Introduction
Anthrax, caused by Bacillus anthracis is an infectious bacterial disease principally of herbivores although a wide range of hosts including carnivores and humans are affected to a lesser extent. It represents one of the most important diseases affecting both domestic and wild animals at the wildlife/livestock interface (later referred to as ‘interface’) [Reference Bengis, Kock and Fischer1] in anthrax endemic regions. The wildlife/livestock interface is defined as a direct physical sharing of the same space at the same time or indirect contact through soil, forage and water with which another animal had recently been in contact and left bodily secretions [Reference Bengis, Kock and Fischer1]. In anthrax endemic regions, sporadic outbreaks of the disease occur, causing significant deaths of both wildlife and livestock [Reference Hugh-Jones and Blackburn2–Reference Turnbull5]. However, anthrax does not always occur as a mass overt disease accompanied by high mortalities of animals [Reference Cizauskas6, Reference Lembo7]. Rather, anthrax may occur as isolated outbreaks/cases with a few animals affected that die from the disease. Such isolated outbreaks/cases of anthrax may be missed and remain unreported to relevant authorities. This conundrum has added to the challenges of diagnosis and surveillance of anthrax at the interface and to a better understanding of anthrax epidemiology.
Zimbabwe, alongside other southern African countries, is an anthrax endemic country [Reference Beyer and Turnbull8, 9]. Anthrax outbreaks have been occurring sporadically, but with more frequency in recent years in all animals – domestic livestock and wildlife, although the disease is better documented in cattle than in wildlife [Reference Clegg10]. The Government of Zimbabwe recognises the importance of anthrax, which is a notifiable disease in the country under the Animal Health Act [11]. According to this Act, some rural districts have been designated as anthrax endemic areas (latter called high-risk zones) based on history and incidence of past disease outbreaks. Such areas were identified through Statutory Instruments which were published in the Zimbabwe Government Gazette from time to time in response to the anthrax outbreaks’ situation in the country. However, the last update of anthrax endemic areas by the Government of Zimbabwe was as early as in 1982 (Statutory Instrument 221/1982 under Animal Health (Anthrax Areas) Order, 1982). Meanwhile, in recent years, anthrax has spread to more virgin areas. Chikerema et al. [Reference Chikerema12] found that anthrax outbreaks in cattle between 1967 and 2006 were recorded more frequently in areas generally from the central plateau to the western parts of the country. However, sporadic anthrax outbreaks were also noted to be scattered elsewhere across the country. This pattern largely agreed with later findings of a spatial modelling study on anthrax risk indicators based on environmental variables – mean precipitation, temperature and vegetation cover among others [Reference Chikerema13].
The unequivocal diagnosis of anthrax is principally based on culture and identification of the B. anthracis or by demonstration of the M'Fadyean reaction in polychrome methylene blue-stained peripheral blood smears from animals which had just died from the disease [Reference Gates, Elkin, Dragon, Williams and Barker4, Reference Hirsch, Biberstein, Hirsch, MacLachlan and Walker14, Reference Quinn15]. In highly susceptible hosts like herbivores, the course of anthrax is usually peracute to acute with high mortality in affected individuals, making serology of little value as a method of laboratory diagnosis, except only for the purpose of monitoring vaccine efficacy [16, Reference Ebedes and Fowler17]. However, carnivores and suids have been found to be relatively resistant, produce antibodies to B. anthracis and could even be reactive to unapparent infections in the range [Reference Beyer and Turnbull8, Reference Bagamian18]. Although the length of the reactive immunity in the affected carnivores has not yet been determined, it is suspected to be longer than a year, as compared with a few months in herbivores [Reference Hugh-Jones and Blackburn2, Reference Cizauskas6] thus potentially enabling detection of antibodies to B. anthracis in these animals well after the disease event [Reference Beyer and Turnbull8, Reference Bagamian19]. Anthrax antibodies have been detected in humans at least 2 years after consumption of B. anthracis-infected meat [Reference Turnbull20]. However, the duration of antibody reactivity following exposure by eating contaminated meat in humans is reportedly indefinite [Reference Turnbull20]. The development and application of the ELISA based on the protective antigen (PA) and lethal factor (LF) antigens for the ante-mortem diagnosis of anthrax in livestock, humans and wildlife have been described by Turnbull et al. [Reference Turnbull21], Boyer et al. [Reference Boyer22] and Ndumnego et al. [Reference Ndumnego23]. The justification for the use of these assays as ante-mortem diagnostic tests in the surveillance and determination of the extent of spread of anthrax in the range has been corroborated by Bagamian et al. [Reference Bagamian19].
