Arterial hypertension and metabolic disorders remain the main risk factors for cardiovascular diseases and are associated with high morbidity and mortality rates worldwide( Reference Landsberg, Aronne and Beilin 1 , Reference Mills, Bundy and Kelly 2 ). This indicates that a better understanding of the underlying mechanisms causing hypertension and metabolic impairment is essential for effective interventions and prevention strategies.
The excessive consumption of fat and sugar, usually described as the westernised lifestyle, has contributed to the growing incidence of metabolic disorders such as dyslipidaemia and type 2 diabetes( Reference Moreira, Texeira and Ferreira 3 ). It has been established that metabolic disorders, especially during pregnancy and early life, enhance susceptibility to the development of cardiometabolic diseases( Reference de Brito Alves, Nogueira and de Oliveira 4 – Reference Costa-Silva, Simoes-Alves and Fernandes 7 ). For example, offspring from dams exposed to a high-fat diet during pregnancy exhibit hypertension and metabolic alterations in adult life( Reference Longo, Refuerzo and Mann 8 , Reference Vidal-Santos, Macedo and Santana 9 ).
Maternal dyslipidaemia is a serious metabolic disorder with a limited therapeutic approach. It has been described as an important risk factor for fetal death, preterm birth and with adverse effects on offspring development( Reference Jiang, Jiang and Xu 10 , Reference Palinski, Nicolaides and Liguori 11 ). Increasing evidence suggests that a maternal dysmetabolic condition, such as maternal dyslipidaemia, may compromise placental functionality and impair the fetus–placental supply through elevated oxidative stress( Reference Loy, Kns and Jm 12 ), pro-inflammatory markers( Reference Roberts, Riley and Reynolds 13 ) and altered lipid and glucose handling( Reference Musial, Vaughan and Fernandez-Twinn 14 ).
Previous studies have shown that the increase in pro-inflammatory markers and imbalance in oxidative stress can alter the mechanisms of blood pressure (BP) control, such as: (i) decrease in baroreflex sensitivity( Reference Monteiro, Franca-Silva and Alves 15 ), (ii) respiratory chemosensitivity dysregulation( Reference Del Rio, Moya and Parga 16 – Reference Nunes, Ribeiro and Franca-Silva 18 ) and (iii) sympathetic hyperactivity( Reference Chan and Chan 19 , Reference Chobanyan-Jurgens and Jordan 20 ). It has been also established that elevated sympathetic activity is involved in the generation of hypertension( Reference Simms, Paton and Pickering 21 , Reference Folkow 22 ) and is associated with metabolic disorders( Reference Mancia, Bousquet and Elghozi 23 – Reference Corry and Tuck 25 ).
It is reported that the sympathetic nervous system may be modulated via central and peripheral respiratory chemoreceptors, evoking reflex responses of sympatho-excitation and tachypnoea( Reference Franchini and Krieger 26 , Reference Zoccal, Simms and Bonagamba 27 ). However, it remains unknown whether a sympathetic hyperactivity, in part modulated by raised respiratory chemosensitivity, could be a potential mechanism underlying maternal dyslipidaemia-induced hypertension and metabolic disorders.
Thus, in the present study, we examined the effects of maternal dyslipidaemia during pregnancy and lactation on the cardiorespiratory physiology and glucose–lipid biochemical parameters in male offspring. Our hypothesis was that a sympathetic hyperactivity associated with higher respiratory chemosensitivity could be a potential hidden basis for the development of arterial hypertension and metabolic disorder in adult male offspring from dams exposed to a dyslipidaemic (DLP) diet during pregnancy and lactation.
Methods
Experimental procedures were performed in accordance with the revised guide for the care and use of laboratory animals( Reference Bayne 28 ). The experimental protocol was submitted and approved by the institutional animal care and use committee (CEUA-UFPB protocol 014/2016) of the Federal University of Paraiba, Brazil.
Animals and experimental design
In all, ten virgin female Wistar rats (Rattus norvegicus) were maintained at room temperature (22±1°C) with a controlled light–dark cycle (dark 06.00–18.00 hours). A standard laboratory chow diet (52 % carbohydrate, 21 % protein and 4 % lipids; Purina Agriband) and water were given ad libitum. At 90 d of age, rats were subjected to dietary manipulations: control (CTL group, n 5) and DLP diets (DLP group, n 5) were offered ad libitum during 1 week. Then, the rats were mated in a special cage overnight (one female for one male) and the pregnant females were transferred to individual cages. The CTL diet was prepared according to the American Institute of Nutrition( Reference Reeves, Nielsen and Fahey 29 , Reference de Brito Alves, Nogueira and Cavalcanti Neto 30 ) and the DLP diet was purchased from Rhoster® Company. The diets (Table 1) were offered during the pregnancy and lactation periods. At 24 h after birth, the offspring were randomly adjusted to eight pups (four male and four female) per litter to ensure standardised nutrition until weaning. At weaning (postnatal day 22), the female and male offspring were housed separately (four per cage) and had free access to a normal diet until the beginning of the experimental phase. The CTL and DLP experimental groups were formed with two rats from each litter. We first examined the impact of maternal dyslipidaemia on serum glucose and lipid parameters in 30-d-old rats. Then, the male rats were maintained in the protocol to access the long-term effect of maternal dyslipidaemia on biochemical parameters and cardiorespiratory physiology at day 90. Female offspring were not included in the present study.
Table 1 Nutritional composition of the diets (g/100 g diet)
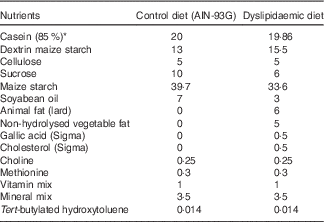
* Casein showed 85 % purity (85 g protein for each 100 g casein).
Effects of the dyslipidaemic diet during pregnancy and lactation in dams
From the 1st day of pregnancy to the end of the lactation, the body weight and food intake of each dam were measured daily using a digital scale (model AS-1000; Marte) having an error range of 0·1 g. Food intake was monitored by measuring the difference between the amount of food put in the cage and the amount remaining after 24 h. The results of body weight and food consumption were compiled and expressed as weekly consumption.
At 24 h after weaning, the dams were fasted overnight and then an oral glucose tolerance test (OGTT) was performed. Then, 24 h after the OGTT, the dams were again fasted overnight and an insulin tolerance test (ITT) was performed after an intraperitoneal injection of insulin (0·75 IU/kg body weight). Blood glucose concentrations were measured before (0 min) and after (30, 60, 90 and 120 min). Last, 48 h after the ITT, the dams were fasted overnight and euthanised by decapitation. The dams had remained on a DLP diet during these four days after weaning. Serum samples were collected for quantification of total cholesterol (TC), TAG and HDL with commercial kits (Bioclin). The LDL and VLDL values were calculated by the use of previously described formulas( Reference Traill 31 ).
