INTRODUCTION
Recently, nutritionists have been searching for natural strategies with low cost and easy application in order to improve animal performance (Durmic & Blache Reference Durmic and Blache2012). Antibiotics and ionophores have very successful results for reducing energy and protein losses in the rumen (McGuffey et al. Reference McGuffey, Richardson and Wilkinson2001). However, the use of antibiotics in animal feeds is facing reduced social acceptance due to the potential appearance of residues in animal products (Russell & Houlihan Reference Russell and Houlihan2003). In addition, their use has been banned in the European Union since 2006 (Official Journal of the European Union 2003). For these reasons, there is interest in using medical plants and plant extracts as alternatives, based on their potent properties and complex bioactivity (Durmic & Blache Reference Durmic and Blache2012). In Mexico, there are native trees that can be used as an alternative feed when forages are scarce and of poor quality in the dry season (Palma et al. Reference Palma, Delgado, Rodríguez and Aguirre1995). However, the use of plants or their extracts as feed additives is restricted by their secondary compound content (Salem et al. Reference Salem, Kholif, Elghandour, Hernandez, Domínguez-Vara and Mellado2014c): although the ideal concentrations can modify and support the utilization of nutrients in the rumen (Salem et al. Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a, Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camachob, Reference Salem, Kholif, Elghandour, Hernandez, Domínguez-Vara and Melladoc), an inverse relationship between secondary compound concentration and animal performance has been observed (Vasta & Luciano Reference Vasta and Luciano2011; Salem et al. Reference Salem, Olivares, Lopez, Gonzalez-Ronquillo, Rojo, Camacho, Cerrillo and Mejia2011a, Reference Salem, Gado, Colombatto and Elghandour2013).
Some plant extracts contain secondary metabolites with active substances and molecules, released in the rumen during fermentation, that have the ability to change the activity of rumen microbial fermentation and stimulate appetite and digestion, due to their antimicrobial activity against some ruminal bacterial species. Some of these active compounds have antioxidant, anti-inflammatory, antiseptic or antiprotozoal properties, inhibiting ruminal ammonia nitrogen (N) release (Kamel Reference Kamel, Garnsworthy and Wiseman2001; Wallace et al. Reference Wallace, McEwan, McIntosh, Teferedegne and Newbold2002; Mejía-Hernández et al. Reference Mejía-Hernández, Salem, Elghandour, Cipriano-Salazara, Cruz-Lagunas and Camacho2014; Salem et al. Reference Salem, Kholif, Elghandour, Hernandez, Domínguez-Vara and Mellado2014c). This activity is due to the action of secondary metabolites such as tannins, saponins and essential oils. Moreover, it is known that microbial protein is an important part of the N flow of post-digestive tracts in ruminants, so that the excretion of purine derivatives (PD) and flow of purine bases into the duodenum have been used as a parameter to estimate the microbial protein flow in ruminants (Balcells et al. Reference Balcells, Guada, Peiro and Parker1992).
The use of exogenous enzymes in animal feed as an additive improves the nutritional value of tree foliage due to the occurrence of solubilization in the dietary fibre. Morgavi et al. (Reference Morgavi, Nsereko, Rode, Beauchemin, McAllister and Wang2000) showed that the use of exogenous enzymes has the potential to improve the quality of fodder trees used as natural additives for ruminant feeding. Exogenous enzymes can stimulate increases in the total number of viable bacteria, increasing fibre digestion and improving the ability of rumen bacteria to ingest and degrade feed and secondary metabolites. It could also increase the amount of crude protein (CP) available for microbial metabolism, which may increase fibre digestibility and the metabolizable energy (ME) density of the diet (Salem et al. Reference Salem, Hassan, Khalil, Gado, Alsersy and Simbaya2012). The exogenous fibrolytic enzymes can work synergistically with exogenous rumen microbial enzymes and thus could increase the digestion and nutritive value of fibrous diets (Morgavi et al. Reference Morgavi, Beauchemin, Nsereko, Rode, McAllister, Iwaasa, Wang and Yang2001).
