature swing adsorption, electrical swing adsorption; (3) cryogenic separation: it is a distillation process operated under very low temperatures (close to −170 °C) and high pressure (around 80 bar). Therefore, for the production of very pure CH4 this technology can be used; (4) membrane technology (Ibrahim et al., Reference Ibrahim, El-Naas, Zhang and Van der Bruggen2018) under various operation pressure (100 mbar–30 bar) and temperature conditions (17–35 °C) (Vogler et al., Reference Vogler, Braasch, Buse, Hempel, Schneider and Ulbricht2013; Falbo et al., Reference Falbo, Tasselli, Brunetti, Drioli and Barbieri2014; Žák et al., Reference Žák, Bendová, Friess, Bara and Izák2018; Tantikhajorngosol et al., Reference Tantikhajorngosol, Laosiripojana, Jiraratananon and Assabumrungrat2019).
The objective was to study the feasibility of combining a novel photocatalytic desulphurization facility with a hollow fibre CO2 adsorption module for biogas upgrading under ambient conditions for promoting in situ livestock biogas applications such as power generation and so on. The acceptable H2S in the biogas for livestock biogas applications was the lower, the better.
Materials and methods
Dairy farm biogas for this study
The conventional method of treating piggery wastewater in Taiwan is the three-step piggery wastewater treatment (TPWT) system, involving (1) solid/liquid separation, (2) anaerobic treatment and (3) aerobic treatment (activated sludge basin with a final clarifier) (Su et al., Reference Su, Liu, Shu and Wu1997; Su and Chen, Reference Su and Chen2018). The TPWT system is also applied to all dairy farms for treating their wastewater. Thus, dairy biogas from the wastewater treatment facility of the NTU dairy farm was used for this study. Analytical data showed that the average COD, BOD and SS of the dairy wastewater after solid/liquid separation were 4170 ± 255, 1650 ± 910 and 3890 ± 529 mg/l, respectively.
PDR design
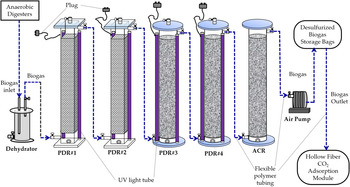
The flowsheet of all processes for biogas production, desulphurization and upgrading is shown in Fig. 1. The PDR was constituted of two acrylic cuboids (130 cm height × 20 cm width × 20 cm length, total volume = 52 litres) and two acrylic cylinders (130 cm height × 20 cm inner diameter, total volume = 41 litres) in series with two UV light tubes (120 cm length, 40 W UV fluorescent blacklight, PULSAR, China) attached to each acrylic cuboid or cylinder (Fig. 2). The aluminium foil sheet was used to cover the surface area of the two acrylic cuboids for reflecting UV light towards the inside of the acrylic cuboids. The two acrylic cuboids were packed with a mixture of Rasching rings (i.e. hollow spherical polypropylene balls) (Sheng-Fa Plastics, Inc., Taiwan) and TiO2-coated light-expanded clay aggregates (LECA) (Su et al., Reference Su, Chang, Chen, Chang and Lee2013) (Fig. 2). The surface of the LECA beads was coated with a mixture of TiO2 anatase powder and concrete. All PDRs used for any time course experiments of this study were packed with Rasching rings and TiO2-coated LECA beads.

Fig. 1. Flowsheet of all processes for biogas production, desulphurization and upgrading.