Lembo et al. [Reference Lembo7] indicated that domestic dogs can be a useful indicator of anthrax in the localities even when the disease is not identified in other species. Thus, the current study aimed at exploring the use of the reactive antibodies to anthrax PA antigen by carnivores and its potential for use as a surveillance tool for determining the ecological presence of B. anthracis. More specifically, the objective was to describe the level of anthrax antibodies that would be detected in domestic dog populations along the wildlife/livestock interface. The second objective was to characterise the distribution of anthrax antibodies at interface and non-interface areas in Zimbabwe in relation to known high and low risk anthrax outbreak areas.
Methodology
Study areas and study design
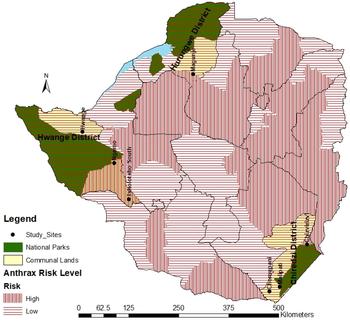
A cross-sectional survey of anthrax reactive antibodies in domestic dogs (Canis lupus familiaris) was carried out at selected interface and non-interface rural sites in Zimbabwe. A two-level study design was employed where, first, study sites were conveniently selected to represent interface and non-interface areas and secondly, individual dogs sampled from households that were randomly selected from the study sites (Fig. 1). Interface sites were those adjacent to wildlife areas, e.g. National Parks where there were known interactions between wildlife and domestic animals. In these sites, domestic dogs are anticipated not only to have access to anthrax infected domestic animal carcasses, but also those of wild animals. Non-interface sites were those distant, at least 15 km away from wildlife areas and where dogs have access to B. anthracis infected domestic animal carcasses only and their access to infected wildlife carcasses was assumed to be absent. The sites were selected in four districts: Chiredzi, Hurungwe, Hwange and Tsholotsho. Four interface sites were selected: Malipati and Chizvirizvi wards in Chiredzi close to Gonarezhou National Park; Ngamo Ward in Tsholotsho and Hwange rural Ward in Hwange District close to Hwange National Park; while non-interface sites were three: Chomupani Ward in Chiredzi, Magunje Ward in Hurungwe and Tsholotsho South Ward in Tsholotsho Districts (Fig. 1, Tables 1 and 2).

Fig. 1. Map of Zimbabwe showing study sites.
Table 1. Distribution of sampled dogs according to interface type, anthrax risk level, sex and age

Table 2. Distribution of anthrax seroprevalence in dogs according to interface type, site, anthrax risk, sex and age

The study areas were further classified as falling under anthrax endemic or high risk zones according to Zimbabwe Government (Animal Health (Anthrax Areas) Order, 1982, Statutory Instrument 221/1982) updated with additional zones identified by Chikerema et al. [Reference Chikerema12, Reference Chikerema13] based on history of anthrax outbreaks in the respective areas. According to this scheme, study sites located in high risk zones were Ngamo, Chizvirizvi, Tsholotsho South and Magunje wards (Table 3, Fig. 1). Hwange, Malipati and Chomupani wards fell under low risk zones for anthrax.