The livers from both groups were collected for analysis of lipid accumulation. Liver tissue was excised, washed in saline solution (0·9 % NaCl) and fixed in buffered formalin (10 %) for 48 h. Then, the tissues were sectioned in microtome to obtain 8-µm-thick slices. A total of ten sections were stained with Oil Red O (Sigma) for 10 min. Sections were examined under light microscopy (4× and 20×) and representative photos were acquired for each group( Reference Mehlem, Hagberg and Muhl 32 ). Another ten different sections were also stained with haematoxylin–eosin and analysed under a common light microscope (Motic BA 200; Scientific Instrument Company).
Measurement of somatic parameters in male offspring
The body weight was recorded at birth and on the 21st, 30th, 60th and 90th days of age with an appropriate balance (model AS-1000). The body length of the pups was recorded by the measurement of external surfaces (length from nose-to-anus) with callipers (0·01 mm accuracy). The body weights and body lengths of pups were used to determine the anthropometrical parameter of the Lee index (cube root of body weight (g)/nose-to-anus length (cm))( Reference de Brito Alves, Nogueira and de Oliveira 4 ).
Glucose and insulin tolerance test
At 30 and 80 d, the rats were fasted overnight for the OGTT and ITT tests. The OGTT was performed after administration of an oral glucose load (2 g/kg) via gavage. Blood samples were taken from the tail veins before glucose administration and, subsequently, at 15, 30, 60 and 90 min. At 24 h after OGTT, ITT was performed after an intraperitoneal injection of insulin (0·75 IU/kg body weight) and blood glucose concentrations were measured before (0 min) and after (30, 60, 90 and 120 min). All measurements of blood glucose concentration were performed with an Accu-Check glucometer (Bayer).
Biochemistry parameters and oxidative stress assay
At 30 and 80 d, offspring from both groups were anaesthetised with ketamine (80 mg/kg) and xylazine (10 mg/kg), and blood samples (approximately 2 ml) were collected by plexus retro-orbital disruption and centrifuged at 300 g , 25°C, during 15 min( Reference de Brito Alves, Nogueira and de Oliveira 4 ). This experiment was performed 48 h after the glucose homoeostasis experiments. Serum samples were collected for the quantification of TC, HDL, VLDL, LDL, TAG and glucose with commercial kits. The concentration of malondialdehyde (MDA), an end product of lipid peroxidation, was measured as an indicative of oxidative stress. In this assay, MDA reacts with thiobarbituric acid to produce a red-coloured complex. Briefly, 400 μl of perchloric acid (7 %) was added to 250 μl of serum, mixed and centrifuged at 600 g , 4°C, during 20 min. The supernatant was collected, added to 400 μl of thiobarbituric acid (0·6 %), heated at 100°C during 1 h and read at 532 nm. A standard curve of MDA was constructed and the results were expressed as nmol of MDA/ml( Reference Cavalcanti, Alves and de Oliveira 33 ).
Surgical procedure for blood pressure record in male rats at 90-d-old
At least 4 d after the plexus retro-orbital disruption experiments, BP measurements were performed as previously described( Reference de Brito Alves, Nogueira and de Oliveira 4 ). For this experiment, eight rats used in each group come from four different litters. In brief, male (CTL, n 8; DLP, n 8) rats at 90 d old were anaesthetised with ketamine (80 mg/kg) and xylazine (10 mg/kg) to insert polyethylene catheters in the femoral artery and vein. The catheters were tunnelled through the back of the neck and ketoprofen (5 mg/kg) was injected subcutaneously. The offspring underwent a period of surgical recovery of 24 h until the BP record experiments began. Arterial pressure (AP) and heart rate (HR) were recorded in conscious animals connecting the arterial catheter to a pressure transducer (LabChart 7 Pro; ADInstruments).
Measurements and analysis of arterial blood pressure, ventilation and sympathetic tonus
The pulsatile AP was recorded for 40–50 min under basal conditions, and the values of the systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP) and HR were calculated by selection of 10 min for each animal. This same period was used for electrocardiogram (ECG) and spectral analysis.
After 40 min of cardiovascular recordings, measurements of the respiratory frequency (RF) were also performed by using the whole-body plethysmographic method( Reference Malan 34 ). Air flow was suspended for short periods (3 min), and breathing was captured by a pressure differential transducer connected to a signal amplifier (ML141 Spirometer; PowerLaband ADInstruments).
After recording the baseline ventilation measurements, the respiratory and cardiovascular responses to CO2 were induced by flushing a hypercapnic gas mixture (7 % CO2, 21 % O2 and N2 balance; Linde Gas) into the plethysmographic chamber, as previously described by our research group( Reference de Brito Alves, Nogueira and de Oliveira 4 ). RF, tidal volume (VT) and ventilation minute (VE) were determined in an air-conditioned room (control baseline) before and after the hypercapnic challenge. A period of 10 s was selected for the determination of mean RF. VT was calculated using the formula described previously by Malan( Reference Malan 34 ) and VE acquired through product RF×VT. The cardiovascular analysis was performed during the flow of the hypercapnic gas mixture. A period of 30 s of BP and HR was selected at 1, 2, 3, 4 and 5 min of gas flow. The cardiorespiratory reflex was assessed by the difference between the baseline and response observed after hypercapnia stimulus (Δ).
A period of 10 min after hypercapnic stimuli, a peripheral chemoreflex was activated through the intravenous injection of potassium cyanide (KCN; 40 mg/100 ml per rat; Merck) in accordance with previous reports( Reference Franchini and Krieger 26 , Reference de Brito Alves, Nogueira and Cavalcanti Neto 30 ). The difference between MAP, HR and RF baseline and the peak increased after the stimulus (ΔMAP, ΔHR and ΔRF) was assessed for, respectively, sympathetic, parasympathetic and respiratory components.
Last, 10 min after the peripheral chemoreflex activation, the contribution of the sympathetic vascular tone to cardiovascular system was evaluated by an intravenous injection of the ganglionar blocker (hexamethonium; 30 mg/kg; Sigma-Aldrich®). The sympathetic tonus was calculated by the difference between the MAP after the blocker and the baseline MAP.
All of the data were analysed offline with the use of appropriate software (LabChart 7 Pro; ADInstruments), and at the end of the research period the animals were euthanised with 0·5 ml overdose of ketamine (endovenously). All protocols performed for the BP and respiratory parameter experiments are shown in Fig. 1.
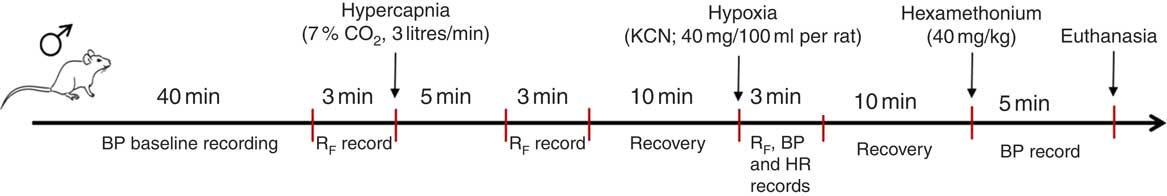
Fig. 1 Schematic protocol performed to record arterial blood pressure and ventilatory parameters. BP, blood pressure; RF, respiratory frequency; HR, heart rate.