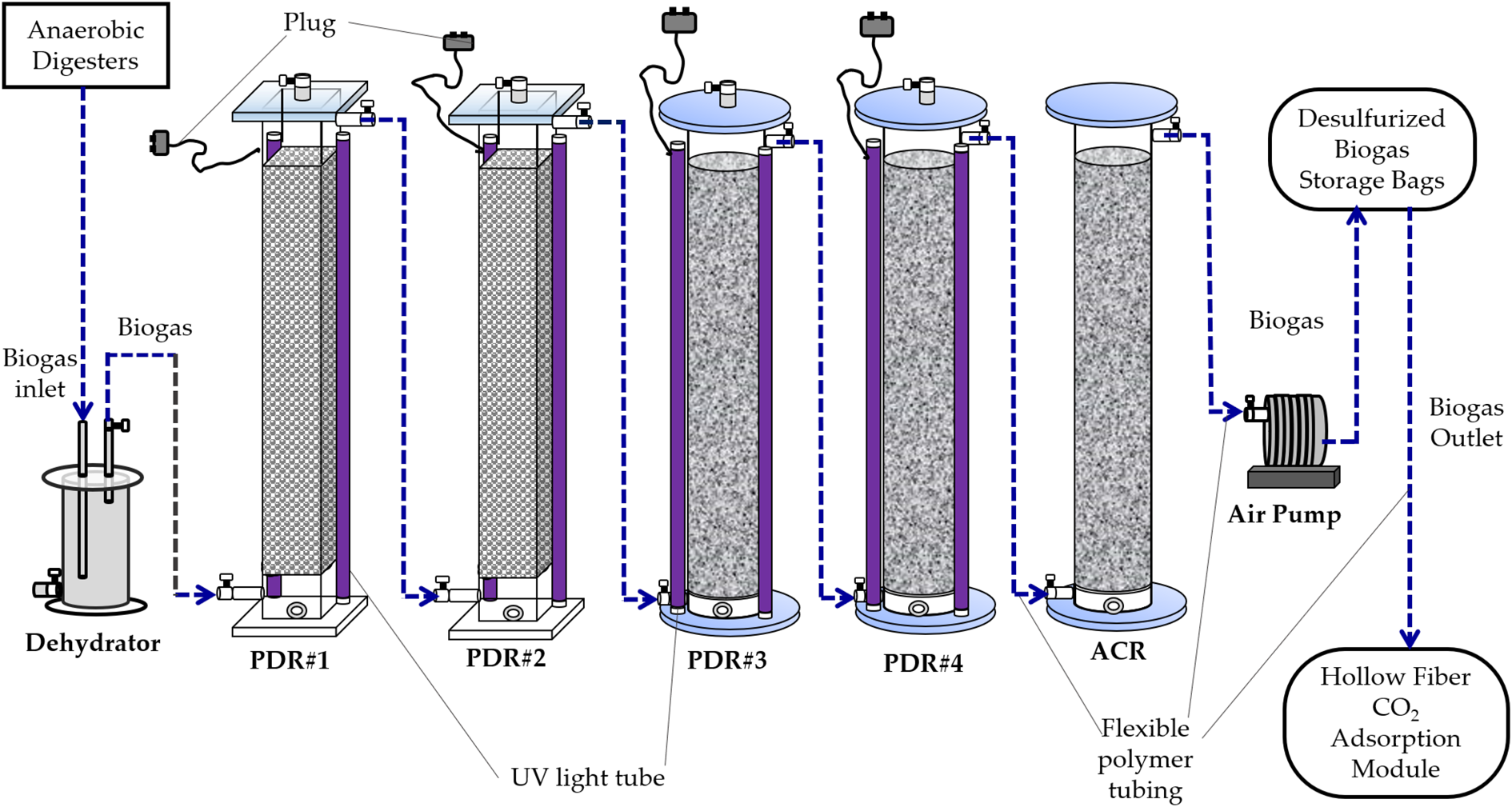
Fig. 2. Sketch and flowchart of the photocatalytic desulphurizing reactor (PDR) in coordination with a biogas upgrading system consisting of an activated carbon reactor (ACR) and a hollow fibre carbon dioxide (CO2) adsorption module.
Untreated biogas entered into the acrylic dehydrator (40 cm height × 19 cm inner diameter, total volume = 11 litres) and then the biogas inlets of the PDRs in sequencing (Fig. 2). There was a 30 cm inlet tubing inside the dehydrator (Su and Hong, Reference Su and Hong2020). Biogas was mixed with a trace amount of air inside the dehydrator. The acrylic PDRs are directly connected to the biogas outlet tubing of the dehydrator (Fig. 2).
Biogas upgrading system design
Activated carbon reactor (ACR) design
The ACR was constituted of an acrylic cylinder (130 cm height × 20 cm inner diameter, total volume = 41 litres). The ACR was packed with a mixture of coconut shell activated carbon pellets (diameter = 5 mm) and Rasching rings (i.e. hollow spherical polypropylene balls) (Sheng-Fa Plastics, Inc., Taoyuan, Taiwan). The ACR was applied to remove impurities and excessive moisture of the desulphurized biogas. It was followed by the four acrylic PDRs (Fig. 2). The operation pressure of the activated carbon filtration reactor was under ambient pressure.
