A breast-feeding mother secretes about 200–300 mg of calcium into breast milk every day and compensatory mechanisms to conserve calcium such as reduced urinary calcium excretion and increased maternal bone mineral turnover are active during lactation(Reference Vargas Zapata, Donangelo and Woodhouse1–Reference Holmberg-Marttila, Leino and Sievanen3). Studies from the west have shown a 4–7 % significant reduction of maternal bone mineral density (BMD) at lumbar spine and hip regions during 3–6 months of lactation(Reference Chan, Nelson and Leung4–Reference Affinito, Tommaselli and di Carlo6). Women who breast-fed for a longer time had a higher bone loss(Reference More, Bettembuk and Bhattoa7). Restoration of bone mineral density is known to occur following resumption of menstruation(Reference Ritchie, Fung and Halloran2, Reference Chan, Nelson and Leung4, Reference More, Bettembuk and Bhattoa7–Reference Krebs, Reidinger and Robertson9).
The afore-mentioned studies have been reported from developed countries, where women have moderate to high dietary intakes of calcium. The consequences of low calcium intake during lactation are not sufficiently studied. It is possible that magnitude and determinants of lactation-related bone changes are different in undernourished women consuming low calcium in their diets when compared with the well-nourished women consuming diets rich in calcium. A study in lactating Brazilian women with calcium intakes of about 460 mg/d suggested that the net balance in bone calcium turnover was positively associated with dietary calcium intake(Reference O'Brien, Donangelo and Zapata10), while other Western studies in women consuming over 800 mg of calcium per day have indicated that bone changes during lactation were not influenced by maternal dietary calcium intakes(Reference Cross, Hillman and Allen11, Reference Kalkwarf, Specker and Bianchi12).
Indian women from low socio-economic group subsisting on cereal-based low-calcium diets(13) breast-feed their infants for a prolonged period of time, often until 2 years of age. In addition, their calcium requirements during pregnancy and lactation remain high because of childbearing at a young age, probably before attainment of peak bone mass (PBM).
Studies from India have demonstrated the poor bone health of women from low socio-economic group as indicated by early fractures and a high prevalence of osteoporosis as early as 40 years of age(Reference Shatrugna, Shetty and Gopalan14–Reference Gupta, Samuel and Kurian16). Prolonged lactation with poor dietary intake of calcium and other nutrients in these women might strain the calcium balance and contribute to bone thinning and early osteoporosis. A descriptive longitudinal study was therefore planned to map the BMD changes during lactation using a sensitive technique of dual-energy X-ray absorptiometry.
Subjects and methods
Sample size
Assuming 95 % CI and 80 % power, standard deviation of BMD at lumbar spine − 0·1 g/cm2, correlation coefficient of spine BMD at two time points − 0·91 and expected difference in spine BMD of 2 %, the required sample size was calculated to be thirty-four. Assuming 20 % loss to follow-up, it was decided to enrol forty women.
Subjects
Forty consecutive women who attended the post-partum follow-up clinic in a large Government Maternity Hospital (Hyderabad, India) were enrolled for the afore-mentioned study during the period of June–September 2002. All the women had established breast-feeding, had undergone tubectomy and visited the clinic for their first follow-up within 1 month post-partum. Since only women who had undergone tubectomy were enrolled in the study, there were no women with parity 1. Parity of the study group women ranged from 2 to 5. None of the women were consuming medications that are known to affect bone metabolism. The study was approved by the institutional ethics committee. Informed consent was obtained from all the subjects.
Study design
Subjects were examined at four time points during the study period – within 1 month after delivery (baseline), and at 6, 12 and 18 months after delivery. At each time point, the following were assessed – maternal anthropometry (height at baseline and weight), infant's weight and length, maternal bone parameters at hip, lumbar spine, forearm and whole body, maternal body composition including lean mass, fat mass and per cent fat. Blood was drawn for estimation of biochemical parameters at each time point. Dietary intakes were assessed at two time points in a subsample of 50 % women – once during the 3–6 months post-partum and second time – at about 12 months post-partum. Information regarding resumption of menstruation and introduction of complementary foods was collected at each time point.
