The potential association of eating frequency (EF) with metabolic risk factors has long been of interest. Several lines of evidence have indicated that people who eat frequently tend to have better blood lipid profiles than those who follow a gorging diet. In a review of randomised cross-over trials in adults, a dose–response relationship between EF and total cholesterol and LDL-cholesterol concentrations has been reported, with clinically significant reductions (>5 %) in total cholesterol and LDL-cholesterol concentrations being observed when at least six meals were consumed per d( Reference Bhutani and Varady 1 ). Additionally, a limited number of cross-sectional studies in free-living adults have consistently shown that a higher EF is associated with lower total cholesterol and LDL-cholesterol concentrations( Reference Titan, Bingham and Welch 2 – Reference Edelstein, Barrett-Connor and Wingard 4 ). However, whether the same association exists in younger populations (i.e. children and adolescents) has not been evaluated.
Many epidemiological studies conducted in children and adolescents have shown an inverse association between EF and adiposity measures( Reference Ritchie 5 – Reference Kontogianni, Farmaki and Vidra 18 ), with some exceptions( Reference Thompson, Ballew and Resnicow 19 – Reference Huang, Howarth and Lin 23 ). However, the results should be interpreted cautiously with regard to methodological limitations. First, although the assessment of EF has relied on a series of self-report questions in many studies( Reference Barba, Troiano and Russo 7 , Reference Antonogeorgos, Panagiotakos and Papadimitriou 8 , Reference Cassimos, Sidiropoulos and Batzios 11 – Reference Neutzling, Taddei and Gigante 15 , Reference Toschke, Kuchenhoff and Koletzko 17 , Reference Ferreira and Marques-Vidal 21 ), none of them has examined or reported the validity of the questions. Only a few studies( Reference Ritchie 5 , Reference Lioret, Touvier and Lafay 16 , Reference Thompson, Ballew and Resnicow 19 ) have assessed EF on the basis of information on actual dietary habits (using dietary records) over a sufficient number of days, which should be important because the day-to-day variation in an individual's EF can be relatively large( Reference Longnecker, Harper and Kim 24 ). Additionally, interpreting the literature on EF is complicated by the fact that there is no consensus about what constitutes a snack, a meal or an eating occasion. While some researchers have relied on respondents' self-identification of meals, snacks or eating occasions( Reference Keast, Nicklas and O'Neil 6 – Reference Antonogeorgos, Panagiotakos and Papadimitriou 8 , Reference Cassimos, Sidiropoulos and Batzios 11 – Reference Toschke, Kuchenhoff and Koletzko 17 , Reference Ferreira and Marques-Vidal 21 , Reference Nicklas, Yang and Baranowski 22 ), others have attempted to use more objective criteria( Reference Ritchie 5 , Reference Kontogianni, Farmaki and Vidra 18 – Reference Jennings, Cassidy and van Sluijs 20 , Reference Huang, Howarth and Lin 23 ) to overcome concerns over definitional differences. Furthermore, the apparent inverse relationship between EF and adiposity measures observed in many studies is likely to be an artifact and in large part can be attributed to the under-reporting of EF concomitant with the under-reporting of energy intake (EI), particularly by obese or overweight subjects( Reference McCrory, Howarth and Roberts 25 , Reference Bellisle, McDevitt and Prentice 26 ). For example, one study( Reference Huang, Howarth and Lin 23 ) found that when subjects with implausible EI were eliminated from the analytical sample, the inverse relationship between EF and BMI percentile no longer existed among children and adolescents. However, previous studies( Reference Ritchie 5 – Reference Toschke, Kuchenhoff and Koletzko 17 , Reference Thompson, Ballew and Resnicow 19 , Reference Ferreira and Marques-Vidal 21 , Reference Nicklas, Yang and Baranowski 22 ) have not taken into account such a potential reporting bias, with some exceptions( Reference Kontogianni, Farmaki and Vidra 18 , Reference Jennings, Cassidy and van Sluijs 20 , Reference Huang, Howarth and Lin 23 ). Taken together, concerns about these methodological limitations clearly bring into question the direction of the relationship between EF and adiposity measures and whether a relationship even exists. Thus, more robust studies are needed to clarify this issue.