Hollow fibre CO2 adsorption cartridge set for biogas upgrading
The hollow fibre CO2 adsorption cartridge set was constituted of one filtration cartridge (47 cm length × 2″ outer diameter) (AuraMat-TP-HC-A-B, Aura Material Inc., Hsinchu, Taiwan) and five independent hollow fibre CO2 adsorption cartridges (47 cm length × 2″ outer diameter) (AuraMat-TP-CO2-A2, Aura Material Inc., Hsinchu, Taiwan) in parallel. For each time course experiment under various biogas flow rate, only one independent cartridge was used individually for CO2 adsorption from desulphurized biogas. The operation pressure of the CO2 adsorption cartridge was under ambient pressure. The size of all biogas tubing including biogas inlet, biogas purging and biogas outlet tubing was 1/4″ tubing. Biogas flow meters (Tohama 10B; Yeong Shin Co. Ltd, Hsinchu, Taiwan) were installed at the inlet and outlet of the CO2 adsorption cartridge set. The pore size of the hollow fibre surface and adsorption interface was less than 1 μm and 0.5−0.8 nm, respectively.
Hollow fibre CO2 adsorption module for biogas upgrading
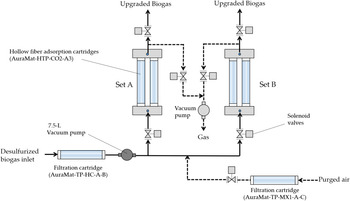
The hollow fibre CO2 adsorption module (AuraMat-HFDCO2-CH4-1L-VC, Aura Material Inc., Hsinchu, Taiwan) (60 cm width × 75 cm length × 115 cm height, power voltage = 220 V/60 Hz) was constituted of two sets, A and B, of hollow fibre CO2 adsorption cartridges (40 cm length × 3″ outer diameter) (AuraMat-HTP-CO2-A3, Aura Material Inc., Hsinchu, Taiwan) in parallel. The size of all biogas tubing including biogas inlet, biogas purging and biogas outlet tubing was 1/2″ tubing. Each set of adsorption cartridge was constituted of five hollow fibre CO2 adsorption cartridges in series. Two gas sampling ports were installed at the biogas inlet and outlet tubing for periodical biogas sampling. Desulphurized biogas was introduced into the module through a filtration cartridge (AuraMat-TP-HC-A-B, Aura Material Inc., Hsinchu, Taiwan) and the CO2 adsorption cartridge sets by an explosion-proof vacuum pump (maximum flow rate = 7.5 litres/min, No. N 87 TTE EX, DRF Corporation, Taipei, Taiwan) inside the module. Another filtration cartridge (AuraMat-TP-MX1-A-C, Aura Material Inc., Hsinchu, Taiwan) for filtering air was also integrated inside the CO2 adsorption module. The operation pressure of the CO2 adsorption cartridge was under ambient pressure.
The operation process of the hollow fibre CO2 adsorption module was as follows in sequence: desulphurized biogas from the storage bags, filtration cartridge (AuraMat-TP-HC-A-B), 7.5 litre vacuum pump, solenoid valves, hollow fibre adsorption cartridges (AuraMat-HTP-CO2-A3), solenoid valves and then discharging biogas (Fig. 3). Moreover, the regeneration process of the hollow fibre CO2 adsorption module was as follows in sequence: purged air, filtration cartridge (AuraMat-TP-MX1-A-C), solenoid valves, hollow fibre adsorption cartridges (AuraMat-HTP-CO2-A3), solenoid valves, vacuum pump and then discharging gas (Fig. 3).

Fig. 3. Flow chart of the hollow fibre carbon dioxide (CO2) adsorption module including operation process (in solid lines) and regeneration process (in dashed lines).
When hollow fibre CO2 adsorption cartridges of Set A was saturated, the inlet biogas was automatically shifted to the set B following the operation process for biogas upgrading (i.e. CO2 adsorption process). Simultaneously, the hollow fibre CO2 adsorption cartridges of Set A following the regeneration process was automatically regenerated. Similarly, when hollow fibre CO2 adsorption cartridges of Set B was saturated, the inlet biogas was automatically shifted to Set A for biogas upgrading. Thus, Sets A and B were operated alternatively.