Electrocardiogram analysis
ECG recordings were processed by computer software (ECG analysis module for LabChart Pro; ADInstruments) for automatic detection of the R waves and beat-by-beat calculation of RR interval. For detection of ECG, 12 ms was used to determine typical QRS width and for R waves at least 100 ms apart. For ECG analysis, 70 ms was used for the maximum PR and 90 ms for maximum RT.
Spectral analysis
The cardiovascular autonomic evaluation was performed using the frequency domain analysis of the SAP and pulse interval (PI) by an appropriate software program (CardioSeries-v.2.4; www.danielpenteado.com). The spectra were integrated in the low-frequency (LF, 0·2–0·75 Hz) and the high-frequency (HF) bands (0·75–3 Hz). To assess the sympathovagal index, the LF:HF ratio of the variability was calculated. In addition, the spontaneous baroreflex sensitivity was calculated through a sequence method.
Statistical analysis
Each experimental group included at least one or two animals from each litter. Bartlett’s test was performed to evaluate data homogeneity as to respiratory, cardiovascular and biochemical parameters, and statistical results supported the use of unpaired Student’s t tests. Two-way ANOVA and Bonferroni’s post hoc test were used for analysis of food intake, body weight, OGTT and ITT during pregnancy and lactation. Statistical analysis was conducted with GraphPad Prism 5 program for Windows (GraphPad Software®, Inc.). The values are presented as means with their standard errors. Values of P<0·05 were considered as statistically significant.
Results
Food intake, body weight and metabolic profile in dams exposed to a dyslipidaemic diet during pregnancy and lactation
As observed in Fig. 2, after weaning, DLP dams presented significantly raised serum levels of TC (4·0-fold), TAG (2·0-fold), VLDL+LDL (7·7-fold) and reduced HDL-cholesterol (2·4-fold) when compared with CTL dams. We also observed that the atherogenic indexes TAG/HDL, TC/HDL and VLDL+LDL/HDL were all increased in the DLP dams (P<0·05, Fig. 2).
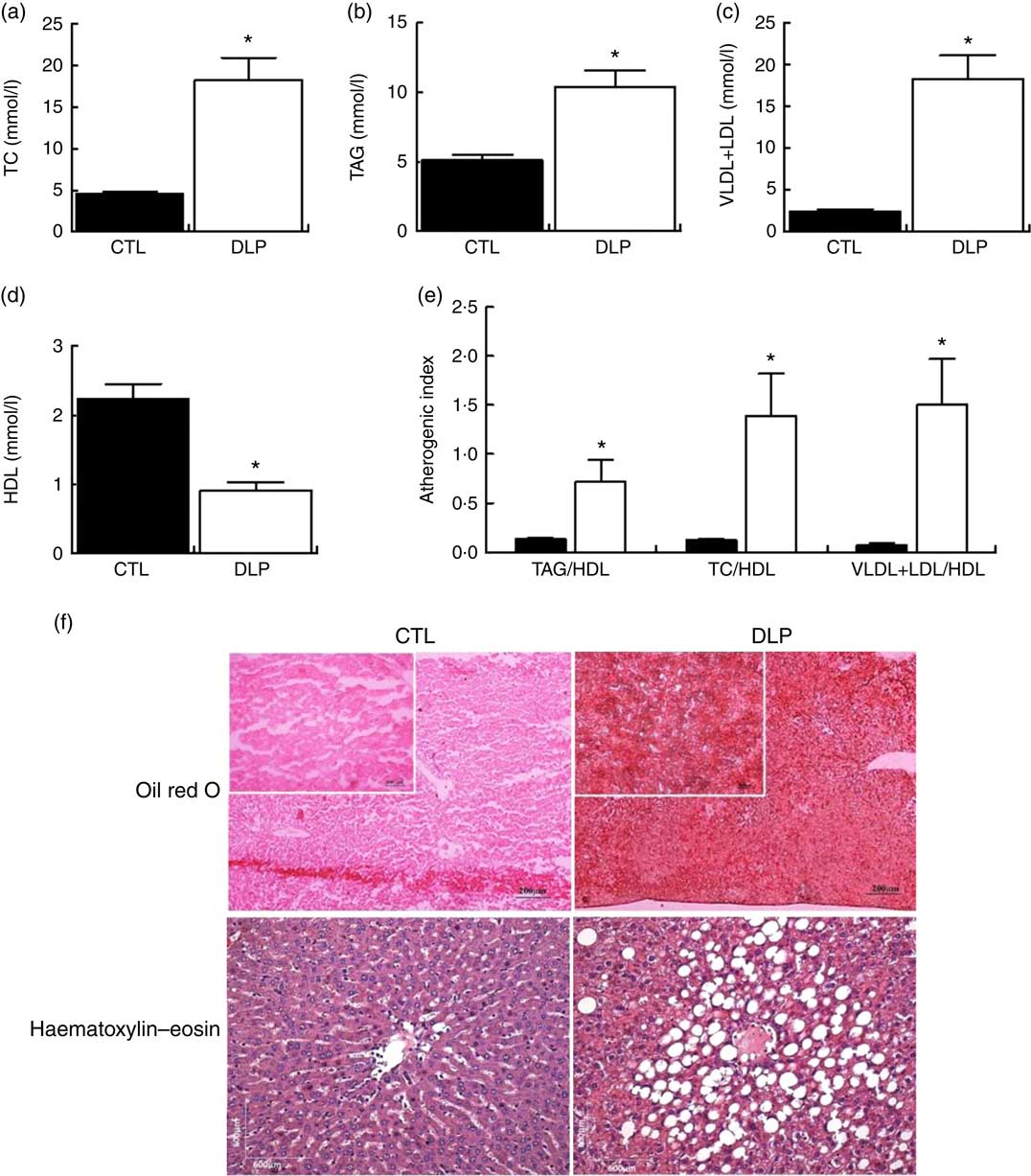
Fig. 2 Fasting serum total cholesterol (TC) (a), TAG (b), VLDL+LDL (c) and HDL (d), atherogenic index (e), hepatic (f) and intestine (g) lipid accumulation in dams that received a control (CTL) or dyslipidaemic (DLP) diet during pregnancy and lactation. Groups: CTL group (n 5) and DLP group. Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using unpaired Student’s t test.
After weaning, the DLP dams exhibited higher hepatic lipid accumulation than the CTL dams (Fig. 2). We measured the food intake and body weight during pregnancy and lactation. Despite the reduced food intake in the last 2 weeks of lactation, there were no differences in the body weight and total weight gain between the CTL and DLP dams (online Supplementary Fig. S1).
In order to evaluate whether the DLP diet during pregnancy and lactation would alter the regulation of glucose homoeostasis, we conducted OGTT and ITT at the end of lactation. As shown in Fig. 3, the DLP dams exhibited higher serum glucose in 15 (9·7 (sem 1·7) v. 6·2 (sem 0·4) mmol/l, P<0·05) and 30 min (9·2 (sem 0·8) v. 6·3 (sem 0·5) mmol/l, P<0·05) of OGTT when compared with CTL. In agreement, the DLP dams presented greater AUC 90 min after OGTT (P<0·05, Fig. 3). Similarly, the DLP dams also exhibited higher serum glucose after intraperitoneal insulin injection in 30 (3·4 (sem 0·16) v. 2·2 (sem 0·21) mmol/l, P<0·05) and 120 min (3·12 (sem 0·56) v. 1·5 (sem 0·19) mmol/l, P<0·05) and consequently greater AUC 120 min after the injection of insulin (P<0·05, Fig. 3).