Therefore, the primary aim of the present cross-sectional study was to examine the associations of EF with adiposity measures and plasma lipid concentrations as well as blood pressure in British children and adolescents. EF was objectively defined based on dietary information obtained from a 7 d weighed dietary record. The secondary aim was to examine the impact of exclusion of misreporters of EI on the associations.
Subjects and methods
Survey design
The present cross-sectional study was based on data from the National Diet and Nutrition Survey (NDNS): Young People Aged 4 to 18 Years. Data from the NDNS were obtained from the UK Data Archive, University of Essex. Full details of the rationale, design and methods of the survey have been described elsewhere( Reference Gregory and Lowe 27 , Reference Murakami, McCaffrey and Livingstone 28 ). Briefly, the sample was randomly selected from 132 randomly selected postal sectors within mainland Great Britain. Eligibility was defined as being aged 4–18 years. Selection of one eligible person per private household was done at random. Data collection was conducted during a 12-month period (January to December 1997). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the National Health Service Local Research Ethics Committee covering each of the postal sectors. Verbal informed consent was obtained from all the subjects and their parents/guardians. Verbal consent was witnessed and formally recorded. Additionally, written informed consent for blood pressure measurement and blood sampling was obtained from the subjects, their parents/guardians or both, depending on the age of the subjects.
Assessment of metabolic risk factors
All anthropometric measurements were performed in duplicate by trained fieldworkers, and the mean value of two measurements was used in the analysis. Height (to the nearest 0·1 cm) and weight (to the nearest 0·1 kg) were measured while the subjects were barefoot and wearing only light clothes. BMI (kg/m2) was calculated as weight (kg) divided by height (m) squared and converted to age- and sex-specific z-scores according to British growth reference data( Reference Cole, Freeman and Preece 29 ). For subjects aged ≥ 11 years, waist circumference was also measured at the midpoint between the iliac crest and the lower rib (to the nearest 0·1 cm). Waist:height ratio (WHtR) was calculated as waist circumference divided by height. Blood samples were collected after an overnight fast and analysed for total cholesterol and HDL-cholesterol and TAG concentrations( Reference Gregory and Lowe 27 ). We calculated the concentration of LDL-cholesterol using the Friedewald equation( Reference Friedewald, Levy and Fredrickson 30 ), except when the concentration of TAG exceeded 4 mmol/l. Systolic and diastolic blood pressures were measured, with the subjects seated, using the Dinamap 8100 oscillometric monitor after the subjects had been sitting quietly for 30 min. The three measurements were taken at pre-set 1-min intervals. The first measurement was discarded, and the mean of the following two measurements was calculated and used in the analysis.
Assessment of dietary intake
Dietary intake data were collected using 7 d weighed dietary records. A detailed description of the procedure has been published elsewhere( Reference Gregory and Lowe 27 , Reference Murakami, McCaffrey and Livingstone 28 ). Briefly, the subjects, the parents or both, depending on the age of the subjects, were asked to keep a weighed record of all food and drinks consumed by the subjects, both in and out of the home, over seven consecutive days. They were supplied with a set of digital food scales and recording diaries and given both written and verbal instructions by trained interviewers on how to weigh and record items in the diaries. When weighing was not possible (e.g. eating out), the subjects were asked to record as much information as possible. Trained interviewers visited the households of the subjects at least twice during the recording period and checked the completeness of food recording. All the collected diaries were checked by trained nutritionists in terms of coding, recorded weights and descriptions of items consumed. Estimates of daily intake of foods, energy and selected nutrients were calculated based on the Food Standards Agency nutrient databank( Reference Smithers 31 ), which is based on McCance and Widdowson's composition of foods series( 32 ) and manufactures' data where applicable. For all dietary variables, mean daily values over 7 d were used in the analysis. Values of nutrient intake were energy adjusted using the density method (i.e. percentage of energy for energy-providing nutrients and amount per 10 MJ of energy for dietary fibre).