Time-course experiments of photocatalytic biogas desulphurization and hollow fibre CO2 adsorption for biogas upgrading
Photocatalytic biogas desulphurization
The four connected acrylic PDRs under various biogas flow rates carried out photocatalytic desulphurization (0.5, 1, 1.5, 3, 4 and 5 litres/min). The un-desulphurized biogas flew through the inlets and outlets of the PDR#1, #2, #3 and #4, sequentially. Gas samples were taken in triplicates from the inlet of the PDR#1 and the outlet of PDR#4 to perform gas chromatography (GC) analysis and to determine H2S, O2, NH3 and SO2 on-site using a portable gas detector and detector tubes with a gas-sampling pump. The initial inlet H2S in biogas was 4760 ± 594−5554 ± 82 and 4558 ± 672−5554 ± 80 mg/m3 under the flow rate of 0.5−5 litres/min in Tables 1 and 2, respectively. The acrylic PDRs were flushed with water (41 litres/each reactor) from the top of the PDRs and then soaked for a 2 h duration when some sulphur appeared on the surface of TiO2-coated LECA beads after completion of the time course experiments under various biogas flow rates. This process was repeated until most sulphur was washed out from the surface of LECA beads.
Table 1. Data of biogas desulphurization by the photocatalytic desulphurizing reactor (PDR) (n = 30)

Data presented as mean ± s.d. H2S, hydrogen sulphide; CO2, carbon dioxide; CH4, methane; N2, nitrogen; v/v, proportion of volume of component to volume of sample; Removal, (inlet data–outlet data)/inlet data; n, sample size; NS, not significant.
Table 2. Continuous data of biogas desulphurization by the regenerated desulphurizing reactor (PDR) (n = 45)
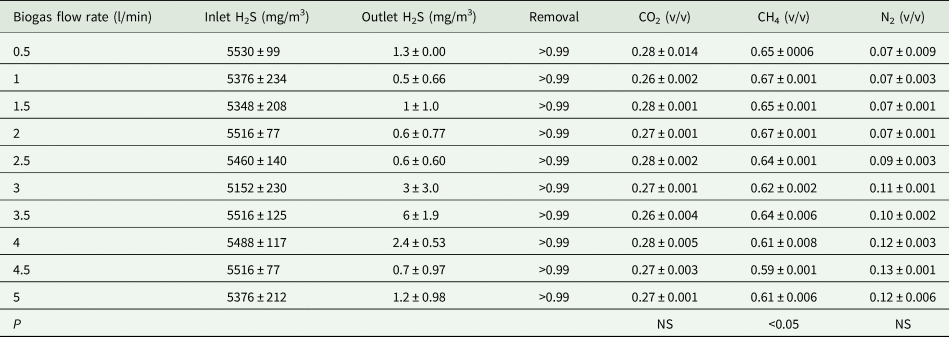
Data presented as mean ± s.d. H2S, hydrogen sulphide; CO2, carbon dioxide; CH4, methane; N2, nitrogen; n, sample size; NS, not significant; v/v, proportion of volume of component to volume of sample; Removal, (inlet data–outlet data)/inlet data.
Biogas upgrading using independent hollow fibre CO2 adsorption cartridge set
Desulphurized biogas was introduced into the biogas filtration cartridge (AuraMat-TP-HC-A-B, Aura Material Inc., Hsinchu, Taiwan) and then the independent hollow fibre CO2 adsorption cartridges#1–#5 (AuraMat-TP-CO2-A2, Aura Material Inc., Hsinchu, Taiwan) through a 1/4 HP air pump for time-course experiments of CO2 removal from the desulphurized biogas. Biogas samples were taken periodically only from the inlet and outlet of the biogas filtration cartridge as the control set. Moreover, all other biogas samples were taken periodically from the inlet of the biogas filtration cartridge and outlet of the independent hollow fibre CO2 adsorption cartridges under various biogas flow rates as the experimental sets. Biogas samples were taken from the sampling ports with 1/8″ silicone tubing of the independent hollow fibre CO2 adsorption cartridge set using a manual gas sampler (Vac-U-Tube, SKC Inc., PA, USA) connecting with 1 litre gas sampling bags (SKC Cat. No. 232-01, SKC Inc., PA, USA). The operation process of the time course experiments was described as follows: biogas samples were taken at 10 min intervals in an hour using the adsorption CO2 cartridge#1 from the inlet of the filtration cartridge and the outlet of the adsorption cartridge#1 under the outlet biogas flow rate of 1 l/min (i.e. inlet biogas flow rate about 2 litres/min). The rest of the time-course experiments were carried out using the independent adsorption cartridge#2–#5 under the outlet biogas flow rates of 1.5, 2, 2.5 and 3 litres/min, respectively, at 10 min intervals in an hour. The contents, CH4, CO2 and N2, of all biogas samples were determined by using GC with a thermal conductivity detector (GC/TCD).