Customary medical care and nutrition advice were provided to all the subjects. The women breast-fed as long as they wished. They were advised to start energy-dense complementary foods at infant's 6 months of age as per the WHO guidelines.
Procedures
Anthropometry
Maternal weight was measured without footwear to the nearest 0·1 kg on lever-type SECA balance (Hamburg, Germany). Heights were measured to the nearest 0·1 cm with a stadiometer (SECA, Birmingham, UK). Infant's weight was measured with lever-type SECA mechanical baby beam balance (Hamburg, Germany), and length was measured with an infantometer made in-house at our Institute. Quality control checks were carried out every 3 months.
Bone mineral density measurements
BMD measurements were carried out using dual-energy X-ray absorptiometry (Hologic QDR 4500 W, Waltham MA, USA) at anteroposterior lumbar spine (L1–L4), hip and forearm as well as whole body including body composition. All scans and analyses were carried out according to the manufacturer's instructions by a trained technician. The scanner was calibrated daily and its performance was monitored as per the quality assurance protocol. No sign of scanner drift was observed during the study period.
The in vivo precision (CV %) was 1 % for lumbar spine and hip BMD and < 1 % for whole-body bone mineral content (BMC) measurements. Manufacturer's normative data were used as a reference range.
Biochemical parameters
A fasting blood sample was drawn in the morning between 09.00 and 10.00 hours in all the subjects and the estimations of biochemical parameters were carried out using standard procedures. Hb was estimated by the cyanmethaemoglobin method. Serum albumin(Reference Gustafsson17), serum tartrate-resistant acid phosphatase(Reference Schiele, Arthur and Floc'h18) and serum bone-specific alkaline phosphatase(Reference Cadeau and Malkin19) estimations were carried out on the same day.
The serum was processed and preserved, and estimation of serum calcium was done within a week of sample collection using atomic absorption spectroscopy. Breast milk sample (1–2 ml) was expressed manually into a sterile tube and calcium levels were estimated using atomic absorption spectroscopy.
Dietary calcium intakes
Dietary calcium intakes were estimated by 24 h recall method by a trained nutritionist. The nutrient intakes were determined using published food composition data(20).
Statistical analyses
SPSS Windows version 14.0 was used for statistical analysis. Descriptive statistics were calculated for all the bone parameters at different sites and time points, anthropometric measurements of mother and offspring, nutrient intakes and demographic variables like age and parity. Prevalences were calculated at different points for biochemical parameters. Z scores of height for age and weight for age were also calculated. Mean values of bone parameters at different time points were compared by repeated-measures ANOVA with post hoc test for each site. Per cent change from baseline was calculated as ((final value − baseline value) × 100)/(baseline value) for each bone parameter and site. Regression models were built to study the relationship of per cent change in BMD at different skeletal sites as dependent variables and height, weight, age, parity, lean mass, fat mass, time of baseline measurements since delivery and duration of post-partum amenorrhoea as independent variables.
Results
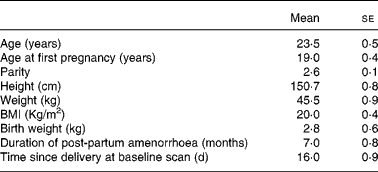
A total of forty women were enrolled for the study. However, thirty-six women completed follow-up till 18 months. The reasons for loss to follow-up were a change of residence (in two) and cessation of breast-feeding consequent to child's illness (in two). Characteristics of the women who were lost to follow-up were not different from those of the study women. Some women had metal jewellery that interfered with the scan area and thus the BMD measurements. The jewellery added about 1 g of mineral and therefore such scans were not included in the study. Therefore, whole-body measurements were available in thirty-two, lumbar spine measurements in thirty-five and forearm measurements in twenty-eight women. Measurements at the hip were available in all the women. The women enrolled in the study lived in urban slums and belonged to the low socio-economic group. All of them had singleton full-term deliveries. Twenty-eight women had vaginal deliveries, where as eight had Caesarean sections. The characteristics of the subjects are presented in Table 1. It is important to note that these women had their first child at 19 years of age, completed childbearing at 23 years and had a tubectomy done after the birth of the index child (parity of 2·6). Maternal height, weight and birth weight of the babies were comparable to the data from the low socio-economic group from India and much lower when compared with well-nourished women and infants from the west. The mean duration of post-partum amenorrhoea was 7 months with a wide variation.
