Background
Medication review is a cognitive pharmaceutical service (CPS) (Benrimoj et al., Reference Benrimoj, Feletto, Gastelurrutia, Martinez Martinez and Faus2010) provided by community pharmacists in a range of countries (Barbanel et al., Reference Barbanel, Eldridge and Griffiths2003; Emmerton et al., Reference Emmerton, Shaw and Kheir2003; McLean et al., Reference McLean, Gillis and Waller2003; Bunting and Cranor, Reference Bunting and Cranor2006; Mehuys et al., Reference Mehuys, Van Bortel, De Bolle, Van Tongelen, Annemans, Remon and Brusselle2008; García-Cárdenas et al., Reference García-Cárdenas, Sabater-Hernández, Kenny, Martínez-Martínez, Faus and Benrimoj2013). One of the earliest funded services is the Medicines Use Review (MUR) service introduced in England in 2005, for which there is relatively little evidence to support either its effectiveness (Wright, Reference Wright2016) or cost-effectiveness (Centre for Policy on Ageing, Reference Centre for Policy2014). A recent systematic review and meta-analysis of randomised controlled trials of medication review suggested that an isolated medication review has minimal effect on clinical outcomes, no effect on quality of life and lacks evidence of economic outcomes, although studies have shown a decrease in the number of drug-related problems (DRP), more changes in medication, more drugs with dosage decrease and a greater decrease or smaller increase of the number of drugs used (Huiskes et al., Reference Huiskes, Burger, van den Ende and van den Bemt2017).
Studies which have focussed on specific conditions, however, have consistently shown positive outcomes. For example, in patients with asthma, studies in several countries have shown that pharmacists can identify problems with medicines and intervene to improve outcomes (García-Cárdenas et al., Reference García-Cárdenas, Armour, Benrimoj, Martinez-Martinez, Rotta and Fernandez-Llimos2016), and that there is a need for such intervention. A study in Denmark (Herborg et al., Reference Herborg, Soendergaard, Jorgenaen, Fonneabaek, Hepler, Holst and Froekjaer2001) and more recent studies conducted in India (Gajanan et al., Reference Gajanan, Fernandes, Avuthu and Hattiholi2015) and Vietnam (Nhu Nguyen et al., Reference Nhu Nguyen, Huynh Thi Hoai and Chavannes2018) found that patients had poor knowledge of asthma. A study in Germany found that the most common advice given to asthmatic patients was education about their medicines (Schulz et al., Reference Schulz, Verheyen, Mühlig, Müller, Mühlbauer, Knop-Schneickert, Petermann and Bergmann2001). Although many well-designed studies (Narhi et al., Reference Narhi, Airaksinen, Tanskanen and Erlund2000; Cordina et al., Reference Cordina, McElnay and Hughes2001; Herborg et al., Reference Herborg, Soendergaard, Jorgenaen, Fonneabaek, Hepler, Holst and Froekjaer2001; Schulz et al., Reference Schulz, Verheyen, Mühlig, Müller, Mühlbauer, Knop-Schneickert, Petermann and Bergmann2001; Barbanel et al., Reference Barbanel, Eldridge and Griffiths2003; Emmerton et al., Reference Emmerton, Shaw and Kheir2003; McLean et al., Reference McLean, Gillis and Waller2003; Saini et al., Reference Saini, Krass and Armour2004; Mangiapane et al., Reference Mangiapane, Schulz, Mühlig, Ihle, Schubert and Waldmann2005; Bunting and Cranor, Reference Bunting and Cranor2006; Haahtela et al., Reference Haahtela, Tuomisto, Pietinalho, Klaukka, Erhola, Kaila, Nieminen, Kontula and Laitinen2006; Armour et al., Reference Armour, Bosnic-Anticevich, Brillant, Burton, Emmerton, Krass, Saini, Smith and Stewart2007; Mehuys et al., Reference Mehuys, Van Bortel, De Bolle, Van Tongelen, Annemans, Remon and Brusselle2008; García-Cárdenas et al., Reference García-Cárdenas, Sabater-Hernández, Kenny, Martínez-Martínez, Faus and Benrimoj2013) have been carried out in asthma, very few provided evidence of effectiveness (Armour et al., Reference Armour, Bosnic-Anticevich, Brillant, Burton, Emmerton, Krass, Saini, Smith and Stewart2007; García-Cárdenas et al., Reference García-Cárdenas, Sabater-Hernández, Kenny, Martínez-Martínez, Faus and Benrimoj2013). A study conducted by Armour et al. (Reference Armour, Reddel, Lemay, Saini, Smith, Bosnic-Anticevich, Song, Alles, Burton, Emmerton, Stewart and Krass2012) assessed the feasibility and sustainability of a CPS for patients with asthma, but did not assess consistency and replicability. According to the Oxford Dictionary, consistency is the quality of achieving a level of performance, which does not vary greatly in quality over time; replicability represents the ability of a scientific experiment or trial to be repeated to obtain a consistent result.