Biogas upgrading using hollow fibre CO2 adsorption module with automatic regeneration
The hollow fibre CO2 adsorption module had to be warmed up at least 2.5 h and then another 2.5 h for filling up biogas inside the cartridges of the CO2 adsorption module before starting any time-course experiments. Desulphurized biogas was introduced into the biogas filtration cartridge (AuraMat-TP-HC-A-B, Aura Material Inc., Hsinchu, Taiwan) and then through three independent hollow fibre CO2 adsorption cartridges (AuraMat-HTP-CO2-A3, Aura Material Inc., Hsinchu, Taiwan) of the Set A or B through an explosion-proof vacuum pump (maximum flow rate = 7.5 litres/min, No. N 87 TTE EX, DRF Corporation, Taipei, Taiwan) inside the adsorption module for time-course experiments of CO2 removal from the desulphurized biogas. Biogas samples were taken at 10 min intervals in an hour from the inlet and the outlet of the CO2 adsorption cartridge module under the outlet biogas flow rates of 1−5 litres/min (i.e. inlet biogas flow rate about 2−10 litres/min). The hollow fibre CO2 adsorption cartridges of Sets A and B were operated alternatively and regenerated automatically based on the signals on the control panel. Biogas samples were taken at 10 min intervals in an hour from the inlet and outlet of the adsorption cartridge module under various outlet biogas flow rates. The contents, CH4, CO2 and N2, of all biogas samples were determinedby using GC/TCD.
Analysis
H2S determination in biogas samples
The gas samples were used to measure the concentrations of H2S from the inlets and outlets of the PDRs on-site using a portable multi-gas detector (ISC MX series, Industrial Scientific Co., PA, USA). When the concentrations of H2S were over the detection limit of the portable multi-gas detector (sensor: H2S: 0–697 mg/m3, SO2: 0–393 mg/m3, O2: 0–0.30 (v/v), NH3: 0–348 mg/m3), a gas sampling pump (GV-100C gas sampling pump; Gastec Co., Japan) with H2S detector tubes (H2S = 14–5575 mg/m3) (Gastec Co., Kanagawa, Japan) was applied for H2S detection.
CH4, CO2 and nitrous oxide determination in biogas samples
Biogas samples from the inlets and outlets were collected in 1 litre Tedlar® bags (SKC, PA, USA) with a single polypropylene fitting. This fitting contained a Teflon® syringe port lined septum and a hose connection, which functioned as a shut-off valve for incoming and outgoing gas. Meanwhile, a 500 ml gas collector (GL Sciences Inc., Tokyo, Japan) was used to withdraw gas samples from the inlets and outlets of the PDRs. Biogas samples were analysed for their composition by GC (Master GC, DANI Instruments, Marlborough, MA, USA), which was equipped with a TCD and Carboxen 1010 PLOT capillary column (30 m × 0.53 mm × 0.25 μm film thickness; Supelco Analytical of Sigma-Aldrich Co., PA, USA) (Su and Chen, Reference Su and Chen2018). Calibration curves of CH4, CO2 and nitrogen gas were obtained by the external standard method, and the calibration curves correlation coefficient was >0.9974.
Statistical analysis
Time-course experiments were conducted in triplicate. One-way analysis of variance was performed using Origin 9.1 software to compare the results using Tukey's test with a significance level of 0.05. The linear regression analysis was applied between biogas flow rate and removal efficiency of H2S or CO2 in each gas when the removal efficiency was significantly different.
Results and discussion
Biogas desulphurization using PDR
The inlet H2S in untreated biogas was 4558 ± 672−5554 ± 80 mg/m3 and the outlet H2S in desulphurized biogas was 0−21 ± 36.0 mg/m3. Moreover, the H2S removal efficiency was about 0.99−1.00 (v/v) under various biogas flow rates (Table 1). Analytical results of biogas samples showed that the gas contents (v/v), CH4, CO2 and N2, of desulphurized biogas, was 0.58 ± 0.016−0.65 ± 0.017, 0.27 ± 0.019−0.32 ± 0.092 and 0.08 ± 0.085−0.13 ± 0.036, respectively (Table 1). CH4 content in the desulphurized biogas was >0.69 (v/v) and the N2 content was <0.14 (v/v). The desulphurizer (PDR) with over air introduction can result in higher N2 content but lower CH4 content. Thus, the operation of the PDRs had to be controlled under low air conditions to assure a certain content of CH4 in the desulphurized biogas.