A hypothesis was developed to explain the synergetic effects resulting from the combination of Salix babylonica (SB) extract and direct-fed enzyme on animal performance: optimal doses of secondary metabolic compounds from plant extracts with optimum doses of enzymes may have cumulative effects on animal performance. Rivero et al. (Reference Rivero, Salem, Gado, González-Ronquillo, Pliego, Peñuelas and Odongo2012, Reference Rivero, Salem, Ronquillo, Cerrillo-Soto, Camacho, Gado and Peñuelas2013) tested the effect of administering 30 ml SB extract in combination with 10 g ZADO® (i.e. an exogenous enzyme cocktail) on the performance of Suffolk lambs. Their results indicated that ZADO® enzyme (EZ) and SB as single or combined feed additives promoted growth performance without altering animal health or affecting cellular immune response or blood chemistry.
Therefore, the aim of the present study was to determine the effects of administering SB extract or/and exogenous enzymes on performance of lambs fed maize silage and concentrate as a basal diet.
MATERIALS AND METHODS
Animals and treatments
Sixteen male Suffolk lambs (29 ± 2·0 kg body weight) were divided into four treatments to be fed the control diet (300 g/kg concentrate (160 g CP/kg, 13·4 MJ ME/kg dry matter (DM) + 700 g/kg maize silage (80 g CP/kg, 11·7 MJ ME/kg DM)), SB (30 ml extract/animal/day), EZ (10 g ZADO®/animal/day) or a mixture EZSB (10 g ZADO® + 30 ml extract/animal/day). The extract was administered orally to individual lambs, once daily, with a 30 ml syringe before feeding, while the enzymes were provided in 100 g concentrate DM before feeding to assure their intake. Animals were housed in individual cages and offered concentrate and maize silage (Table 1) twice daily (08·00 and 16·00 h) for 60 days, with 10 days of adaptation to the diet. Feed and water intake were recorded every day. Each animal's weight was recorded every 15 days, after feed and water removal and before adding a new feed or water (shrunk weight) to calculate the average daily gain (ADG, g/day) and feed conversion (FC, g/day). Animals were housed in individual metabolic cages for 7 days in which faeces, urine and feed samples were collected daily. Sulphuric acid (50 ml/l) was added to the urine to keep the pH <3·0 in order to prevent proliferation of bacterial pathogens that could potentially destroy any PD present in each sample collected, then samples were frozen (−20 °C) for later analysis.
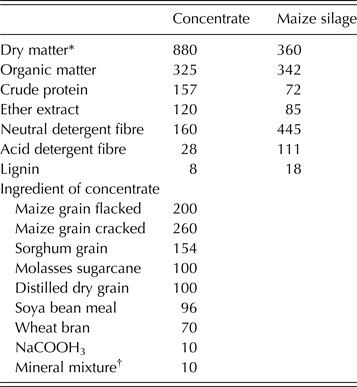
Table 1. Chemical composition of maize silage and concentrate mixture as well as the ingredients of the concentrate (g/kg DM)

* Dry matter expressed as g/kg fresh feed.
† Mineral/vitamin premix (25) (vitamin A (12 000 000 IU), vitamin D3 (2 500 000 IU), vitamin E (15 000 IU), vitamin K (2·0 g), vitamin B1 (2·25 g), vitamin B2 (7·5 g), vitamin B6 (3·5 g), vitamin B12 (20 mg), pantothenic acid (12·5 g), folic acid (1·5 g), biotin (125 mg), niacin (45 g), Fe (50 g), Zn (50 g), Mn (110 g), Cu (12 g), I (0·30 g), Se (200 mg), Co (0·20 g).
The commercial enzyme product, ZADO® is an enzyme cocktail obtained from Ruminococcus flavefaciens, recently developed by the laboratory of Rumen Ecology Centre, Animal Production Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt. ZADO® is a powdered multi-mix of cellulases, xylanases, protease and α-amylase enzymes, in addition to the related anaerobic bacteria which produce these enzymes (Khattab et al. Reference Khattab, Gado, Kholif, Mansour and Kholif2011), coated with starch and glycol. ZADO® is given to animals directly before feeding and is active immediately after feeding. The main actions are on rumen kinetics and improvements in how effectively the rumen microflora can utilize feed ingredients, and should be reflected in the animal's performance in terms of either milk or meat production (Khattab et al. Reference Khattab, Gado, Kholif, Mansour and Kholif2011; Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013).