In Italy, although the Government (Legge Reference Italian69/2009 e D.LGS 153/2009) approved the provision of CPS in 2009, no services are delivered by community pharmacies. In contrast to many other countries, Italian community pharmacists are not permitted to keep patient records of medication dispensed, hence reviewing medicines is less feasible. Moreover, Italian pharmacists do not receive training in clinical pharmacy as part of their undergraduate training, and postgraduate training in this area is also not widely available. The Italian Pharmacists’ Federation (FOFI), recognising the extent to which Italian pharmacy had failed to move forward with other countries in developing CPS, identified the need for different types of evidence to be obtained locally before any service could be commissioned. FOFI collaborated with academic researchers to develop a programme of studies to fulfil this need. The Italian Medicines Use Review (I-MUR) project began in 2010 and took five years to complete. The I-MUR developed was a structured approach to medication review, based on the English service but with several key differences. Based on evidence of the benefits of CPS in asthma, this condition was selected for the programme and the I-MUR designed specifically for patients with asthma. It was constructed using mostly closed questions to enable the community pharmacists providing the intervention to easily gather the data essential for demonstrating the type of evidence which could ultimately support the continuation of such services.
The I-MUR programme involved three phases: Phase 1 (intervention testing), 2 (evaluation) and 3 (cluster randomised controlled trial). Phases 1 and 3, in which pharmacists delivered the I-MUR, were conducted in 2012–2013 and 2014–2015, respectively. Phase 1 was a non-randomised study with no follow-up, conducted in four cities in Northern Italy which identified the potential for benefit. This study aimed to determine whether pharmacists were able to undertake the process of completing an I-MUR with asthmatic patients and upload data onto a web platform. Both pharmacists and patients involved in Phase 1 were excluded from Phase 3, which was a cluster randomised control trial including an economic analysis, conducted in 15 regions across the whole of Italy. Phase 3, reported elsewhere (Manfrin et al., Reference Manfrin, Thomas and Krska2017), demonstrated that the I-MUR was both effective and cost-effective. Results from the evaluation (Phase 2) which obtained the views of pharmacists, patients and GPs on the I-MUR service provided during Phase 1 have also been published (Manfrin and Krska, Reference Manfrin, Tinelli, Thomas and Krska2018).
However, for a service to be commissioned and funded, there should be demonstrable capacity to benefit (need), consistency and replicability of the structures and processes which contribute to clinical effectiveness should be assured (Donabedian, Reference Donabedian1980). As no previous studies had been undertaken in Italy of CPS, the collection of process data was an important aspect of evaluating delivery of the I-MUR intervention. For an intervention to become standard practice, a degree of consistency is needed and for a particular outcome to be achieved, the structures and processes should be similar. Previous studies have shown that delivery of a medication review intervention varies between individual pharmacists (Krska and Avery, Reference Krska and Avery2008; Hinchliffe, Reference Hinchliffe2011). We therefore examined key process measures which could contribute to the effectiveness of the I-MUR intervention to assess the potential consistency of delivery, in the absence of formal fidelity testing, which is regarded as the extent to which a test duplicates the actual conditions or task performed; the closer the match, the higher the fidelity of the test. We also assessed the potential need for the service (potential to benefit) by review of the problems patients reported with their asthma and their medicines.