Adsorption and photocatalytic decomposition of DMS and DMDS using an improved type of silica bead inner supported with TiO2 (TiO2/SiO2) were investigated by Nishikawa and Takahara (Reference Nishikawa and Takahara2001). Although photocatalytic decomposition of DMDS using the inner-supported bead was below 0.53 (v/v) for the same condition as DMS, the removal of DMDS in the lighting up condition from the start using the bead was about 1.00 (v/v) for 50 h. It was suggested that DMDS was treated completely by the composite effects of adsorption and photocatalytic decomposition (Nishikawa and Takahara, Reference Nishikawa and Takahara2001). The LECA bead of the PDR was proven without any H2S adsorption characterization (Su et al., Reference Su, Chang, Chen, Chang and Lee2013, Reference Su, Chen and Chang2014; Su and Chen, Reference Su and Chen2015; Su and Hong, Reference Su and Hong2020). Thus, H2S removal of the biogas was completely photocatalytic oxidation without any adsorption.
Biogas desulphurization using regenerated PDR
When the concentrations of outlet H2S increased, some yellowish elemental sulphur was observed on the surface of some TiO2-coated LECA beads inside the PDRs. In the meantime, inlet biogas was switched off, and tap water (41 litres/reactor) was introduced from the top of the PDRs soaking for a 2 h duration before draining the soaking water. This process was repeated until the sulphur was washed out from the surface of LECA beads. Comparison of the H2S removal efficiency from biogas before (Table 1) and after PDR regeneration (Table 2) was made to achieve optimal operation parameters. The data of statistical analysis showed that there was no significant difference in the contents of CH4, CO2 and N2 in the desulphurized biogas by using the PDR for desulphurization under the biogas flow less than 5 litres/min (Table 1). The PDR is only applied to remove H2S, thus, the contents of CH4, CO2 and N2 should remain constant.
After PDR regeneration, inlet H2S in untreated biogas was 5152 ± 230−5530 ± 99 mg/m3 and the outlet H2S in desulphurized biogas was 0.48 ± 0.66−6.27 ± 1.86 mg/m3. Moreover, H2S removal efficiency was higher than 0.99 (v/v) under various biogas flow rates, i.e. 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5 and 5 litre/min (Table 2). Analytical results of biogas samples showed that the gas contents (v/v), CH4, CO2 and N2, of desulphurized biogas, were 0.59 ± 0.001−0.67 ± 0.001, 0.26 ± 0.002−0.28 ± 0.014 and 0.07 ± 0.001−0.13 ± 0.001, respectively (Table 2). Thus, sulphur on the surface of LECA beads might be dissolved in the tap water in the form of elemental sulphur or sulphate ion (Su and Hong, Reference Su and Hong2020). The photocatalytic oxidation of H2S at the gas/solid interface was investigated using TiO2 as the photocatalyst and in situ Fourier transform infrared spectroscopy analysis by Kataoka et al. (Reference Kataoka, Lee, Tejedor-Tejedor and Anderson2005). A malodorous compound, H2S was removed via a photocatalytic process under ambient conditions. H2S was oxidized to sulphate species on the surface of TiO2 without producing a noticeable gaseous intermediate, e.g. sulphur dioxide (SO2) (Kataoka et al., Reference Kataoka, Lee, Tejedor-Tejedor and Anderson2005). The results of this study showed that there was no SO2 detected in the outlet biogas of the PDR. Thus, the results of this study were consistent with the finding of the study of Kataoka et al. (Reference Kataoka, Lee, Tejedor-Tejedor and Anderson2005). However, the data of statistical analysis showed that there was a significant difference in the contents of CH4 in the desulphurized biogas by using the regenerated PDR under the biogas flow less than 5 litres/min (P < 0.05) (Table 2). The results implied that some sampling or analysing errors may occur in those gas samples.
The linear regression analysis was not applied between biogas flow rate and removal efficiency of H2S in each gas (Tables 1 and 2) because the removal efficiency was not significantly different.