Enzyme activities in the enzyme preparation were determined for endoglucanase (Robyt & Whelan Reference Robyt and Whelan1972), α -amylase activity (Bernfeld Reference Bernfeld1955), protease activity (Lin et al. Reference Lin, Gary and Robert1969) and xylanase activity (Robyt & Whelan Reference Robyt and Whelan1972) by catalysing hydrolysis of xylan from oat spelt: the reducing groups liberated were determined using alkaline copper reagent (Table 2).
Table 2. Composition of exogenous enzymes of ZADO®

* One unit (U) is defined as the amount (g) of enzyme needed to release 1 μmol xylose per minute from 5 mg/ml xylan solution (pH 5·5 and 37 °C).
† One unit (U) is defined as enzyme needed to produce 1 mg glucose from starch in 1 h by 1 g ZADO® (pH 4·6 and 40 °C).
‡ One unit (U) is defined as enzyme activity required to release 1 μmol reducing sugar from 4 mg/ml Na carboxymethyl cellulose in 1 min (pH 5·5 and 37 °C).
§ One unit (U) is defined as enzyme needed to produce 1 μmol amino acids from protein in 1 min (pH 5·5 and 37 °C).
Preparation of maize silage
Whole maize plants were cut at the beginning of grain development, growth stage (GS) 71) according to the BBCH scale (Lancashire et al. Reference Lancashire, Bleiholder, Langelüddecke, Stauss, Van Den Boom, Weber and Witzen-Berger1991): kernels were at the blister stage and c. 16% DM. Maize plants were chopped into lengths of 0·5–1·0 cm. Plants were ensiled and compacted with a tractor, covered with black plastic and tyres, and allowed to ferment for a further 1 year before feeding to sheep in the present study.
Preparation of extract
Preparation of the extract was as described by Salem (Reference Salem2012). Briefly, SB leaves collected from five different willow trees were blended in a Wiley mill. One kg of leaves in 8 litres of methanol/ethanol/distilled water (10/10/80, v/v/v) was kept at room temperature for 48 h before being placed in a water bath for 60 min at 30 °C. The solution was filtered with gauze, discarding the solid fraction and liquid fraction was retained at 4 °C.
For determination of active chemical constituents, subsamples of SB (100 g) were soaked in 150 ml of methanol, acetone and hexane (1 : 1 : 1 v/v/v) solvent at room temperature for 24 h. The crude extract was filtered through Whatman No.1 and over active charcoal to remove chlorophyll. The extract was concentrated in a vacuum to 20 ml and lyophilized to obtain the dried extract: 10 ml of the extract was used in gas chromatography–mass spectrophotometry (GC–MS; Varian Saturn 2100 T 3900 GC/MS mass selective detector connected to a RTX 6890 Gas Chromatograph, NY, USA) analysis. Separation was carried out in a capillary column, RTX 5MS (50 ml/l phenyl methyl polysiloxane) 30 m long, 0·25 mm internal diameter and 0·25 m film thickness. The column temperature was kept at 50 °C for 6 min and programmed to increase to 300 °C at a rate of 5 °C per min. The flow rate of helium (the carrier gas) was 1 ml/min with a split vent flow of 20 ml/min. The flow rate setpoint was adjusted by increments of 0–0·01 ml/min. An aliquot (1 μl) of the solvent containing the extract of S. babylonica was injected into the GC column with the injector heater at 300 °C. The MS was operated in full scan mode (40–650 m/z at a rate of two scans per second) with electron impact ionization (EI mode) at 70 electron volts (eV) at an ion source temperature of 230 °C. The relative proportion of constituents was expressed as mg/g of peak area normalization. Identification of extract components was based on direct comparison of the retention times and mass spectral data, computer search matching with the National Institute of Standards and Technology (NIST) MS Search 2·0 library, and by comparison of the fragmentation patterns of mass spectra data with those reported in the literature MS.