Table 3. Survey logistic regression analysis of the distribution of anthrax seroprevalence according to interface, anthrax-risk level, sex and age group of dogs

On average each ward had about 30 villages and each village 30 households, giving an average of 900 households per ward. In turn this gave a total of 6300 households across the seven wards in this survey. Based on available resources, approximately 30 households per ward, representing about 3.3% of the total, were selected by a simple random procedure. At least one dog was sampled from the identified household with a bias towards dogs older than 2 years. It was postulated that such dogs were more likely to have been exposed to anthrax if the disease occurred in the area. However, dogs younger than 2 years were also sampled in the absence of older dogs at identified households. Where a selected household had no dogs present, another household was conveniently selected from the list. Blood from study dogs was collected by cephalic venipuncture between September and December 2015. The dog was restrained by its owner and 4 ml of blood was drawn into a plain vacutainer tube and cold-preserved overnight till separation of serum. A total of 186 sera samples were collected from the dogs.
Laboratory testing
Dogs were screened for PA-reactive antibodies using the conventional PA ELISA. The 83-kDa PA was prepared as previously described in Hahn et al. [Reference Hahn24] and was obtained from Dr Wolfgang Beyer. Briefly, Escherichia coli BL21-CodonPlus-RIL cells (Stratagen, LaJolla, USA) harbouring the plasmid pREP4 and pQE-30 (Qiagen, Venlo, Netherlands) encoding rPA83 (recombinant PA 83) were grown, purified and dialysed against phosphate buffered saline [Reference Hahn24]. Sera were analysed for specific immunoglobulins using ELISA as previously described by Hahn et al. [Reference Hahn24] with modifications as described by Ndumnego et al. [Reference Ndumnego23]. Ninety-six well-microtitre plates were coated with anthrax antigen (0.5 µg PA) and incubated at 4 °C for 18 h. The plates were washed with a washing buffer made up of 0.05% Tween in phosphate buffered saline (PBS). This was followed by incubation at room temperature for 1 h with 200 µl of 10% skimmed milk in PBS as the blocking buffer. The plates were then washed twice. The serum samples and controls were set up in duplicates and diluted once at 1:50 with 10% skim milk in 0.05% PBST solution as the diluent, in a single point measurement for antibody reaction to the B. anthracis PA antigen. In each ELISA plate, duplicate wells were respectively, dedicated to known positive control serum from a vaccinated goat [Reference Ndumnego25] and negative control serum from a domestic dog from Pretoria, South Africa which was pre-screened for anthrax-reactive antibodies. Also six blank wells containing only blocking solution were also provided for per plate.
Following incubation at room temperature for 30 min with the lid on, the plates were washed five times and horseradish peroxidase-conjugated rabbit protein A/G [Reference Peng, Simons and Becker26] (Thermo Scientific, Rockford, USA) diluted 1:10 000 in skim milk diluent following reconstitution were added to the test and control wells and the plates incubated for 30 min at room temperature. After washing the plates five times, the enzyme substrate 2,2′-azino-di-(3-ethyl-benzthiazoline sulfonate (ABTS)) was added to the plate and allowed to develop in dark at room temperature for 40 min with the lid closed. Absorbance readings were taken at 405 nm using a Biotek powerwave XS2 reader (Winooski, USA). For each plate, the cut-off point for positive samples was the sum of mean of the negative control sera readings plus three standard deviations. Values equivalent to or higher than the cut off points were taken as positive results, while values below the cut off points represented negative results.
Data capture and analysis
The demographic data of the dogs sampled in this study was captured in Microsoft Excel software in which it was summarised and coded. Data captured included age, sex of dog, low or high anthrax risk area, interface type, geographical location (ward, district) and geographical coordinates – longitudes and latitudes. The results (numbers that were positive and negative) in the serological tests were also captured. Data analysis was performed in Stata Version SE/11 for Windows (Stata Corp., College Station, TX, USA). Individual animal seroprevalence estimates with 95% confidence intervals were computed using the survey data analysis command in Stata, with adjustment for study sites (wards) which represented the strata and the primary sampling units were households. Survey Data Analysis in Stata was used to perform logistic regression analysis according to Dohoo et al. [Reference Dohoo, Martin and Stryhn27]. The dependent variable was the seropositive status of dogs (0 = negative, 1 = positive) while independent variables were study site, type of interface, sex and age group of dogs, as well as anthrax risk levels (low and high risk) (Table 3). The odds ratio (OR) was used to evaluate the association between seropositivity and the epidemiological variables.