Establishment of the number of eating occasions
Data from the 7 d dietary records were also used to calculate the average number of eating occasions per d, i.e. EF. Eating occasions were defined as any occasion when any food or drink was consumed( Reference Ritchie 5 , Reference Lioret, Touvier and Lafay 16 , Reference Kontogianni, Farmaki and Vidra 18 , Reference Jennings, Cassidy and van Sluijs 20 , Reference Hartline-Grafton, Rose and Johnson 33 – Reference Yannakoulia, Melistas and Solomou 35 ). If two eating occasion occurred in ≤ 15 min, both were counted as a single eating occasion; when two eating occasions were separated >15 min, both were considered to be distinct eating occasions( Reference Kontogianni, Farmaki and Vidra 18 , Reference Thompson, Ballew and Resnicow 19 , Reference Yannakoulia, Melistas and Solomou 35 , Reference Ma, Bertone and Stanek 36 ). EF was calculated based on all eating occasions, except for those providing < 210 kJ of energy. This calculation method has been used in several previous studies( Reference Hartline-Grafton, Rose and Johnson 33 – Reference Ruidavets, Bongard and Bataille 37 ) and was chosen to avoid giving undue weight to eating occasions that only included water, low-energy beverages or small quantities of foods.
Assessment of non-dietary variables
The socio-economic status of the head of the household (i.e. occupational social class) was reported and used as a proxy for children's social class. The following three categories were used: (1) manual (i.e. skilled manual, partly skilled and unskilled occupations: social classes III manual, IV and V); (2) non-manual (i.e. professional, managerial, technical and skilled non-manual occupations: social classes I, II and III non-manual); (3) unclassified.
For subjects aged ≥ 7 years, a 7 d physical activity diary was used concurrently with the dietary record. A detailed description of the procedure has been published elsewhere( Reference Gregory and Lowe 27 , Reference Murakami, McCaffrey and Livingstone 28 ). Briefly, the subjects were asked to provide information on the time spent being active from a list of prompted moderate-, vigorous- and very-vigorous-intensity activities. Information on activities that were not already listed and sleep was also provided. Trained interviewers checked the completeness of recording at least twice during the recording period. Subsequently, time spent daily on sleep and very light-, light-, moderate-, vigorous- and very vigorous-intensity activities was computed for each day of recording. The number of hours spent per d on each activity was multiplied by the metabolic equivalent value of that activity (derived from a published table)( Reference Ainsworth, Haskell and Herrmann 38 ), and all metabolic equivalent-h products were summed to obtain a total metabolic equivalent-h score for the day. The score was then divided by 24 h to give a physical activity level value and classified into four categories (sedentary, low active, active and very active) according to the US Dietary Reference Intakes( 39 ). For subjects aged ≤ 6 years, for whom activity diaries were not collected, the ‘active’ level was assigned based on a result on total energy expenditure measured by the doubly labelled water in the NDNS feasibility study( Reference Rennie, Jebb and Wright 40 ).
Evaluation of energy intake reporting
We calculated each subject's estimated energy requirement (EER) using equations published from the US Dietary Reference Intakes( 39 ). The subjects were identified as acceptable reporters (AR), under-reporters or over-reporters of EI based on their ratio of EI to EER (EI:EER), according to whether their ratio was within, below or above the 95 % confidence limits of the expected ratio of 1·0. Based on a published equation( Reference Huang, Howarth and Lin 23 ), AR were defined as having EI:EER in the range of 0·72–1·28, under-reporters those with EI:EER < 0·72 and over-reporters as those with EI:EER >1·28. A detailed description of the procedure has been published elsewhere( Reference Murakami, McCaffrey and Livingstone 28 ).