Laboratory tests
Samples of feed and faeces were weighed, dried in a forced air oven (60 °C for 48 h) and ground in a Wiley mill (3 mm). Conventional analysis of feed and faecal samples was carried out according to AOAC (1990) for DM (#934·01), ash (#942·05), N (#954·01) and ether extract (EE, #920·39). The neutral detergent fibre (NDF, Van Soest et al. Reference Van Soest, Robertson and Lewis1991), acid detergent fibre (ADF) and lignin (AOAC 1990; #973·18) analyses were conducted using an ANKOM200 Fibre Analyser unit (ANKOM Technology Corporation, Macedon, NY, USA). Neutral detergent fibre was assayed with α-amylase in the NDF. Both NDF and ADF are expressed without residual ash. Urine samples were subjected to determination of N (AOAC 1990) and of PD and creatinine in urine according to the method described by Balcells et al. (Reference Balcells, Guada, Peiro and Parker1992).
Calculations and statistical analysis
Microbial nitrogen (MN) was determined in mmol/day using the following equation (Belenguer et al. Reference Belenguer, Yañez, Balcells, Ozdemir-Baber and Gonzalez-Ronquillo2002):
where PD, purine derivatives (mmol/day), MN, microbial nitrogen (g/day). Metabolizable protein (MP) was determined by Alderman & Cottrill (Reference Alderman and Cottrill1993):
The growth performance data were analysed with the PROC MIXED procedure of SAS (2002) in which a completely randomized design was used with the statistical model Y ij = μ + T i + E ij, where Y ij is every observation of the jth lamb assigned to ith treatment, T i is the treatment effect, and E ij is the residual error. Comparisons of results were performed using Tukey's test at P < 0·05 (Steel & Torrie Reference Steel and Torrie1980).
RESULTS
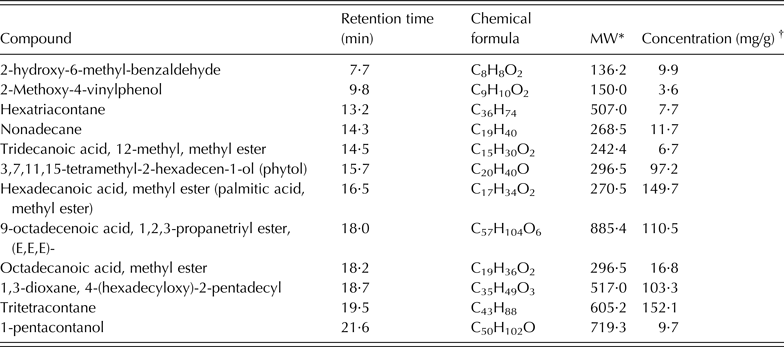
About 59 compounds were identified in S. babylonica extract; all of them were C10–C60 compounds: tritetracontane (an aliphatic hydrocarbon: 15·2 mg/g), 9-octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E, a trioleoylglycerol: 11·1 mg/g), hexadecanoic acid-methyl ester (a saturated fatty acid: 10·5 mg/g), 1,3-dioxane-4-(hexadecyloxy)-2-pentadecyl (a heterocyclic organic compound: 10·3 mg/g) and phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol: 9·7 mg/g). There were also some aliphatic hydrocarbons such as nonadecane (1·2 mg/g) and hexatriacontane (0·8 mg/g), and carboxylic acid in the form of oxygenated hydrocarbons (Table 3).
Table 3. Principal chemical constituents identified in Salix babylonica leaf extracts by GC/MS analysis (adapted from Salem et al. Reference Salem, Salem, Gonzalez-Ronquillo, Camacho and Cipriano2011b)

* MW, molecular weight of the compound (g/mol).
† Concentration based on the total areas of the identified peaks.
Analysis of enzyme activity showed greater content (U/g) of α-amylase and protease with a reasonable content from cellulase and xylanase (Table 2).