Results
The distribution of the sampled dogs according to interface type, sex and age is presented in Table 1 and their seroprevalence according to interface type, sex, age and risk zones are shown in Table 2. A total of 186 dogs were sampled with the majority (86.8%, 118/186) being from interface sites. Of the sampled dogs, 51.1% (95/186) were males and 48.9% (91/186) were females. Most (71%, 132/186) of the sampled dogs were more than 2-years old.
At the animal level, 96 out of 186 dogs tested were seropositive giving an overall seroprevalence of 51.6%. Overall, sites from the non-interface areas recorded a significantly (P < 0.001) higher (72.1%) anthrax seroprevalence compared with those from the interface (41.5%). The results demonstrated a strong association (χ 2 = 14.3, P < 0.001) between anthrax seropositivity and interface type and overall, the non-interface recorded a significantly higher odds (OR = 3.2, 1.6 < OR < 6.2, P < 0.001) of a dog being anthrax seropositive than the interface. In the interface areas, Hwange Ward (8.8%) recorded a significantly (P < 0.001) lower seroprevalence compared with the other three sites which recorded a seroprevalence ranging from 37% to 65.6% and there was no significant difference among them (Ngamo and Malipati P = 0.64; Ngamo and Chizvirizvi P = 0.27). Tsholotsho South site (seroprevalence of 90%) recorded the highest seroprevalence for the non-interface sites, which was significantly (P = 0.03) higher than that of Magunje (58.8%), another non-interface site (Table 2).
There was no significant difference (P = 0.67) in seroprevalence between male and female dogs respectively, at 53.7% and 49.5%. Similarly there was no significant difference in anthrax seroprevalence between dogs less than 2 years and those more than 2 years (P = 0.84) (Table 2).
Low anthrax-risk sites (42.5%) had a significantly (P = 0.04) lower seroprevalence compared with high anthrax-risk sites (58.5%). High risk sites in the non-interface area recorded an overall significantly (P = 0.02) higher seroprevalence (70.4%, 38/54) compared with high risk sites in the interface areas (46.2%, 24/52). Similarly, low risk sites in the non-interface area recorded an overall significantly (P = 0.03) higher seroprevalence (78.6%, 10/14) compared with low risk sites in the interface area (36.4%, 24/66). Dogs from Hwange and Tsholotsho South had the least and highest odds of being anthrax seropositive, respectively (Table 3). Dogs from Thsolothso South were more than 90-times (OR = 96.5, 13.5 < OR < 690.8) more likely to be seropositive compared with those from Hwange.
Discussion
There was a high proportion of positive antibody reaction to anthrax PA antigen in dogs across all study sites, thus indicating prior exposure to the pathogen despite last recorded anthrax outbreaks varying between sites from a few weeks to 20 years before the present study. There were seropositive dogs in both previously identified endemic (high) and low risk areas for anthrax although seroprevalence was expectedly higher in endemic areas [Reference Chikerema13]. A significantly higher proportion of dogs in non-interface sites reacted positively than those in interface sites. According to Hampson et al. [Reference Hampson28] and Lembo et al. [Reference Lembo7], location was a significant predictor of whether a dog would be antibody positive. Pastoralist areas where anthrax-related livestock cases and deaths had been reported regularly were associated with higher seroprevalences in domestic dogs [Reference Lembo7]. Our results demonstrated a strong association between anthrax seropositivity and interface type (location). Bagamian et al. [Reference Bagamian19] suggested that, since they are often not found in national parks and have a limited home range in comparison with other carnivores; domestic dogs are not ideal as sentinels for anthrax risk assessment in wildlife. During the present study, data collection on domestic dogs’ access into national parks and their roaming distance in the parks was not done. Nonetheless, the demonstration of the presence of antibody reaction to anthrax in domestic dogs at the interface can be relevant for wildlife conservation [Reference Beyer and Turnbull8, Reference Hampson28, Reference Hugh-Jones and de Vos29] since this point to B. anthracis contamination of the environment shared between domestic livestock and wildlife.