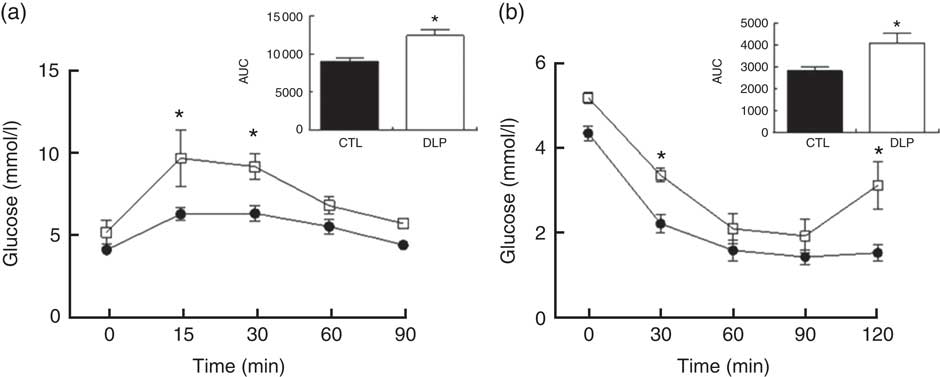
Fig. 3 Oral glucose tolerance test (a) and insulin tolerance test (b) in dams that received a control (CTL) or dyslipidaemic (DLP) diet during pregnancy and lactation. Groups: CTL group (n 5, ![]() ) and DLP group (n 5,
) and DLP group (n 5, ![]() ). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
Together, these results show that excessive consumption of saturated trans-fatty acids and cholesterol during pregnancy and lactation induce a maternal dysmetabolic condition characterised by dyslipidaemia and altered glucose tolerance and insulin sensitivity.
Short-term effect of maternal dyslipidaemia on somatic growth and biochemical parameters in juvenile male offspring
Fig. 4 shows the somatic growth curves from birth to 90 days. Body weight in the rats exposed to maternal dyslipidaemia was lower at birth (5·2 (sem 0·08) v. 5·6 (sem 0·1) g, P<0·05, Fig. 4) and weaning (39·5 (sem 0·7) v. 44·5 (sem 0·6) g, P<0·05, Fig. 4) when compared with the CTL rats. From weaning to 60 d, the body weight was similar between groups. However, at 90 d, the DLP rats exhibited significantly higher body weight (362 (sem 4·7) v. 336 (sem 4·9) g, P<0·05, Fig. 4) in comparison with the CTL rats. Despite the changes in body weight, no change was observed in body length (P>0·05; Fig. 4) and Lee index (P>0·05; Fig. 4).
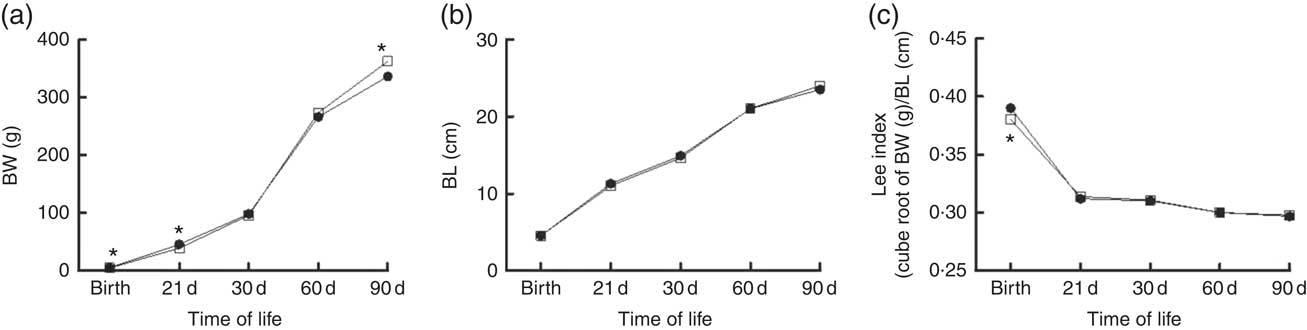
Fig. 4 Body weight (BW) (a), body length (BL) (b) and Lee index (c) in male offspring from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 20, ![]() ) and dyslipidaemic group (DLP, n 20,
) and dyslipidaemic group (DLP, n 20, ![]() ). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
Regarding the short-term effects of maternal dyslipidaemia on glucose handling in male offspring (30 d after birth), despite the fact that the DLP offspring exhibited increased glucose at 15 min (4·3 (sem 0·3) v. 3·5 (sem 0·19) mmol/l, P<0·05, Fig. 5) and a tendency for increased AUC of OGTT (P=0·06; Fig. 5), the fasting serum glucose concentration was similar between groups (P>0·05, Fig. 5). Serum lipid profiles of the male offspring reveal that serum TC and VLDL cholesterol concentrations were increased in DLP rats (P<0·05; Fig. 5). The TAG and HDL-cholesterol concentrations were not different between CTL and DLP offspring (Fig. 5).
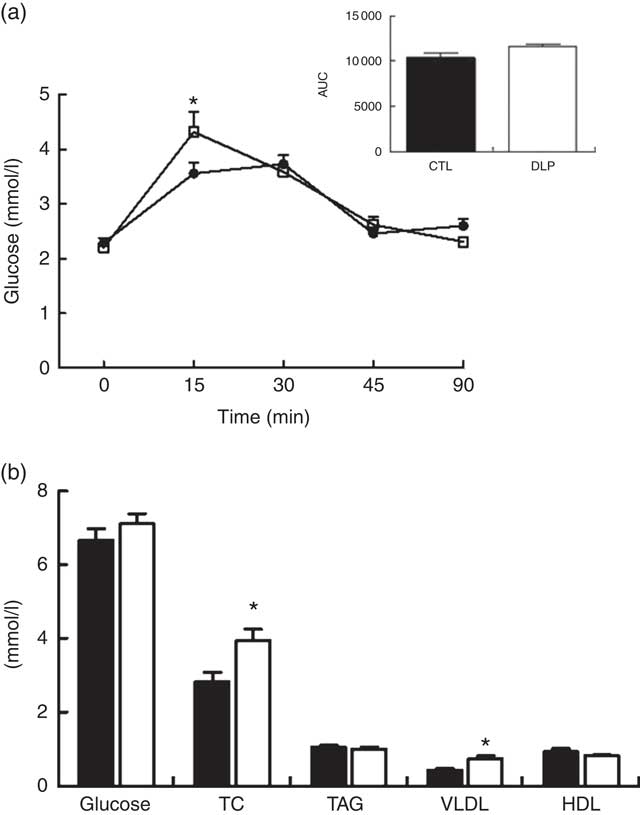
Fig. 5 Oral glucose tolerance test (a) and fasting serum of glucose, total cholesterol (TC), TAG, VLDL and HDL (b) in male offspring at 30 d old from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 8) and dyslipidaemic group (DLP, n 8). Values are means with their standard errors represented by vertical bars. a: ![]() , CTL;
, CTL; ![]() , DLP; b:
, DLP; b: ![]() , CTL;
, CTL; ![]() , DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
, DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
Long-term effect of maternal dyslipidaemia on the biochemical and cardiorespiratory parameters in male rat offspring
Investigating the long-term effects of maternal dyslipidaemia on glucose handling (80 d of age), insulin sensitivity and the serum lipid profile, no difference was observed between CTL and DLP male offspring (P>0·05; Fig. 6).