Analytical sample
Of the 2672 potentially eligible people identified for the study, 2127 (80 % of the eligible sample) participated in the survey. We excluded subjects with missing information on the variables examined (n 182 for anthropometric data; n 1254 for blood sample data; n 222 for blood pressure data; n 426 for dietary intake data; n 125 for physical activity data; and some subjects had more than one missing value). We further excluded underweight subjects (i.e. BMI ≤ 3rd percentile of the age- and sex-specific growth reference data( Reference Cole, Freeman and Preece 29 ); n 48), because they accounted for less than 3 % of the overall population, as well as comprised a group that may be malnourished and at risk of other clinical conditions (although inclusion of these subjects did not change the results materially (data not shown)). The final study sample comprised 1636 subjects aged 4–18 years (61 % of the eligible sample) for adiposity measures, 847 for blood lipid profiles and 1606 for blood pressure.
Statistical analyses
All statistical analyses were carried out for children aged 4–10 years and adolescents aged 11–18 years separately, using the SAS statistical software (version 9.2; SAS Institute, Inc.). Separate analyses carried out for boys and girls revealed similar patterns of associations of EF with metabolic risk factors, and tests for interaction with sex were not significant (data not shown). Therefore, we present results for both sexes combined. For the investigation of the association between EF and selected characteristics, EF was categorised at tertile points based on distribution. Linear regression analyses were carried out to investigate the associations of EF (independent variable) with BMI z-score, WHtR, total cholesterol, HDL-cholesterol and LDL-cholesterol concentrations, TAG concentration, and systolic and diastolic blood pressures (dependent variables). EF was analysed continuously in the main analysis after confirming the linearity of relationships using tertile categories. Using the PROC REG procedure, we calculated the adjusted regression coefficients (with standard errors) of variation of each of metabolic risk factors by one increase of EF. Potential confounding factors considered were age, sex, social class, physical activity levels, intakes of protein, fat, total sugar and dietary fibre, and EI:EER. For the analysis of blood lipid profiles and blood pressure, BMI z-score was also included as a potential confounding factor. These potential confounding factors were selected based on a comprehensive literature review of epidemiological studies on this topic( Reference Titan, Bingham and Welch 2 – Reference Huang, Howarth and Lin 23 ). EI was not included as a potential confounding factor not only because we considered it to be a potential causal factor for EF and adiposity measures but also because there was a strong correlation between EI and EI:EER (Pearson's r: 0·73). We made adjustment for the intakes of macronutrients as we intended to investigate the associations between EF and metabolic risk factors independently of macronutrient composition. Further adjustment for SFA intake (instead of total fat intake) in the analysis of blood lipid profiles did not change the results materially (data not shown). Similarly, further adjustment for Na and K intake in the analysis of blood pressure did not change the results materially (data not shown). The analyses were conducted not only for the entire population but also for AR.
Data have not been weighted to take into account known sociodemographic differences between responders and non-responders, not only because the impact of this adjustment, applied as a weighting factor, for nutritional variables was extremely small and not significant( Reference Gregory and Lowe 27 ) but also because we were only interested in relationships between variables, rather than estimates of prevalence( Reference Murakami, McCaffrey and Livingstone 28 ). All reported P values are two tailed, and P values < 0·05 were considered to be statistically significant.
Results
The mean value of BMI z-score was 0·38 in children (n 818) and 0·47 in adolescents (n 818; Table 1). The mean values of total cholesterol and LDL-cholesterol concentrations were, respectively, 4·24 and 2·75 mmol/l in children (n 324) and 4·02 and 2·57 mmol/l in adolescents (n 523). The mean value of EF was 4·9 times/d in children and 4·7 times/d in adolescents. The percentages of AR and under-reporters were 80 and 19 % in children and 47 and 52 % in adolescents, respectively (only six children (0·7 %) and three adolescents (0·4 %) were classified as over-reporters).