Although limited to seven wards in three rural districts in a country of 62 rural districts, these findings suggest that anthrax was more widespread in Zimbabwe than officially recognised. This tally with the findings of Chikerema et al. [Reference Chikerema13] where spatial modelling of the disease based on environmental factors found that the range of anthrax risk areas was potentially more than officially recognised. Hampson et al. [Reference Hampson28] and Hugh-Jones and de Vos [Reference Hugh-Jones and de Vos29] underscored the importance of environmental factors in influencing the distribution of anthrax. In support of earlier findings by Chikerema et al. [Reference Chikerema12, Reference Chikerema13], the findings of this research calls for a review and updating of the anthrax spatial distribution vis-à-vis designation of endemic areas in Zimbabwe by the responsible animal health authorities. This would be through improved anthrax surveillance and reporting as outlined by OIE [16] and results of research surveys to date. This should help animal health authorities in the planning and allocation of resources for better control of the disease [Reference Coffin30], as anthrax is a notifiable disease in Zimbabwe requiring mobilisation of state resources for its control.
While serology is routinely utilised to investigate zoonoses, it is less used for anthrax which is characterised by rapid disease progression leading to death [Reference Bagamian19]. In the case of wild carnivores, there is often little evidence of predators or scavengers dying from anthrax or anthrax-related pathology or illness [Reference Hampson28]. However, there are few instances where anthrax maybe incriminated in the decimation of wild carnivore species such as the case of African wild dogs (Lycaon pictus) documented by Woodroffe and Ginsberg [Reference Woodroffe and Ginsberg31]. This is supported by serologic investigations where antibody prevalence is higher in carnivores and omnivores (63–100%) than herbivores (0–46%) [Reference Bagamian19]. Hence, serosurveillance of carnivores and omnivores often helps to identify anthrax risk zones. In accordance with previous studies [Reference Lembo7, Reference Hampson28], domestic dogs were used to assess their potential value as an indicator of anthrax risk in livestock and wildlife. Cleaveland et al. [Reference Cleaveland, Meslin and Breiman32] and Hampson et al. [Reference Hampson28] detailed features that make domestic dogs useful sentinel hosts of livestock and human infections, especially in developing countries, namely that they were ubiquitous, susceptible to a wide range of emerging human infections, free-roaming scavengers, generally accessible for safe handling and sampling. This is corroborated in this study where positive antibody reaction to anthrax occurred in the absence of recent reported disease outbreaks in the respective areas. The anthrax outbreak in Magunje in 2015 happened in free-range domestic pigs a few weeks after the sampling of the dogs in the area (Table 2). Prior to the outbreak in domestic pigs in 2015, the last anthrax outbreak in the area was in 2012. Other areas had last recorded anthrax outbreaks more than two decades back (Malipati 1995, Chomupani 1995, Hwange 1994), while Chizvirizvi and Ngamo had last reported anthrax in 2013. The findings support occurrence of either frequent sub-lethal infections [Reference Bagamian19] or missed lethal but isolated cases in animals [Reference Hugh-Jones and de Vos29, Reference Beyer33]. These would include missed anthrax cases in both livestock and wildlife at the interface; with the latter being more frequently missed [Reference Cizauskas6, Reference Bagamian19, Reference Turnbull20, Reference Hugh-Jones and de Vos29, Reference Cleaveland, Meslin and Breiman32].
The standard OIE [16] surveillance procedures for anthrax are based on the detection of B. anthracis organisms in peripheral blood smears of unopened recently dead animals. Culturing for B. anthracis can also be done in old suspect carcasses or potentially contaminated environmental samples. Anthrax surveillance in domestic dogs could be made use of as an adjunct to these standard procedures in further evaluating the extent of ‘silent’ anthrax circulation in areas where the disease status is uncertain. In addition, longitudinal serological studies could produce additional information on the dynamics of ‘silent’ circulation of anthrax in dog populations providing key insights on the unknown pathogen ecology and disease epidemiology between outbreaks.