Aims
The aims of this study were to assess the consistency and replicability of I-MUR by:
(i) comparing the demographics, medications (active ingredients) used, self-reported adherence and problems with asthma and medicines of patients involved in Phases 1 and 3 of the I-MUR programme;
(ii) quantifying and comparing the types of pharmaceutical care issues (PCIs) and actions taken by pharmacists during the provision of I-MUR in both phases.
Methods
Selection and recruitment of pharmacies, pharmacists and patients
Local pharmacy organisations in each of the four regions (Phase 1) and 15 regions (Phase 3) invited all community pharmacists to participate (using phone calls and emails). For those who expressed interest in the study, a selection process was undertaken to ensure that pharmacists all met pre-specified inclusion criteria, which included a private room, internet connection and provision of some services beyond dispensing from their pharmacy. Patients were recruited by the pharmacists on the basis of either a diagnosis or prescription of medicines for asthma. Further details of all inclusion and exclusion criteria for pharmacies, pharmacists and patients have been published elsewhere (Manfrin et al., Reference Manfrin and Krska2015).
The I-MUR intervention
The I-MUR involves a face-to-face consultation between pharmacist and patient in a private room. The I-MUR was generally based on the English MUR template, but a new more systematic, structured interview template was developed, specifically for asthma patients, which used closed questions allowing quantitative data to be gathered easily. Questions in the template covered: asthma symptoms, medicines used (active ingredients), problems and adherence. The first version of I-MUR was developed by A.M. for Phase 1, and was validated by eight Italian non-participating community pharmacists. The results of the evaluation (Phase 2) provided the opportunity to add two questions to the I-MUR instrument before using it in Phase 3. All pharmacists in both Phases (1 and 3) received training regarding asthma physiopathology and clinical pharmacology from respiratory physicians (1 h). A.M. provided 3 h of training in Phase 1 and 4 h in Phase 3. The difference in the length of training was due to higher complexity of Phase 3. The training provided was in pharmaceutical care, in particular how to identify PCIs, which could have an impact on the use of medicines and/or asthma control and to provide appropriate advice to patients and recommendations to their GPs. The latter were based on the individual pharmacists’ clinical judgements using all the data gathered during the I-MUR. Pharmacists were also trained in the use of the I-MUR template to gather data and in uploading it onto the web platform. Use of this system enabled pharmacists to enter patient-level data in Italy, which was then downloaded for analysis in the United Kingdom, thus avoiding the complexities of paper-based data collection. In order to provide the I-MUR, the pharmacists had to request details of all medicines patients were using, due to the lack of patient medication records. For each patient, pharmacists recorded: medicines used, responses to closed questions on asthma, problems and adherence, PCIs they identified and actions/recommendations they made.
Data analysis
Medications (active ingredients) were classified using the World Health Organization (WHO) anatomic, therapeutic, chemical (ATC) classification system. The frequency of use of three major drug classes, non-steroidal anti-inflammatory drugs (NSAIDs), ace-inhibitors (ACE-Is) and β-blockers not recommended in asthmatic patients was determined. The number of PCIs identified and actions taken during the I-MUR service provision were classified by the pharmacists using the methods of Krska et al. (Reference Krska, Jamieson, Arris, McGuire, Abbott, Hansford and Cromarty2002) before adding to the web platform. This classification system, which has been used in medication review studies in England (Krska and Avery, Reference Krska and Avery2008), was deemed sufficiently simple for use by the Italian pharmacists.