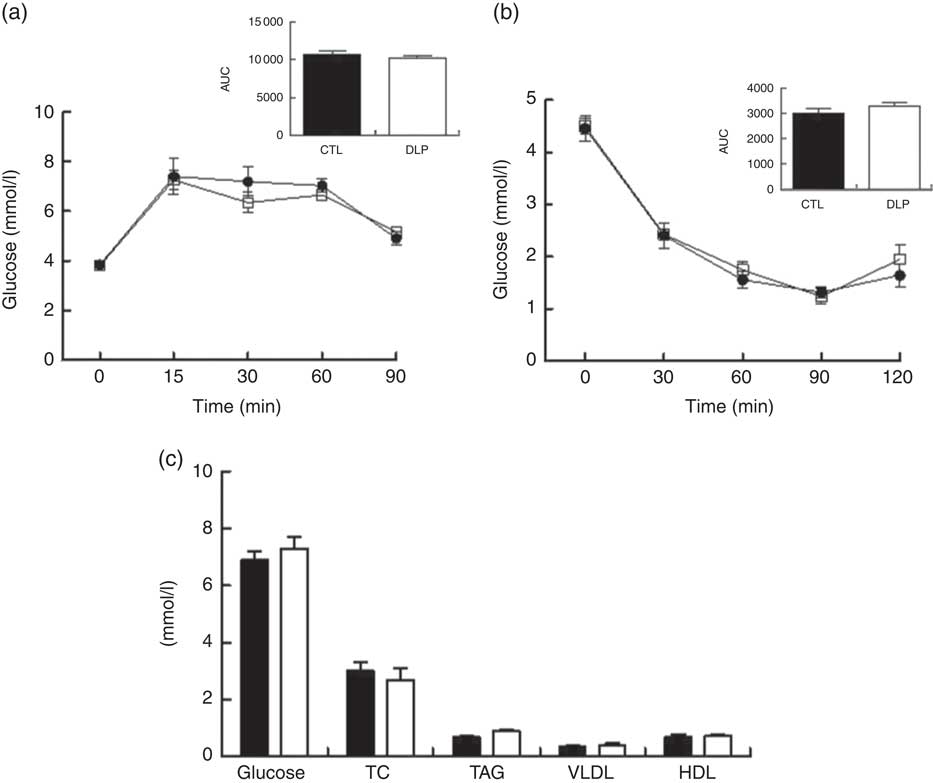
Fig. 6 Oral glucose tolerance test (a), insulin tolerance test (b) and fasting serum of glucose, total cholesterol (TC), TAG, VLDL and HDL (c) in male offspring at 80 d old from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control (CTL, n 8) and dyslipidaemic group (DLP, n 8). Values are means with their standard errors represented by vertical bars. a and b: ![]() , CTL;
, CTL; ![]() , DLP; c:
, DLP; c: ![]() , CTL;
, CTL; ![]() , DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
, DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test.
On examining male offspring at 90 d, we found that the DLP rats displayed an increase in SAP, DAP and MAP in comparison with the CTL group (P<0·05; Table 2). The arterial hypertension observed in our model was not associated with tachycardia, as evidenced by the similar baseline HR between the CTL and DLP groups (P>0·05, Table 2). Although BP and HR are interrelated components of the cardiovascular system, resting HR and BP do not necessarily increase at the same rate in hypertensive condition. The inverse proportion between BP and HR may be due to increased stiffness of the large arteries, such as the aorta and large elastic arteries. Spectral analysis using baseline BP recordings demonstrated that the LF range of SAP and the LF:HF ratio of the PI were augmented in the DLP group (P<0·05; Table 2). In relation to spontaneous baroreflex sensitivity, both the CTL and DLP rats exhibited similar baroreflex gain (P>0·05, Table 2). In addition, all ECG measures analysed, such as RR, PR, QRS and QT intervals and P duration, were not different between the CTL and DLP groups (P>0·05, Table 2). Representative baseline recordings of pulsatile arterial pressure (PAP), MAP and HR in male rat offspring are shown in the online Supplementary Fig. S2.
Table 2 Baseline parameters of blood pressure in male offspring at 90 d old from control and dyslipidaemic dams (Mean values with their standard errors)
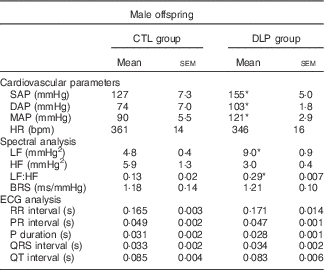
SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; HR, heart rate; bpm, beats per min; LF, low-frequency band; HF, high-frequency band; BRS, spontaneous baroreflex sensitivity; ECG, electrocardiogram. * P<0·05.
Under the baseline condition, RF, VT and VE values were similar between the CTL and DLP male offspring (P>0·05; Fig. 7). Hypercapnia (CO2 7 %) produced an increase in RF, VT and VE values in both the CTL and DLP groups (online Supplementary Fig. S2). In comparison with CTL offspring, the DLP rats exhibited higher values of ΔRF (Fig. 6) and ΔVE (Fig. 7), but no difference in ΔVT (Fig. 7) values after hypercapnia. Breathing recordings of representative rats from the CTL and DLP groups during the basal and hypercapnic conditions are shown in the online Supplementary Fig. S3. In addition, during the 5 min of hypercapnic gas flushing, the MAP (Fig. 7) was augmented in the DLP group when compared with the CTL group, but no difference in HR was observed (Fig. 7).