Table 1 Baseline characteristics of the study women (n 36)
(Mean values with their standard errors)
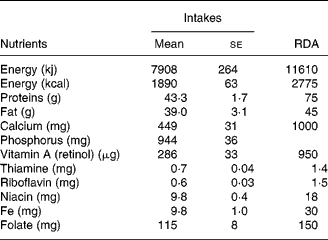
Dietary intakes
Dietary intakes are presented in Table 2. There were no significant differences in the nutrient intakes during early and later part of lactation and therefore, pooled values are given in Table 2. Diets were typically cereal based with very low intake of protective foods such as milk and milk products, flesh foods, fish, fruits and vegetables. Intake of all the major nutrients was below the RDA(21) for lactating women.
Table 2 Dietary intakes of the study subjects (n 18)
(Mean values with their standard errors)
Duration of breast-feeding
All the women except three breast-fed their infants throughout the study period of 18 months. One stopped breast-feeding after 6 months and two stopped after 1 year. Five women practised exclusive breast-feeding during the first 6 months post-partum. Commonly, small sips of water and animal milk feeds were offered during first 6 months, and semi-solid and solid foods were offered in the second half of infancy.
Changes in bone and body composition parameters during lactation
The baseline values of bone and body composition parameters and changes at four time points during the post-partum period are presented in Table 3 and Fig. 1. It may be observed that different sites responded differently during the period of the study. Femoral neck BMD showed a significant reduction at 6 months with partial recovery of BMD at 18 months. Changes in femoral neck bone area (BA) and BMC during this period were not statistically significant. Hip BMD reduced significantly at 6 months with gradual recovery to the baseline value by 18 months. Hip BA did not show significant changes, whereas hip BMC showed significant reduction at 6 months with return to baseline at 12 months itself. Forearm BMD showed reduction at 1 year with complete recovery by 18 months.
Table 3 Changes in maternal bone, body composition and biochemical parameters and infant growth during 18 months post-partum
(Mean values with their standard errors)

BA, bone area in cm2, BMC, bone mineral content in g; BMD, bone mineral density in g/cm2; lean mass and fat mass in kg; weight and weight of infant in kg; length of infant in cm; BSAP, serum bone-specific alkaline phosphatase in IU/l; TRAP, serum tartrate-resistant acid phosphatase in IU/l. WAZ, weight for age Z score; LAZ, length for age Z score.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).

Fig. 1 Bone mineral density (BMD) changes (per cent) at different skeletal sites and whole body during 18 months post-partum; n 36 (![]() , femoral neck;
, femoral neck;![]() , hip), 35 (
, hip), 35 (![]() , lumbar spine), 32 (
, lumbar spine), 32 (![]() , whole body) and 28 (
, whole body) and 28 (![]() , forearm). values are means, with standard errors represented by vertical bars. There was a significant reduction in BMD at hip as well as the whole-body bone mineral content (WB-BMC) at 6 months with complete recovery by 18 months. Femoral neck (FN) BMD reduced significantly at 6 months with a partial recovery at 18 months. Forearm BMD reduced significantly at 12 months with recovery at 18 months. Loss of lumbar spine BMD at 6 months was not statistically significant and the BMD increased significantly at 12 and 18 months when compared with the baseline.
, forearm). values are means, with standard errors represented by vertical bars. There was a significant reduction in BMD at hip as well as the whole-body bone mineral content (WB-BMC) at 6 months with complete recovery by 18 months. Femoral neck (FN) BMD reduced significantly at 6 months with a partial recovery at 18 months. Forearm BMD reduced significantly at 12 months with recovery at 18 months. Loss of lumbar spine BMD at 6 months was not statistically significant and the BMD increased significantly at 12 and 18 months when compared with the baseline.