Comparisons between the data obtained from Phases 1 and 3 were made using three non-parametric techniques. (χ 2) for independence (Pearson’s χ 2) was used when comparing the relationships between two categorical variables, and when each of these variables could have had more than two or more categories. Fisher’s exact test for independence was used when the frequency was below five. χ 2 test for goodness-of-fit was used with categorical variable when comparing the proportion of cases from a sample with hypothesised of those obtained previously from a comparison population. Owing to the number of hypotheses tested (53 items), the Bonferroni correction was adopted, as suggested by Goldman (Reference Goldman2008), resulting in a level of significance of P=0.0009. The analysis was conducted using Microsoft Excel version 2016 and SPSS version 24.
Findings
Demographic details
In Phase 1, four Italian regions and four specific locations (towns) were involved: Piemonte (Torino), Toscana (Pistoia), Lombardia (Brescia) and Veneto (Treviso). The number of pharmacists enrolled in Phase 1 was 74 and they recruited 895 patients. Phase 3 was powered to detect a clinically significant difference in ATC score, using a large number of pharmacists across Italy, to minimise the effect of inter-pharmacist variation on the primary outcome. Therefore, 201 pharmacists and 816 patients completed Phase 3. The number of regions involved in this phase was 15: Trentino Alto Adige, Lombardia, Sicilia, Puglia, Sardegna, Piemonte, Valle d’Aosta, Veneto, Friuli Venezia Giulia, Toscana, Emilia Romagna, Marche, Abruzzo, Lazio and Campania.
Patients recruited to both studies showed similar gender and age distributions, spanning a large range of ages (Table 1).
Table 1 Patients’ demographic and asthma active ingredients
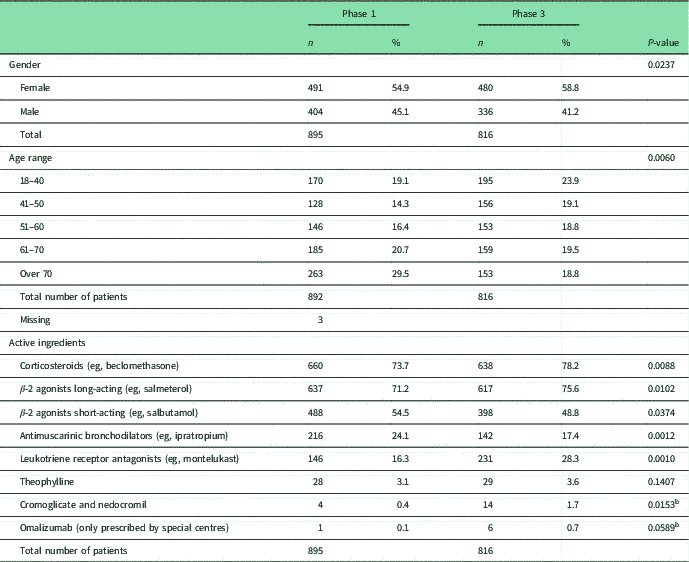
χ 2 with Bonferroni adjustment 0.05/53 0.0009
a Statistical significant difference.
b Fisher’s exact test with Bonferroni adjustment.
Medicines used (as active ingredients)
The median number of all active ingredients used by patients before receiving the I-MUR was 5.0, in both Phases 1 and 3. No statistically significant differences were found across the two populations in the active ingredients used for treating asthma (Table 1), with the most common active ingredients being corticosteroids (73.7%, Phase 1; 78.2%, Phase 2) and long-acting β-2 agonists (71.2%, Phase 1; 75.6%, Phase 2), whereas the least common was omalizumab (0.1%, Phase 1; 0.7% Phase 2). Overall, the proportion of patients using active ingredients considered to be inappropriate in asthmatic patients (ACE-I, β-blockers and NSAIDs) was slightly higher in Phase 3 compared with Phase 1, but the difference was not statistically significant (Table 2).