Biogas upgrading using independent hollow fibre CO2 adsorption cartridge set
The higher the CH4 content, the higher the heat value of biogas. In other words, the lower the CO2 content, the higher the CH4 content in biogas. Analytical data showed that the inlet and outlet CH4 contents (v/v) in the desulphurized biogas under various outlet biogas flow rates (1 to 3 litre/min) were 0.56−0.64 and 0.56 ± 0.006−0.86 ± 0.011, respectively (Table 3). The highest CH4 content (v/v) in the outlet biogas was 0.86 ± 0.011. The inlet and outlet N2 contents (v/v) in the desulphurized biogas were 0.10–0.16 and 0.14 ± 0.009−0.25 ± 0.172, respectively (Table 3). The lowest N2 content (v/v) in the outlet biogas was 0.14 ± 0.009. The amount of introducing air might be reduced through an opening of the dehydrator. Moreover, the inlet and outlet CO2 contents (v/v) in the desulphurized biogas were 0.25–0.28 and 0.01 ± 0.004–0.28 ± 0.007, respectively (P < 0.05) (Table 3). The data of statistical analysis showed that there was a significant difference in the difference of CH4/N2 or removal of CO2 in the upgraded biogas by using the independent hollow fibre cartridges under the biogas flow less than 3 litres/min (Table 3). The results implied that different biogas flow rates affect the removal efficiency of CO2, which was related to the removal efficiency of CH4 and N2 by using simple hollow fibres CO2 adsorption cartridges.
Table 3. Carbon dioxide (CO2) removal of the desulphurized biogas by independent hollow fibre cartridges (n = 30)

Data presented as mean ± s.d. CH4, methane; N2, nitrogen; n, sample size; NS, not significant; R 2, coefficient of determination; v/v, proportion of volume of component to volume of sample; Removal or difference, (inlet data–outlet data)/inlet data.
The highest removal efficiency (v/v) of CO2 was 0.97 ± 0.014 under the outlet biogas flow rate of 1 litre/min (Table 4). The removal efficiency of CO2 decreased with increased desulphurized biogas flow rates (P < 0.05). Experimental results showed that the hollow fibre adsorption cartridge was capable of removing CO2 from the desulphurized biogas and promote CH4 concentrations, however, the cartridge had to be regenerated once an hour after the CO2 adsorption process. Thus, an integrated hollow fibre CO2 adsorption module significantly removed CO2 in biogas and was more feasible for commercial pig farm use.
Table 4. Carbon dioxide (CO2) removal of the desulphurized biogas by a hollow fibre CO2 adsorption module (n = 30)

Data presented as mean ± s.d. CH4, methane; N2, nitrogen; n, sample size; NS, not significant; R 2, coefficient of determination; v/v, proportion of volume of component to volume of sample; Removal or difference, (inlet data–outlet data)/inlet data.
Biogas upgrading using a hollow fibre CO2 adsorption module
The hollow fibre CO2 adsorption module comes with an automatic regeneration function. Analytical data showed that the inlet and outlet CH4 contents (v/v) in the desulphurized biogas under various outlet biogas flow rates (1 to 5 litre/min) were 0.61–0.63 and 0.70 ± 0.045–0.84 ± 0.006, respectively (Table 4). The highest CH4 content (v/v) in the outlet biogas was 0.84 ± 0.006. The inlet and outlet N2 contents (v/v) in the desulphurized biogas were 0.09–0.11 and 0.13 ± 0.005–0.14 ± 0.007, respectively (Table 4). The lowest N2 content (v/v) in the outlet biogas was 0.13 ± 0.005. Moreover, the inlet and outlet CO2 contents (v/v) in the desulphurized biogas were 0.27–0.29 and 0.03 ± 0.005–0.18 ± 0.057, respectively (Table 4). The highest removal efficiency (v/v) of CO2 was 0.90 ± 0.018 under the outlet biogas flow rate of 1 litre/min (Table 4). The removal efficiency of CO2 decreased with increased desulphurized biogas flow rates (P < 0.05). Experimental results showed that the hollow fibre adsorption module was capable of removing CO2 from the desulphurized biogas and promote CH4 concentrations, however, the module was automatically regenerated once an hour after the CO2 adsorption process.