At lumbar spine, the loss of BMC and BMD at 6 months post-partum was not statistically significant and there was a significant improvement in these parameters at 12 and 18 months when compared with the baseline value. BA at lumbar spine did not change during this period.
There was a significant reduction in whole-body BMC at 6 months with a gradual recovery by 18 months. Surprisingly, maternal weight, lean mass and fat mass did not show significant change in spite of prolonged lactation. Growth faltering of infants was observed with progressive decline in weight for age and length for age Z scores from 6 to 18 months.
Regression analysis
Regression models were constructed with per cent change in BMD at 6 months at each skeletal site as dependent variable, and baseline height, weight, lean mass, fat mass, age, parity, time of baseline measurements since delivery and duration of post-partum amenorrhoea as independent variables (Table 4). It was observed that baseline lean mass was the most important determinant of bone preservation or gain in BMD at femoral neck, hip as well as whole body, explaining 27, 50 and 25 % of variance, respectively, whereas baseline weight was the most important determinant of per cent gain in lumbar spine and fat mass was the most important determinant of per cent gain in forearm BMD explaining 18 and 19 % of variance, respectively.
Table 4 Regression models of per cent change in bone mineral density bone mineral density (BMD) at different skeletal sites at 6 months with height, weight, age, parity, duration of post-partum amenorrhoea, baseline fat mass and lean mass, and time of baseline measurements since delivery as independent variables

FN, femoral neck.
Biochemical parameters
The mean values of Hb, serum protein, serum albumin, serum calcium and serum zinc were normal and did not change from baseline during lactation. Serum phosphorus values reduced when compared with the baseline, throughout the lactation period (from 44 (se 1) mg/l at baseline to 38 (se 1) mg/l at 18 months, P < 0·05). As expected, breast milk calcium values showed significant reduction from baseline at 12 and 18 months (from 236 (se 14) mg/l at baseline to 148 (se 8) mg/l at 18 months). Serum bone-specific alkaline phosphatase did not show a significant change during lactation when compared with the baseline value. On the other hand, serum tartrate-resistant acid phosphatase showed a significant reduction during lactation when compared with the baseline value (7·8 (se 0·4) IU/l at baseline to 5·7 (se 0·5) IU/l at 18 months).
Discussion
This is the first study from India that investigates the lactation-related bone changes among undernourished women consuming diets with gross inadequacy of all the important nutrients, especially calcium.
The overall nutritional status of the study women as indicated by their height, weight, BMI and dietary intakes was comparable to that reported by National Nutrition Monitoring Bureau surveys, which are large-scale nutrition surveys of the low socio-economic population from India(13). The mean birth weight of their infants also corresponded to the mean birth weight reported for the low-income group population from India (Table 1).
The women breast-fed their babies throughout the study period with inadequate complementary feeds. Early introduction of animal milk in small quantities may have reduced the period of lactation amenorrhoea to 7 months in contrast to the earlier studies reported from the same population where it was 11 months(Reference Prema, Naidu and Neelakumari22). This practice of early introduction of animal milk to infants may not have affected growth in the first 6 months as indicated by their weight for age Z scores at baseline and at 6 months, but it may have hastened the return of menstruation. The effect of poor complementary feeding on growth of infants was apparent after 6 months of age.
The mean values of bone parameters were much lower than those reported for Western women of similar age group, indicating poor bone health of the study women(Reference More, Bettembuk and Bhattoa7, Reference Kalkwarf, Specker and Bianchi12). When the changes in bone parameters were studied at 6, 12 and 18 months of lactation, it was observed that the women suffered from a significant loss of BMD at femoral neck (4·6 % loss) at 6 months. There were no signs of recovery at 12 months, with only a partial recovery at 18 months (Table 3; Fig. 1). This finding is not in agreement with similar studies reported from the west. Most of the Western studies reported restoration of femoral neck BMD by 18 months post-partum(Reference Laskey and Prentice23, Reference Sowers, Corton and Shapiro24). On the other hand, loss of BMD at hip showed complete recovery at 18 months in the present study.