Table 2 Pharmaceutical care issues (PCIs) and active ingredients
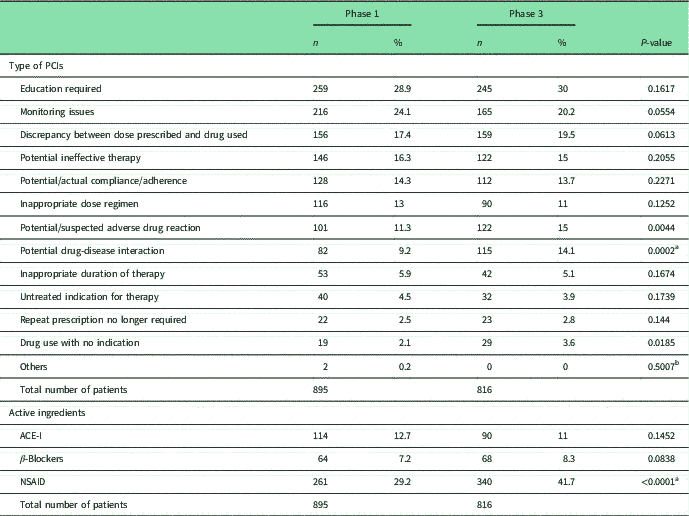
χ 2 with Bonferroni adjustment 0.05/53 0.0009
ACE-I=angiotensin-converting-enzyme inhibitors; NSAID=non-steroidal anti-inflammatory.
a Statistical significant difference.
b Fisher’s exact test with Bonferroni adjustment.
Self-reported adherence and problems with medicines
Patients’ self-reported adherence to medications was low in both phases: 51.4% (n=460) in Phase 1 and 45.8% (n=374) in Phase 3 (P=0.0214; not statistically significant). Around a quarter of patients in both studies perceived they had problems with their asthma medications, whereas around three-quarters considered they knew how to use them, considered they were working and were effective (Table 3).
Table 3 Patients’ perceptions of their medications
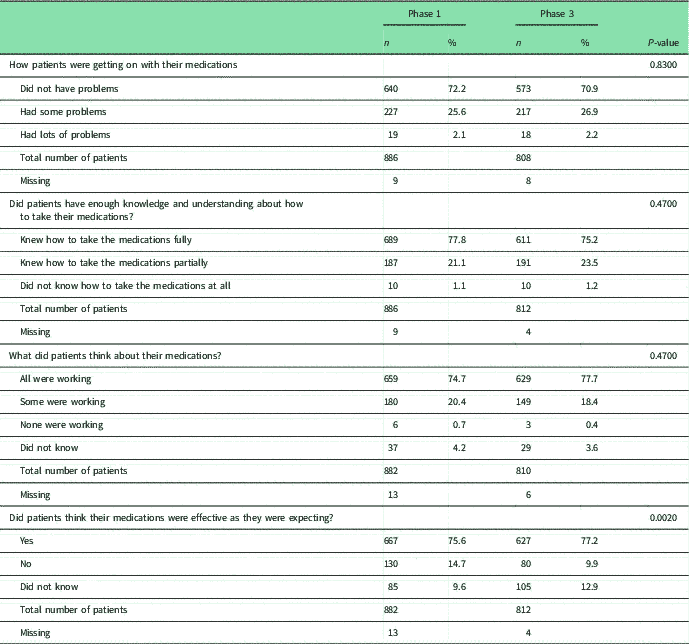
χ 2 with Bonferroni adjustment. 0.05/53 0.0009
a Statistical significant difference.
b Fisher’s exact test with Bonferroni adjustment.
PCI identified and actions taken
Despite patients perceiving they knew how to use medicines, in Phase 1 pharmacists identified at least one PCI in 543 (60.7%) patients, a mean of 2.4 per patient among those with a PCI. In Phase 3, 64.6% of patients (527/816) had a PCI with the mean being 2.5 per patient. The three most common PCIs in both phases were the same: education required, monitoring issues and discrepancy between dose prescribed and drug used (Table 2).
In both Phases, the most common type of action taken by pharmacists was to provide drug information to patients. Overall, there were very few differences between the two studies in the types of actions taken by pharmacists to improve medicines use (Table 4). The frequency with which advice regarding healthy living was provided also showed no differences between the phases. In particular, pharmacists provided advice to stop or reduce smoking to 23 and 25% of patients in Phases 1 and 3, respectively. The need to carry out monitoring was the most frequent type of advice given to GPs in both phases, but was slightly more common in Phase 3, as was the recommendation to change a drug and other advice.