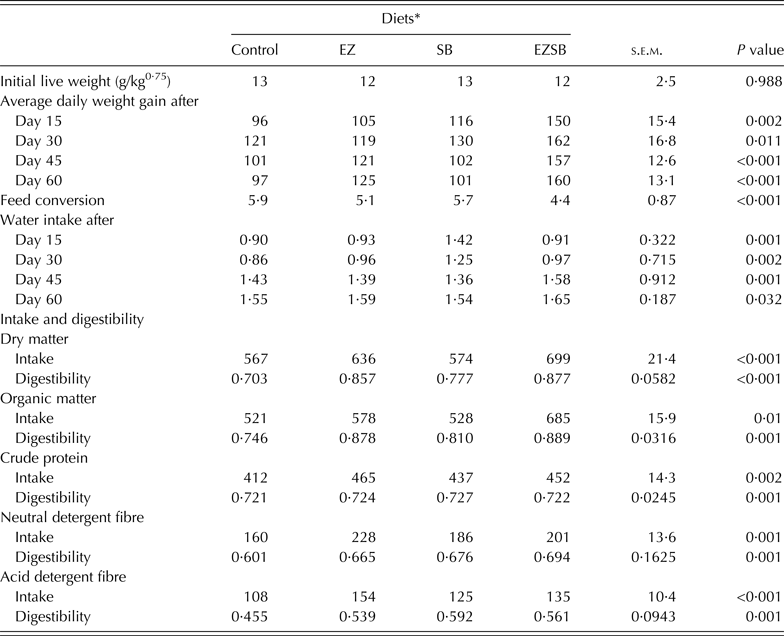
Initial metabolic weight (LW)0·75 of lambs showed homogeneity (P = 0·988) between different treatments. Lambs from treatment EZSB had the greatest (P ≤ 0·01) ADG (g/day) throughout the period of the experiment. However, during the first 30 days, treatment SB had greater (P < 0·05) ADG versus EZ and the control; while the EZ treatment increased (P < 0·05) ADG versus SB and the control during the last 30 days of the experiment. The FC ratios of lambs from the three experimental treatments increased versus the control in the order EZSB followed by EZ and then SB (P < 0·001) (Table 4).
Table 4. Average daily weight gain (g/day), water consumption (l/day), feed intake (g/day) and digestibility (g digested/g ingested) of the diets supplemented with exogenous enzymes and/or Salix babylonica extract in lambs (n = 5)

* Diets supplemented with of exogenous enzyme (EZ, 10 g/h/day), S. babylonica (SB, 30 ml/h/day) extract and their combination (EZSB).
Water consumption increased most (P = 0·001) in SB, followed by EZ during the first 15 days and in EZSB during the second 15 days (i.e. after 30 days) compared to the control. However, during the period from 45 to 60 days, lambs from EZSB consumed more water (P < 0·05) (Table 4).
Intake of DM and organic matter (OM) increased (P < 0·05) in EZSB followed by EZ treatments compared to the other treatments, whereas NDF and ADF intakes were greater (P < 0·001) in EZ treatments. Regarding nutrient digestibility, EZSB increased (P ≤ 0·001) DM and OM digestibilities. However, the SB treatment increased (P = 0·001) ADF digestibility compared to other treatments (Table 4).
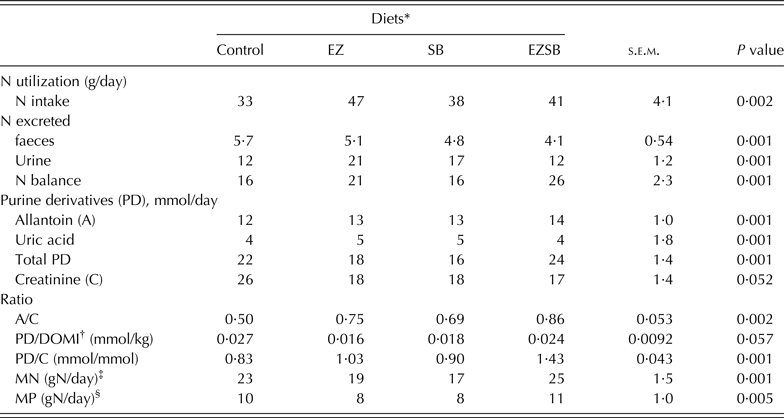
The greatest N intake (P = 0·002) was observed with EZ treatments, with less N excreted in the urine (P = 0·001): the control group had lower N intake but the greatest (P = 0·001) N extraction in faeces. The greatest value for N balance (P = 0·001) was recorded with the EZSB treatment followed by EZ (P = 0·001) (Table 5).