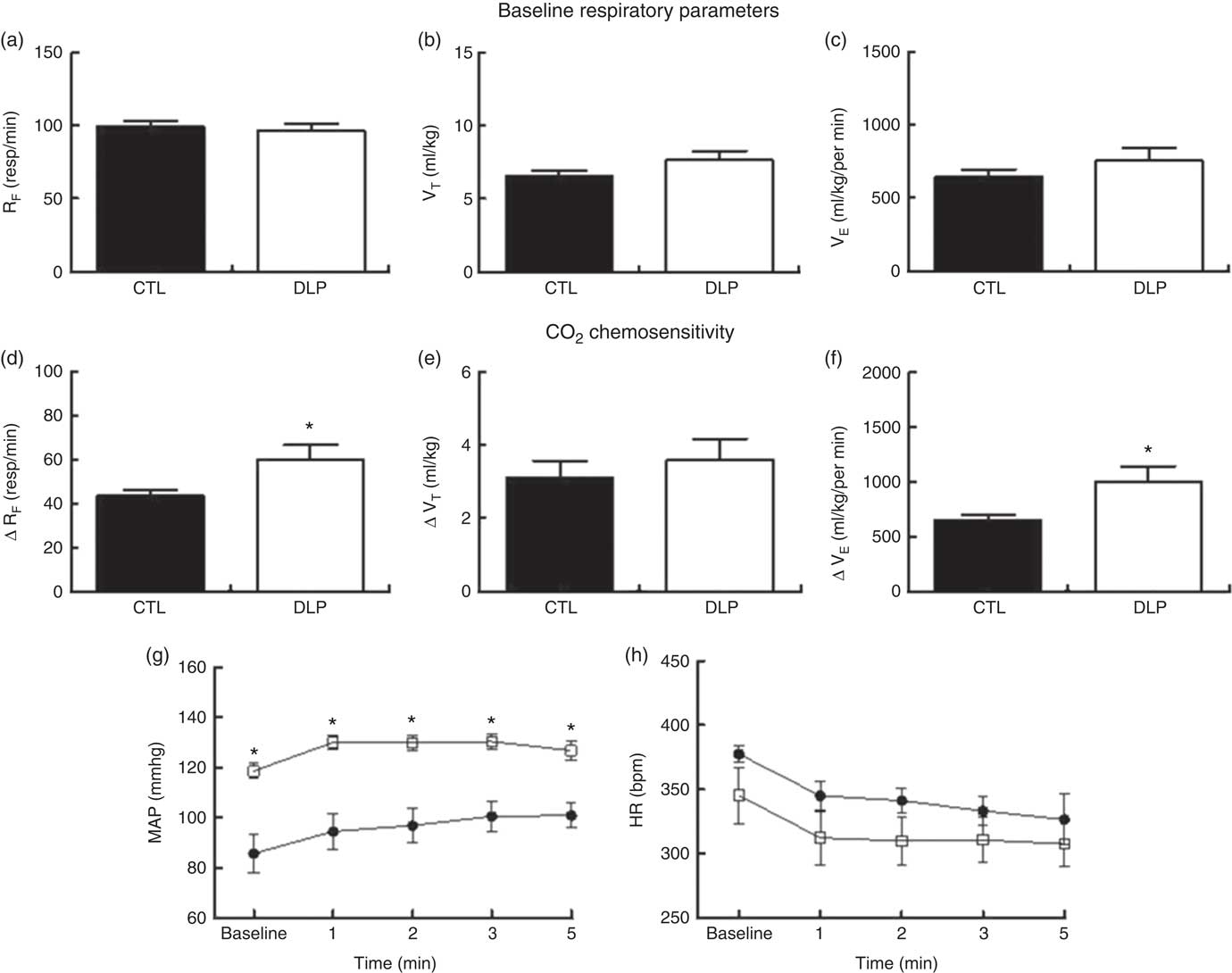
Fig. 7 Respiratory frequency (RF), tidal volume (VT) and ventilation minute (VE) at rest (a–c) and after hypercapnia (7 % CO2) (d–f). During the 5 min of hypercapnia, the values of mean arterial pressure (MAP, g) and heart rate (HR, h) were recorded in male offspring at 90 d old from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 10) and dyslipidaemic group (DLP, n 10). Values are means with their standard errors represented by vertical bars. g and h: ![]() , CTL;
, CTL; ![]() , DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test. bpm, Beats per min.
, DLP. * P<0·05 v. CTL using two-way ANOVA, followed by Bonferroni’s post hoc test or Student’s t test. bpm, Beats per min.
Peripheral chemoreflex activation produced pressor, bradycardic and tachypnoeic responses in both groups (Fig. 8). The increases in MAP (P<0·05; Fig. 8) and RF (P<0·05; Fig. 8) were significantly greater in the DLP group than in the CTL group. In contrast, the magnitude of the decrease in HR showed a tendency towards reduction in the DLP rats (P=0·06, Fig. 8). Together, these results suggest that the DLP offspring exhibited increased sensitisation of cardiorespiratory chemoreceptors.
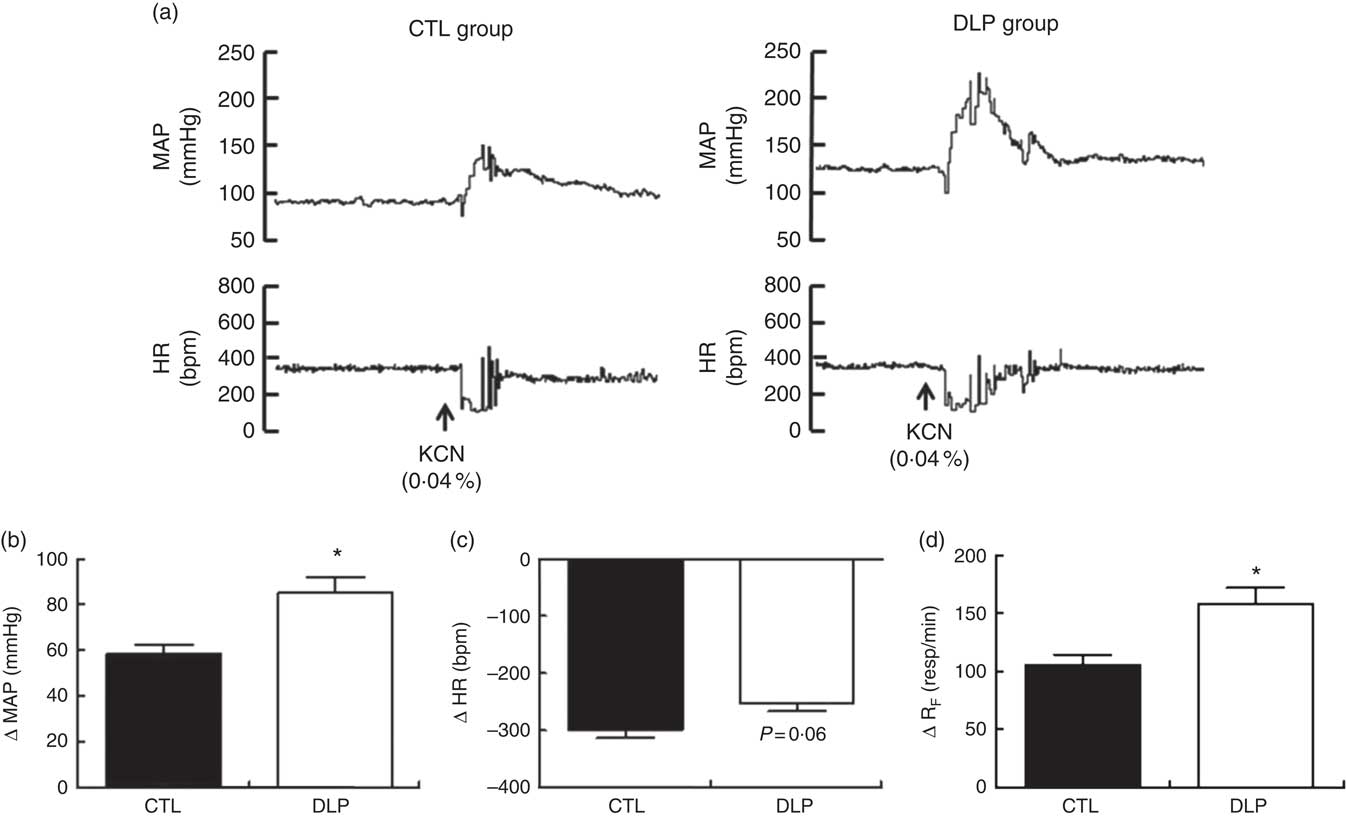
Fig. 8 Representative recording of mean arterial pressure (MAP) and heart rate (HR) during chemoreflex activation (a) and evaluation of variation in MAP (b), HR (c) and respiratory frequency (d) during chemoreflex activation (KCN, 0·04 %) in male offspring at 90 d old from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 10) and dyslipidaemic group (DLP, n 10). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using Student’s t test. bpm, Beats per min.