Table 4 Frequency of advice provided by pharmacists to patients and general practitioners
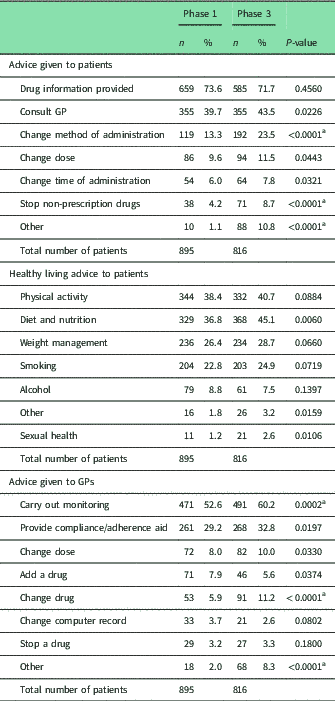
Pearson χ 2 with Bonferroni adjustment 0.05/53 0.0009
a Statistical significant difference.
Discussion
The data obtained in Phase 1 were derived from a large sample of patients, but a relatively small number of pharmacists in only four northern Italian cities, where the cooler temperatures may have an adverse effect on asthma control. In contrast, the findings from Phase 3 involved a similar number of patients but recruitment was spread over 15 out of 20 Italian regions, from the North to the much warmer South, and almost three times the number of pharmacists were involved. The similarities in the prevalence of inappropriate medicines used, such as ACE-I and β-blockers, perceptions of problems, adherence and smoking across the two studies, however, suggests that the results obtained in Phase 1 were confirmed in Phase 3. Furthermore, a total of 53 items were compared in this analysis, and only 15% (8/53) of them presented a statistically significant difference. Thus, these results suggest the need for an intervention, which could provide potential benefits for patients with asthma.
The finding that around 50% of patients were adherent to asthma treatment is in line with the results of a systematic review (Engelkes et al., Reference Engelkes, Janssens, de Jongste, Sturkenboom and Verhamme2015), which found that adult adherence rates to asthma treatment was between 30 and 70%. The relatively high rates of smoking in patients with asthma are reflective of the high national prevalence in Italy (21%) (Lugo et al., Reference Lugo, Zuccaro, Pacifici, Gorini, Colombo, La Vecchia and Gallus2017).
The process measures recorded, PCIs identified and actions taken, during delivery of the intervention, were also consistent between the two phases. One possible reason for such consistency is that the patient population was similar, despite the broad inclusion criteria, but the use of a standardised, structured template and similar training could also contribute to these findings. Given that Phase 3 demonstrated the I-MUR to be both clinically effective and cost-effective, it is reasonable to anticipate that, provided the structures are consistent (training, private area, other pharmacist inclusion criteria met) the processes maintained through the use of a structured interview, the benefits demonstrated in Phase 3 should be achieved in a commissioned service.
The mean number of PCIs per patient of 2.5 and 2.4 in Phases 1 and 3, respectively, and the most common action taken by pharmacists being patient education required in both populations are similar to the studies in other European countries. A Danish study conducted in 2001 (Herborg et al., Reference Herborg, Soendergaard, Jorgenaen, Fonneabaek, Hepler, Holst and Froekjaer2001) found that the most common DRP in asthmatic patients was poor knowledge of asthma, suggesting that, as was found here, patient education was required. A German study (Schulz et al., Reference Schulz, Verheyen, Mühlig, Müller, Mühlbauer, Knop-Schneickert, Petermann and Bergmann2001) reported the most common advice given to asthmatic patients by pharmacists was drug information. This was also the most common recommendation in a study in England of patients with multi-morbidity (Krska et al., Reference Krska, Cromarty, Arris, Jamieson and Hansford2000), which used the same classification system for quantifying PCIs and pharmacist actions. Other studies in multi-morbid patients have, however, found a higher number of DRPs (Brulhart and Wermeille, Reference Brulhart and Wermeille2011).