High seroprevalence was observed in domestic dogs sampled 6 weeks after an anthrax outbreak was detected in zebras and wildebeest [Reference Lembo7]. In our study, data on wildlife anthrax outbreaks in the national parks was not available. A simultaneous serosurvey in wild carnivores could have provided an insight on anthrax occurrence in the national parks and adjacent rural communities. Serology in lions has been shown previously to be a good way of monitoring anthrax activity in an area [Reference Turnbull20]. In the massive wildlife anthrax outbreak in Malilangwe Wildlife Reserve in the country, the finding of seropositive lions (16.7%, 2/12) prior to the outbreak indicated that anthrax was sporadically active in the region [Reference Clegg10] before the large outbreak. A significantly higher post-outbreak seroprevalence (92.3%, 12/13) was recorded in lions [Reference Clegg10]. High antibody titres in lions were also previously linked to recent exposure and were associated with anthrax foci in Namibia [Reference Turnbull34].
At present, knowledge of age and gender bias in anthrax infection is limited to mortality records [Reference Clegg10, Reference Lindeque and Turnbull35]. Limited literature is available on age ratios while there is currently none for gender ratios as part of anthrax serological surveys. In black-backed jackals (Canis mesomelas) exposure to B. anthracis was found to increase significantly with age and all animals older than 6 months were seropositive [Reference Bellan36]. Age seroprevalence in Serengeti African lions (Panthera leo) showed seroconversion at a young age and seropositive animals were found in all age groups [Reference Lembo7]. Seroprevalence patterns of domestic dogs indicated seroprevalence in dogs >1 year of age [Reference Lembo7]. In our study no significant effect of the dog's age and sex was found on the seroprevalence of antibodies against B. anthracis. This suggests that a biased sampling of older dogs for serosurveillance of anthrax might be unnecessary as younger dogs were equally reactive to B. anthracis. However, in our study age was dichotomised and this is a limitation that may result in loss of information and sometimes even inaccurate results [Reference Collins, Ogundimu and Altman37]. Further studies are required on gender and age ratios as part of anthrax serological surveys and such data may determine if exposure is gender and/or age biased.
In conclusion, the serological survey showed that anthrax was present in areas where the disease outbreaks have not been reported over long periods of time. It shows that the B. anthracis activity was more widespread and circulating more frequently than officially appreciated. The study also demonstrated the high potential of domestic dogs as indicators of anthrax circulation in the range. This tool could help understanding anthrax ecology in the apparent absence of disease. It could also potentially be used to determine the status of anthrax in areas where the disease presence or absence is uncertain due to lack of reported outbreaks in livestock species, given the missed cases or under reporting of outbreaks by communities [Reference Hugh-Jones and Blackburn2, Reference Bagamian18]. Such information would augment the surveillance measures for anthrax already outlined by WHO/OIE [9, 16], for better understanding the range of the disease [Reference Bagamian18, Reference Bagamian19, Reference Lindeque and Turnbull35] and hence targeted public health awareness campaigns and preventive livestock vaccinations where warranted.
Acknowledgements
This work was undertaken in the framework of the Research Platform ‘Production and Conservation in Partnership’ (www.rp-pcp.org) and funded by the Ministère Français des Affaires Etrangères through the FSP-RenCaRe project (FSP n°2011/36). The University of Zimbabwe Research Board also contributed financially to this study.
The authors would also like to acknowledge the collaboration in both field and laboratory activities on this project by the Department of Livestock and Veterinary Services, Ministry of Agriculture Farm Mechanization and Irrigation Development, Zimbabwe. In addition, many collaborating stakeholders enabled this research to take place including the cooperation of the communities of Chiredzi, Tsholotsho, Hwange and Hurungwe (Magunje Ward) Districts who readily availed their dogs for blood sampling and for availing more information on incidents of anthrax in their respective areas. Dr Farai Vhori is also acknowledged for his diligent work in helping the main researcher in the field sampling exercise.
Conflict of interest
None.