Biogas upgrading using a regenerated hollow fibre CO2 adsorption module
The hollow fibre CO2 adsorption module was automatically regenerated for 3 h before performing the continuous time-course experiments. Analytical data showed that the inlet and outlet CH4 contents (v/v) in the desulphurized biogas under various outlet biogas flow rates (1–5 litre/min) were 0.63–0.65 and 0.70 ± 0.045–0.86 ± 0.005, respectively (Table 5). The highest CH4 content (v/v) in the outlet biogas was 0.86 ± 0.005. The inlet and outlet N2 contents (v/v) in the desulphurized biogas were 0.10–0.11 and 0.12 ± 0.002–0.13 ± 0.006, respectively (Table 5). The lowest N2 content (v/v) in the outlet biogas was 0.12 ± 0.002. Moreover, the inlet and outlet CO2 contents (v/v) in the desulphurized biogas were 0.25–0.27 and 0.01 ± 0.002–0.18 ± 0.054, respectively (Table 5). The highest removal efficiency (v/v) of CO2 was 0.94 ± 0.028 under the outlet biogas flow rate of 1 litre/min (Table 5). The removal efficiency of CO2 decreased with increased desulphurized biogas flow rates (P < 0.05). Experimental results showed that the hollow fibre adsorption module was capable of achieving more than 0.90 (v/v) of CO2 removal efficiency (i.e. 0.90 ± 0.018–0.94 ± 0.028) from the desulphurized biogas and promote CH4 content (v/v), however, the CH4 content (i.e. 0.84 ± 0.006–0.86 ± 0.005) was still less than 0.90 after CO2 adsorption process. The N2 adsorption cartridge might be needed to remove N2 (i.e. 0.13 ± 0.006–0.14 ± 0.005, v/v) in the desulphurized biogas and significantly promote CH4 content. The hollow fibre CO2 adsorption module has to be modified for enlarging module size by installing more cartridges, i.e. increase the desulphurized biogas loading volume, or rest the automatic regeneration intervals to achieve more than 0.95 (v/v) of CH4 in the upgrading biogas.
Table 5. Carbon dioxide (CO2) removal of the desulphurized biogas by a regenerated hollow fibre CO2 adsorption module (n = 30)

Data presented as mean ± s.d. CH4, methane; N2, nitrogen; n, sample size; NS, not significant; R 2, coefficient of determination; v/v, proportion of volume of component to volume of sample; Removal or difference, (inlet data–outlet data)/inlet data.
The data of statistical analysis showed that there was a significant difference in the removal of CH4, CO2 and N2 in the upgraded biogas by using the hollow fibre module under the biogas flow less than 5 litres/min (P < 0.05) (Tables 4 and 5). The results implied that different biogas flow rates affect the removal efficiency of CO2, which was related to the removal efficiency of CH4 and N2 by using simple hollow fibres CO2 adsorption module. Also, the automatic hollow fibre CO2 adsorption module significantly removed the CO2 in biogas without manual regeneration and operation.
The linear regression analysis was applied between biogas flow rate and removal efficiency of CO2 in each gas (Tables 3–5) because the removal efficiency was significantly different. Since the maximum adsorption limit was set at 1 litre/min of outlet biogas flow rate, the removal efficiency of CO2 for all sets under the outlet biogas flow rate of more than 1 litre/min declined rapidly right after saturation of the hollow fibre adsorption. Results of linear regression analysis showed that the R 2 value of Tables 3–5 was 0.74282, 0.70246 and 0.74621, respectively, when the y-axis was CO2 removal efficiency and the x-axis was outlet biogas flow rate.
Conclusions
The purpose of developing the PDR was intended to desulphurize livestock biogas under ambient conditions and applied with the various biogas applications such as power generation, absorption chillers, or kitchen stoves. The higher the heat value, the more efficient the biogas applications. For onsite application, the loading volume of biogas must be increased with the enlargement of the size of the biogas desulphurization facility and the hollow fibre CO2 adsorption module. Thus, nitrogen gas adsorption cartridges might be needed and installed next to the hollow fibre CO2 adsorption cartridge inside the new hollow fibre CO2 adsorption module for promoting CH4 concentration in the upgraded biogas.
Acknowledgements
We thank Fang-Ching Chang and Phil Pan for their expert technical assistance during the hollow fibre adsorption experiments.
Financial support
This work was funded by grants (No. MOST 105-2623-E-002-002-ET, 108 AS-17.2.1-AD-U3 and 109 AS-15.2.1-AD-U2) awarded from the Ministry of Science and Technology (MOST) and Council of Agriculture (COA), Executive Yuan, Taiwan, respectively.
Conflict of interest
None.
Ethical standards
Not applicable.