To test whether sympathetic tonus was augmented in DLP rats at 90 days, we evaluated the changes in MAP (ΔMAP) after administration of a pharmacological ganglionic blocker in both groups. After the blockage, the DLP rats showed increased ΔMAP (P<0·05; Fig. 9) when compared with CTL rats.
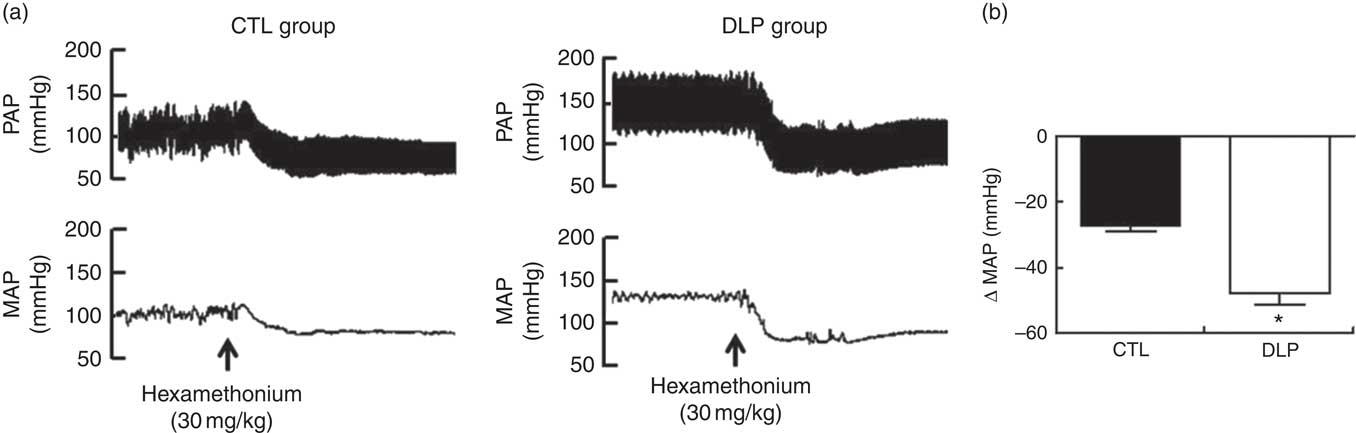
Fig. 9 Representative tracings of pulsatile arterial pressure (PAP) and mean arterial pressure (MAP) after hexamethonium (a) and evaluation of delta change of the MAP (b) in male offspring at 90 d old from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 10) and dyslipidaemic group (DLP, n 10). Values are means with their standard errors represented by vertical bars. *P<0·05 v. CTL using Student’s t test.
Short- and long-term effects of maternal dyslipidaemia on oxidative stress in male offspring
The quantification of serum MDA is summarised in Fig 10. MDA concentrations in DLP rats were increased at 30 d (P<0·05; Fig. 10) and 90 d of age (P<0·05; Fig. 10) when compared with the CTL rats.
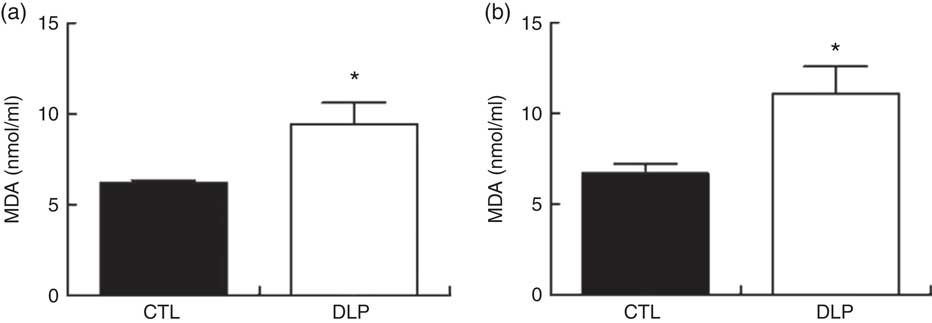
Fig. 10 Serum malondialdehyde (MDA) concentration at 30 d of age (a) and 80 d old (b) in male offspring from dams exposed to maternal dyslipidaemia during pregnancy and lactation. Groups: control group (CTL, n 8) and dyslipidaemic group (DLP, n 8). Values are means with their standard errors represented by vertical bars. * P<0·05 v. CTL using Student’s t test.
Discussion
Previous studies have reported that high dietary fat, cholesterol and sugar intake during pregnancy( Reference Musial, Vaughan and Fernandez-Twinn 14 , Reference El-Sayyad, Al-Haggar and El-Ghawet 35 ) or lactation( Reference Tsuduki, Yamamoto and Hatakeyama 36 ) impair lipid and glucose metabolism in mouse and rat dams.
To the best of our knowledge, here we have demonstrated for the first time that dams fed a DLP diet during pregnancy and lactation periods exhibit high levels of cholesterol, TAG, atherogenic index, compromised glucose tolerance, insulin sensitivity and hepatic steatosis at weaning. These findings suggest that an excessive consumption of a diet rich in saturated trans-fatty acids and cholesterol during pregnancy and lactation may be a useful experimental model for studying the effects of dyslipidaemia in dams and in their offspring.
Similarly to previous studies, we have observed that pups from dams fed a DLP diet had low weight at birth and weaning, but recovered normal body weight at 30 d of age( Reference Musial, Vaughan and Fernandez-Twinn 14 , Reference El-Sayyad, Al-Haggar and El-Ghawet 35 ). Interestingly, a recent meta-analysis of clinical trials found that maternal dyslipidaemia during pregnancy was associated with an increased risk of preterm birth( Reference Jiang, Jiang and Xu 10 ). Together, this evidence reveals that high levels of maternal lipid may increase the risk of low birth weight.
In adult life, DLP rats exhibited higher body weight in comparison with the CTL offspring. Both groups, however, presented a similar Lee index. Body fat composition was not analysed, so it is not possible to state that DLP offspring displayed obesity. Future studies investigating possible development of obesity will be focused in our next research.