Table 5. Nitrogen utilization, purine derivatives and creatinine excretion in lambs fed maize silage and concentrate supplemented with mixture of exogenous enzymes and/or Salix babylonica extract (n = 5)

* Diets supplemented with of exogenous enzyme (EZ, 10 g/h/day), S. babylonica (SB, 30 ml/h/day) extract and their combination (EZSB).
† DOMI, dry organic matter intake.
‡ MN, Microbial nitrogen = (PD/0·52)/(0·92 × 1·97) = X mmol/day.
§ MP, Metabolizable protein (gN/day) = MN × 0·75 × 0·85 (Alderman & Cottrill Reference Alderman and Cottrill1993).
Concentrations of allantoin, total PD, allantoin : creatinine ratio (P = 0·002), and PD/creatinine (P = 0·001) were increased in EZSB compared to the other treatments, while uric acid concentration increased (P = 0·001) in EZ. Both MN (g N/day; P = 0·001) and MP (g N/day; P = 0·005) increased most in EZSB, followed by the control, compared to the other treatments (Table 5).
DISCUSSION
Daily weight gain, nutrient intake and digestibility
A limited number of exogenous enzymes and phytogenic extracts have been commercially introduced onto the market. In the present study, the ADG of lambs throughout the experimental period (i.e. 60 days) was improved by 51·6% with EZSB followed by 13·3% for EZ, and then by 8·2% for SB compared with the control treatment. The different feed additives (i.e. EZ, SB, EZ + SB) improved the intake and digestibility of DM, OM, NDF and ADF compared to the control. These improvements were reflected in the feed conversion ratios, which improved by 25·7% for EZSB, 13·5% for EZ and 3·2% for SB treatments. The increased feed intake, nutrient digestibility and daily gain with the administration of SB extract and/or enzymes may be related to the positive effects of metabolites contained in the extract (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Rivero et al. Reference Rivero, Salem, Gado, González-Ronquillo, Pliego, Peñuelas and Odongo2012: Salem et al. Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a, Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camachob), and/or the action exerted by the enzymes (Rivero et al. Reference Rivero, Salem, Gado, González-Ronquillo, Pliego, Peñuelas and Odongo2012). Researchers have demonstrated that a positive correlation between feeding animals diets supplemented with fibre-degrading enzymes, their performance and modes of action are uncertain (Alsersy et al. in press). Hydrolysis of dietary fibre before ingestion, provision of readily fermentable substrates for ruminal micro-organisms and enhanced synergism in the activity between microbial enzymes in the rumen are possible mechanisms (Morgavi et al. Reference Morgavi, Beauchemin, Nsereko, Rode, McAllister and Wang2004; Holtshausen et al. Reference Holtshausen, Chung, Gerardo-Cuervo, Oba and Beauchemin2011; Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013). Moreover, other positive improvements were observed as a result of administering enzymes, including enhanced microbial colonization of feed by increasing numbers of ruminal fibrolytic microbes (Morgavi et al. Reference Morgavi, Nsereko, Rode, Beauchemin, McAllister and Wang2000) or non-fibrolytic microbes (Colombatto et al. Reference Colombatto, Mould, Bhatt, Morgavi, Beauchemin and Owen2003), increased rate of fibre degradation in the rumen, increased rumen MP synthesis (Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013, Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a; Alsersy et al. in press) and total tract digestibility (Gado et al. Reference Gado, Salem, Odongo and Borhami2011; Khattab et al. Reference Khattab, Gado, Kholif, Mansour and Kholif2011). However, the increased digestion of fibre fractions in the EZ treatment compared to the control may also be related to reduced digesta viscosity (Hristov et al. Reference Hristov, McAllister and Cheng2000) or altered ruminal fermentation (Nsereko et al. Reference Nsereko, Beauchemin, Morgavi, Rode, Furtado, McAllister, Iwaasa, Yang and Wang2002). Previous reports using the same enzyme product have also shown that nutrient digestibility was increased (Gado et al. Reference Gado, Salem, Odongo and Borhami2011; Khattab et al. Reference Khattab, Gado, Kholif, Mansour and Kholif2011; Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013).