The changes in BA and BMC at different skeletal sites showed an interesting pattern. At the spine and total hip, changes in BMC appear to determine the changes in BMD. However, at the femoral neck and forearm, changes in BMD appear to be the result of both an increase in BA and a decrease in BMC, though these changes in BA and BMC are not significant.
Surprisingly, at lumbar spine, no significant reduction in BMD was observed at 6 months, despite the calcium drain in breast milk. This is in contrast to virtually all the studies reported in lactating women as none of these studies have shown the preservation of lumbar spine BMD (LS-BMD) at 6 months of lactation(Reference Chan, Nelson and Leung4–Reference Krebs, Reidinger and Robertson9). There was a wide variation in per cent change in LS-BMD, with some women losing up to 6·7 % of BMD and some women gaining up to 16 % of BMD at 6 months post-partum. Studies from the west indicate that LS-BMD loss occurred even among women having high dietary intake of calcium and shorter duration of lactation(Reference Karlsson, Obrant and Karlsson5–Reference More, Bettembuk and Bhattoa7). In the present study, LS-BMD showed a significant increase at 12 and 18 months when compared with the baseline value (3·6 and 6·3 %, respectively). Biochemical marker of bone resorption (serum tartrate-resistant acid phosphatase) showed a significant reduction at 6 months, whereas bone-specific alkaline phosphatase, which is a marker of bone formation, did not show a significant change when compared with the baseline. It was not possible to measure vitamin D and parathyroid hormone levels in the study participants. Vitamin D insufficiency is associated with secondary hyperparathyroidism and bone loss mainly from cortical sites, similar to the changes observed in the present study(Reference Sigurdssan, Franzsan and Steingrimsdottir25–Reference Duan, Deluca and Seeman27). However, studies investigating biochemical correlates of BMD changes during lactation have shown that calcitrophic hormones such as parathyroid hormone, 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D do not play a central role in calcium mobilisation associated with bone loss of lactation(Reference DeSantiago, Alonso and Halhali28, Reference Sowers, Zhang and Hollis29).
The women in the present study were younger when compared with the women from the Western studies. It may be speculated that the absence of LS-BMD loss in the present study may be because of an attempt to increase the BMD for PBM acquisition at this young age. But this finding is in contrast to a number of studies in adolescent mothers from the west who had a loss of BMD at the hip and spine(Reference Chan, Ronald and Slater30, Reference Sowers, Scholl and Harris31), despite their young age. Western studies in well-nourished adolescents have showed that they attained PBM shortly after puberty(Reference Sabatier, Guaydier-Souquieres and Laroche32, Reference Van Coeverden, De Ridder and Roos33) It needs to be explored whether undernutrition delays the age at PBM consolidation in the Indian context. It is known that undernutrition delays the attainment of adult height to as late as 20 years(Reference Satyanarayana, Radhaiah and Mohan34). Studies investigating the age at PBM development among undernourished populations in India are not available. Cross-sectional data from this Institute suggest that PBM may not be attained till 25–30 years of age even among Indian population from high income group with calcium intakes of about 800 mg/d(35). Different patterns of change at different skeletal sites may be linked to the differences in the age at which PBM is attained at those sites. Studies in Caucasian youth indicated that peak volumetric BMD at femoral neck was reached much earlier than lumbar spine in both men and women(Reference Henry, Fatayerji and Eastell36). A longitudinal study from Sweden also corroborated these findings(Reference Sundberg, Gardsell and Johnell37).
It is remarkable that despite poor dietary calcium intakes of about 450 mg/d and breast milk calcium losses of about 200 mg/d, these women could resist bone loss at lumbar spine at 6 months and could increase LS-BMD by more than 6 % at 18 months. Lack of significant decline in BMD at lumbar spine may reflect the delayed baseline measurements (within 1 month post-partum, not within 0·5 month as used by other studies) and hence, some decrease in BMD may have been missed.