In our study, serum TC and LDL-cholesterol were maintained at a high level in DLP offspring at 30 d of age. It has been shown that impaired glucose tolerance associated with high serum levels of cholesterol, TAG and fatty liver are observed in pups from dams fed a high-fat or high-cholesterol diet during pregnancy or lactation( Reference Tsuduki, Yamamoto and Hatakeyama 36 , Reference Sun, Purcell and Terrillion 37 ). These alterations possibly occur as a consequence of high cholesterol and fat content in the dams’ milk( Reference Tsuduki, Yamamoto and Hatakeyama 36 ).
In agreement with other studies that used maternal high-fat diet as a model( Reference Vidal-Santos, Macedo and Santana 9 ), our study showed that male offspring exposed to maternal dyslipidaemia during pregnancy and lactation developed arterial hypertension at 90 d of age. It should be noted, however, that another study reported that offspring from dams exposed to hypercholesterolaemia during pregnancy did not exhibit hypertension in adulthood( Reference De Assis, Seguro and Helou 38 ). However, in that study, BP was measured in anaesthetised rats, limiting the comparison with our results.
It is well accepted that the autonomic dysfunction, verified by excessive sympathetic tone and vagal withdrawal, alters the vascular reactivity and contributes to the development of hypertension in both hypertensive experimental models and hypertensive patients( Reference Zoccal, Simms and Bonagamba 27 , Reference de Brito Alves, Nogueira and Cavalcanti Neto 30 , Reference Simms, Paton and Pickering 39 , Reference Grassi 40 ).
The LF and HF ranges or the LF:HF ratio represent more accurately the sympathetic activity and autonomic modulation( Reference Barros, De Brito Alves and Nogueira 41 ). In addition to these methods for estimating sympathetic activity, we used an experimental protocol involving a ganglionic sympathetic blockade with hexamethonium. We observed in our study that LF power, LF:HF ratio and delta for arterial pressure variation (∆MAP) after hexamethonium infusion were increased in the DLP offspring. Together, our results support the hypothesis that the arterial hypertension observed in male rats from dams exposed to dyslipidaemia during pregnancy and lactation is associated with sympathetic hyperactivity. It is interesting to note that increased LF power, LF:HF index and great delta arterial pressure variation after hexamethonium infusion are observed in hypertensive rats( Reference Barros, De Brito Alves and Nogueira 41 ) and onset hypertension in men( Reference Carthy 42 ).
It has been reported that a baroreflex dysfunction could lead to a sympathetic over-activity and subsequent development of hypertension( Reference Meguro, Miura and Kimura 43 ). In addition, sympathetic outflow and BP have been shown to be modulated by the rhythmicity of the respiratory nervous system( Reference Dempsey, Sheel and St Croix 44 ). Part of this modulatory effect is influenced by the peripheral and central respiratory chemoreceptors. Activation of these chemoreceptors produces a powerful activation of the cardiorespiratory neuronal network and enhances the sympathetic and respiratory outflow( Reference Prabhakar 45 ).
On analysing spontaneous baroreflex sensitivity, we found no differences between the groups, suggesting that baroreflex control, at least within the narrow range of the physiological variations of BP, is not altered in adult rats exposed to maternal dyslipidaemia. This result differed from another study showing a reduction in the baroreflex control in male offspring from dams fed a westernised diet( Reference Vidal-Santos, Macedo and Santana 9 ).
The critical window relevant for the control of breathing and chemosensory consists of that period when respiratory control is still too immature and incapable of mounting an effective defence against acute respiratory challenges( Reference Bavis and MacFarlane 46 ). A number of environmental factors such as stress stimuli or oxygen levels experienced during early life can elicit, in the short or long term, developmental plasticity in the respiratory control system, which may be exemplified through changes in pulmonary ventilation or the hypoxic/hypercapinc ventilatory response( Reference Bavis and MacFarlane 46 , Reference Hill, Grandgeorge and Bavis 47 ).
Our research group has convincingly demonstrated that nutritional insults during pregnancy and lactation can have an impact on the development of the respiratory phenotype, such as causing changes in baseline ventilation and hypoxic and hypercapnic ventilatory response( Reference de Brito Alves, Nogueira and de Oliveira 4 ). Here, we have demonstrated that cardiovascular and ventilatory reflex responses to hypercapnia and hypoxia intensified significantly in male rats exposed to dyslipidaemia during pregnancy and lactation.
Clinical and experimental studies have shown that high levels of inflammatory markers (e.g. IL-6 and TNF) associated with high reactive O2 species (ROS) are found in the plasma and placenta from pregnant women with metabolic disorders or obesity( Reference Zambrano, Ibanez and Martinez-Samayoa 48 ). Together, pro-inflammatory cytokine and ROS could impair placental functioning and induce adverse effects on the fetus( Reference Roberts, Riley and Reynolds 13 , Reference Myatt and Maloyan 49 ).
A recent study showed that inflammation-induced discharge of PGE2 alters breathing patterns and increases ventilatory response to CO2 ( Reference Samarasinghe, Sands and Skuza 50 ). Interestingly, Samarasinghe and colleagues demonstrated that the ventilatory responses to hypoxia and hypercapnia were intensified in mouse offspring exposed to prenatal inflammation in the final days of gestation( Reference Forsberg, Horn and Tserga 51 ). It was also shown that oxidative stress augments chemoreflex sensitivity in rats exposed to chronic intermittent hypoxia( Reference Morgan, Bates and Rio 17 ). Our finding was that MDA levels, a biomarker used to assess oxidative stress, were increased in the serum of DLP offspring.
Considering the complementary approaches used in the present study, we propose, on the basis of our results, that environmental stress caused by maternal dyslipidaemia increases chemoreceptor sensitivity, which is a primary contribution to sympathetic hyperactivity. Then, these changes may reinforce the development of hypertension in maternal dyslipidaemia-induced hypertension model. It is, however, important to note that we investigated only male offspring. A growing number of studies have related that onset of hypertension evidenced in offspring from maternal nutritional insults dams is sex-dependent. The findings related that female offspring exposed to a nutritional insult during fetal life can be resistant to the development of hypertension in young adulthood( Reference Zambrano, Ibanez and Martinez-Samayoa 48 , Reference Bayol, Farrington and Stickland 52 ). Our study was limited by the lack of pro-inflammatory cytokine and stress oxidative marker measurements in dams and of investigation of the molecular mechanisms associated with our physiological findings. These gaps will be investigated in future studies.
In conclusion, the present study shows that male offspring from DLP dams exhibit hypertension in adult life combined with sympathetic hyperactivity and amplified ventilatory and autonomic responses to hypoxia and hypercapnia.
Acknowledgements
The English text of this paper has been revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip – TESL (Cambridge University).
V. A. B. and M. M. are recipients of the CNPq Fellowship.
K. S. L. G., E. V. d. A. and J. L. d. B. A. designed the research and conducted the experiments; K. S. L. G., E. V. d. A., J. S. A., C. M. B., D. A. G., J. H. C.-S., M. M., H. V., V. A. B. and J. L. d. B. A. analysed the data and performed the statistical analysis; and K. S. L. G., V. A. B., H. V. and J. L. d. B. A. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003014