Although about 59 compounds were identified in S. babylonica, none of the individual active compounds was tested for its effect on animal nutrition. In contrast, many studies with whole crude SB extract (Salem et al. Reference Salem, Olivares, Lopez, Gonzalez-Ronquillo, Rojo, Camacho, Cerrillo and Mejia2011a, Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a) showed improved animal growth performance and nutrient digestion when the extract was administered to animals. These effects may be due to positive impacts of plant secondary metabolites on ruminal micro-organism activity (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Salem et al. Reference Salem, Hassan, Khalil, Gado, Alsersy and Simbaya2012). Administration of plant extracts may increase muscle deposition, with improved meat quality (Mapiye et al. Reference Mapiye, Chimonyo, Dzama, Strydom and Muchenje2010), due to increased amino acid flow to the duodenum (Mueller-Harvey Reference Mueller-Harvey2006). Improved daily weight gain may also be related to the ability of SB extract to reduce the amount of methane produced during fermentation, making more energy available for growth (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011). In fact, about 80–120 g/kg of the digestible energy ingested by ruminants is lost in the rumen as methane (Busquet et al. Reference Busquet, Calsamiglia, Ferret and Kamel2006). Within the rumen, some bacterial species are capable of metabolizing the active compounds of the extract, including phenolics (Varel et al. Reference Varel, Jung and Krumholz1991), alkaloids (Wachenheim et al. Reference Wachenheim, Blythe and Craig1992) and saponins (Hu et al. Reference Hu, Liu, Ye, Wu and Guo2005; Hart et al. Reference Hart, Yañez-Ruiz, Duval, McEwan and Newbold2008), and utilize them as an energy source. These metabolites may also act as catalysts for fibre degradation through increasing access of fibrolytic bacteria to the cell-wall components (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011). Phenylpropanoic acid and phenylacetic acid have been reported to improve cellulose degradation and growth of Ruminococcus albus (Stack & Cotta Reference Stack and Cotta1986).
The increased water consumption in the SB treatment may be due to the astringency of the extract. Mera Álvares (Reference Mera Álvares2004) found that the tannins present in the plants can precipitate the salivary proteins, causing an unpleasant astringent taste in the mouth. Forbes (Reference Forbes1995) reported that animals consume more water to adjust the osmotic balance in the gastrointestinal tract.
Nitrogen utilization and purine derivatives
Both the EZ and EZSB treatments increased N intake, by about 42·1 and 25·5%, respectively, followed by SB treatments with 15·8%, compared to the control. At the same time, EZSB and EZ treatments improved N balance by 64·5 and 36·8% compared to the control. However, plant extracts rich with active metabolites such as tannins can improve N utilization by impacting and binding with plant proteins in the rumen and preventing microbial degradation, causing an increased amino acid flow to the duodenum from the rumen (Mueller-Harvey Reference Mueller-Harvey2006). Besides having a protective effect on protein in the rumen, SB extract has the ability to promote duodenal absorption of amino acids and minimize the excretion of N (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Athanasiadou & Kyriazakis Reference Athanasiadou and Kyriazakis2004). Salem et al. (Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a) stated that S. babylonica extract supplementation to the diet of growing lambs caused a greater ADG with increased DM intake during the experimental period.