The lack of consistency in provision of an intervention found in some studies (Krska and Avery, Reference Krska and Avery2008; Hinchliffe, Reference Hinchliffe2011) is a potential problem for a commissioned service. Greater standardisation of the intervention, using the mechanisms employed here, may help to reduce such variation. Although it must be acknowledged that structured questioning is somewhat contrary to the open-ended approach to medication review advocated in England, which seeks to improve patient-centred consultations (Picton and Wright, Reference Picton and Wright2013), the inclusion of selected standardised questions into the process and recording of these data could help to provide a greater degree of evidence, while still allowing for individualised care.
Strengths and limitations
The data presented here involved 1711 patients and 275 pharmacists, thus the combined data represent one of the largest reports describing a pharmaceutical care intervention. The two studies, Phases 1 and 3, were conducted at different times, in different locations, by different pharmacists and with different patients, yet showed similar findings. The large sample sizes included also support the generalisability of the results achieved using I-MUR in Phase 3.
The data gathered during the I-MUR (medications used, problems, PCIs and actions) were uploaded onto a web platform by the pharmacists who conducted the I-MUR and it was not possible to verify the accuracy of these. The classification of PCIs and actions was dependent on the interpretation of the pharmacists and these were not validated. However, this approach did facilitate the conduct of two large-scale studies at minimal cost and no problems were encountered in use of these classification systems, despite the large number of pharmacists involved.
Conclusions
A structured approach to medication review for asthma has the potential to improve outcomes due to consistency in delivery and there is potential to benefit from the I-MUR across Italy. It may be appropriate to consider using a similar structured approach for other conditions and in other countries. Involving pharmacists in gathering patient-level data may be a useful strategy to help generate the type of evidence suggested as being required to support CPS such as medication review.
Acknowledgements
The authors thank the Italian pharmacists, general practitioners and consultants, patients and all other people who have been involved in the studies. The authors are grateful to the Italian Pharmacists’ Federation (FOFI) who funded Phase 1 and 3 and to Ordine dei farmacisti di Brescia, Pistoia, Torino and Farmarca Treviso who founded Phase 1 as well.
Authors’ Contribution
A.M. is the principal investigator who developed I-MUR, J.K. supervised the work. A.M. and J.K. drafted the paper, A.M. conducted the statistical analysis. Both authors revised the manuscript for intellectual content, read and approved the final manuscript. A.M. acts as the guarantor for the results.
Financial Support
This paper did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The funders of Phase 1 and 3 are reported in the acknowledgements session.
Conflicts of Interest
None.
Ethical Standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Phase 1 was approved by the University of Kent Ethics Advisory Group for Human Participants on 19 September 2012 (ref. no. 020S11/12), Phase 3 was approved by the University of Kent Faculty of Sciences Research Ethics Committee on 18 February 2014 (ref. no. 0281314) and subsequently approved by the Brescia Ethics Committee in Italy on 3 June 2014 (ref. no. 1710-Studio RE I-MUR), which acted also as the coordinating centre in Italy.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Participants consented to the study after full explanation of what was involved was given. Signed consent forms from pharmacists were retained in the university. Signed consent forms from patients were retained by pharmacists in the pharmacies.
Anonymity and Data Storage
Data obtained during I-MUR consultations were coded and stored electronically on a computer system at the University of Kent, in a directory which is password protected. Hard copies of any patient data, if any were collected during the I-MUR, were the responsibility of the participating Italian pharmacists to store in a secure filing cabinet in their pharmacies. All electronic data regarding Phase 1 have been password protected and are accessible only by the researcher. All electronic data regarding Phase 3 have been deposited in the Kent Academic Repository (KAR url: https://www.kent.ac.uk/library/research/kar/), after being treated in accordance with requirements of the Data Protection Act (1998), that is anonymised and stripped of any identifiable references to the participants.