Changes in forearm BMD, whole-body BMC and whole body BMD were transient and showed complete recovery at 18 months. It may be speculated that hormonal interplay and metabolic adjustments in young undernourished women may lead to an internal reorganisation of mineral in such a way that lumbar spine is privileged.
Increased bone turnover in the hypo-oestrogenic phase of post-partum amenorrhoea is known to be qualitatively similar to menopause. But unlike menopause, when metabolically active trabecular bone at lumbar spine shows rapid loss of bone density, lactation was associated with significant increase in lumbar spine BMD in the present study.
Surprisingly, in spite of prolonged lactation and poor dietary intakes of almost all the nutrients including energy, these women did not lose weight, lean mass or fat mass. Data collected from the women showed that they probably conserved energy by opting out of the labour force for a period of 1–1·5 years.
Regression analysis indicates that maternal weight and specifically lean body mass explain major variation in BMD changes at various skeletal sites among undernourished women.
A higher weight may have a positive effect on bone density by either bone-loading effect or it may be an indicator of higher nutrient reserves available for utilisation during the nutritionally demanding phase of lactation. Western studies in well-nourished subjects have not reported the relationship of maternal weight to the lactation-related bone changes. It may be speculated that among undernourished populations maintaining precarious nutrient balance, availability of nutrient reserves becomes important for resisting lactation-related bone changes.
Earlier studies from this Institute demonstrated that in undernourished populations, nutritional status as indicated by body weights and BMI is a major determinant of bone health(Reference Shatrugna, Kulkarni and Kumar15). The present study demonstrates that even during dynamic phase of lactation characterised by hormonal changes and increased calcium demand, nutritional status of women, as indicated by weight, lean mass and fat mass, continuing to be a major determinant of bone changes.
It is interesting to note that in the present study, lean body mass was a major determinant of a preservation of BMD at femoral neck, hip and whole-body BMC. Lean body mass is known to be a major determinant of development and maintenance of BMD at the hip region(Reference Vicente-Rodriguez, Ara and Perez-Gomez38). Muscle mass is the major constituent of the lean body mass and the positive effect of lean mass on BMD is thought to be mediated by its bone-loading effect as well as osteogenic stimulation by muscle force. It is remarkable that these functions of lean mass continue to be important for preservation of bone density at femoral neck and hip during lactation. Other studies have not looked for these relationships. It would be important to see the effect of different lean body mass values at a given BMI on lactation-related bone changes.
The present study thus provides important information regarding the lactation-related bone changes in young Indian women from the low socio-economic group. The study is unique with subjects having grossly inadequate intakes of all the nutrients including calcium, prolonged breast-feeding with little complementary foods and follow-up till 18 months post-partum. The results indicate that at the time of building maternal PBM, maternal weight, lean body mass and fat mass are important determinants of bone preservation during lactation. The study raises important questions regarding calcium balance in lactating women during the growth phase and stimulates further research to explore the relevance of nutrition to the bone changes during lactation.
Acknowledgements
The present study was funded by the Indian Council of Medical Research. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation or writing of this manuscript. The authors declare that they have no conflict of interest. B. K. and V. S. contributed to the study design, subject enrolment, interpretation of the results and the manuscript preparation. N. B. carried out statistical analysis of the data. P. A. K. was responsible for the biochemical estimations, K. U. R. for the bone measurements by dual-energy X-ray absorptiometry and A. C. O. for the estimations of dietary intakes. All the authors reviewed and approved the final version of the manuscript. The authors would like to thank the directors of the National Institute of Nutrition, Hyderabad, India, who provided the facilities and support system to carry out this work. They are grateful to Ms B. Prema Kumai, Ms K. Sundaramma and Mr R. Sambasiva Rao for their help during the course of the study. The present study would not have been possible without the unstinting cooperation of the women who were subjects of the study and the authors are grateful to them.