Increased N intake with both EZ and EZSB treatments may be related to increased nutrient digestibility caused by the addition of enzymes (Gado et al. Reference Gado, Salem, Robinson and Hassan2009; Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013; Alsersy et al. in press), which may be due to creation of a stable enzyme–feed complex (Kung et al. Reference Kung, Treacher, Nauman, Smagala, Endres and Cohen2000; Colombatto et al. Reference Colombatto, Mould, Bhatt, Morgavi, Beauchemin and Owen2003). The current results indicate a better utilization of nutrients, reducing environmental pollution in the soil and the amount of excess N excreted, therefore avoiding transformation of N to the greenhouse gas nitrous oxide (N2O) (Elizondo Salazar Reference Elizondo Salazar2006). The current results are similar to Pinos-Rodríguez et al. (Reference Pinos-Rodríguez, González, Mendoza, Bárcena, Cobos, Hernández and Ortega2002), who indicated that fibrolytic enzymes have a favourable impact on N balance in sheep fed alfalfa or ryegrass hay.
Some authors have established a relationship between the amount of digestible OM intake and the urinary excretion of PD (Balcells et al. Reference Balcells, Fondevila, Guada, Castrillo and Surra1993; Pérez et al. Reference Pérez, Balcells, Cebrian and Martin-Orue1998). However, others found no differences in PD excretion in urine with different intakes of OM (Chen et al. Reference Chen, Chen, Franklin, Orskov and Shand1992). Positive relationships have been described between DM intake and urinary excretion of PD (Vercoe Reference Vercoe1976) or OM digestibility and urinary excretion of PD in sheep (Balcells et al. Reference Balcells, Fondevila, Guada, Castrillo and Surra1993), goat (Lindberg Reference Lindberg1985) and cow (Liang et al. Reference Liang, Matsumoto and Young1994). The values reported in the present study for EZSB are greater than those reported for sheep (18·9–22·3) (Antoniewicz & Pisulewski Reference Antoniewicz and Pisulewski1982; Balcells et al. Reference Balcells, Fondevila, Guada, Castrillo and Surra1993). Salem et al. (Reference Salem, Gado, Colombatto and Elghandour2013) found that feeding steers on ZADO® caused an increase in total PD and allantoin compared to those of the control treatment (i.e. no enzyme addition). However, Gado et al. (Reference Gado, Salem, Robinson and Hassan2009) found no differences between Brown Swiss cows fed the same enzyme preparation (i.e. ZADO®) and those fed no enzymes.
The efficiency of MP synthesis increases with increasing feed intake (ARC 1984). The current results indicate that when lambs’ diets were supplemented with SB extract and enzymes, the quantity of MP available for metabolism and the net energy density of the ZADO® enzyme diet increased. Addition of SB extract was expected to have a positive effect on increasing degradabilities of CP and cell-wall constituents, as well as increasing MP; however, in the present study it had no effect on protein digestibility, with only numerically increased NDF digestibility. This may be related to the concentrations of active compounds and metabolites in the extract (Salem et al. Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a, Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camachob). Salem et al. (Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a) found that administration of SB extract caused an in vitro ruminal MP production from lambs fed increased concentrate diet.
From the current results, it was observed that cumulative positive effects were obtained as a result of combing SB extract with enzymes. The most important factor should be the dose of the additives administered (Jiménez-Peralta et al. Reference Jiménez-Peralta, Salem, Mejía-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Salem et al. Reference Salem, Gado, Colombatto and Elghandour2013). Rivero et al. (Reference Rivero, Salem, Gado, González-Ronquillo, Pliego, Peñuelas and Odongo2012, Reference Rivero, Salem, Ronquillo, Cerrillo-Soto, Camacho, Gado and Peñuelas2013) stated positive effects on Suffolk lamb performance with no effects on blood chemistry when both SB extract and ZADO® enzymes were administered together.
CONCLUSION
The supplementation of S. babylonica extract (30 ml/day) with exogenous enzymes ZADO® (10 g/day), given as an additive in a basal diet of growing lambs, improved their DM and OM digestibility and their live weight. Combination of the two feed additives had a positive impact on the use of N and MP synthesis.
The authors acknowledge the financial support from the IAEA, Vienna, Austria, Research Contract number MEX16307 within the D3·10·27 Coordinated Research Project. First author also wishes to thank the National Council for Science and Technology (CONACYT, Mexico) for the scholarship for her MSc at the Universidad Autónoma del Estado de México.