Table 1 Basic characteristics of the subjects (Mean values and standard deviations or percentages)
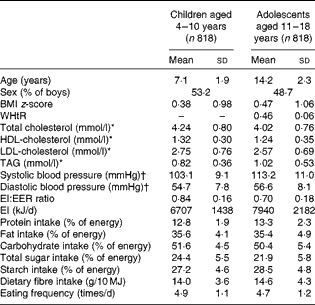
WHtR, waist:height ratio; EI, energy intake; EER, estimated energy requirement.
* n 324 for children and n 523 for adolescents.
† n 793 for children and n 813 for adolescents.
The associations between EF and potential confounding factors are summarised in Table 2. Subjects with a higher EF were more likely to be boys (only adolescents), be in non-manual social class and be physically active (only children). Additionally, EF was positively associated with EI:EER, EI and energy-adjusted intakes of carbohydrate and total sugar and inversely associated with energy-adjusted intakes of protein, fat (only adolescents), starch and dietary fibre.
Table 2 Selected characteristics according to the tertile (T) of eating frequency (EF) (Mean values and standard deviations or percentages)

EI, energy intake; EER, estimated energy requirement.
* Calculated using general linear models for continuous variables and a Mantel–Haenszel χ2 test for categorical variables.
† There were no subjects classified into ‘sedentary’ level.
Table 3 summarises the independent associations between EF and metabolic risk factors. In children, after adjustment for age, sex, social class, physical activity levels, intakes of protein, fat, total sugar and dietary fibre, reported EI:EER and BMI z-score, EF was inversely associated with total cholesterol and LDL-cholesterol concentrations (n 324, P= 0·01 and 0·04, respectively). Conversely, EF was independently and positively associated with BMI z-score in adolescents (P= 0·004). There were no associations between EF and other metabolic risk factors examined. In analyses in which only AR were included, similar results were obtained, except for an inverse association between EF and diastolic blood pressure in children (n 636, P= 0·04).
Table 3 Associations of eating frequency with metabolic risk factors* (Regression coefficients with their standard errors)

AR, acceptable reporters; WHtR, waist:height ratio.
* Adjustment was made for age (years, continuous), sex (boys or girls), social class (manual, non-manual or unclassified), physical activity level (low active, active or very active), protein intake (% of energy, continuous), fat intake (% of energy, continuous), total sugar intake (% of energy, continuous), dietary fibre intake (9/10 MJ, continuous) and BMI z-score (continuous, except for BMI z-score and WHtR). AR were defined as subjects with a ratio of energy intake to estimated energy requirement ranging from 0·72 to 1·28.
† Regression coefficients are the change in metabolic risk factors with one additional eating occasion per d.
Discussion
In the present British cross-sectional study, a higher EF was found to be associated with lower total cholesterol and LDL-cholesterol concentrations in children, while EF was found to be positively associated with BMI z-score in adolescents. There were no associations between EF and other metabolic risk factors examined, including WHtR, HDL-cholesterol concentration, TAG concentration and blood pressure. Similar results were obtained when only subjects with plausible EI were analysed. To our knowledge, this is the first study to examine the association between EF and blood lipid profiles in children and adolescents.
We found that while there was no association between EF and BMI z-score in children, a higher EF was associated with a higher BMI z-score (but not with WHtR) in adolescents. Mixed findings have also been obtained in well-designed previous studies. In a 10-year prospective study of girls, less frequent eating at baseline (9–10 years of age) was found to predict a greater gain in BMI and waist circumference( Reference Ritchie 5 ). Conversely, another prospective study of girls, aged 8–12 years, showed that a higher EF ( ≥ 6 times/d) compared with a moderate EF ( ≥ 4 to < 6 times/d) was associated with a higher increase in BMI z-score between 8 and 12 years of age and 11 and 19 years of age( Reference Thompson, Ballew and Resnicow 19 ). Additionally, a cross-sectional association between EF and BMI z-score and WHtR was observed in centrally obese children aged 9–10 years, after adjustment for energy misreporting( Reference Jennings, Cassidy and van Sluijs 20 ). In a cross-sectional analysis where only plausible energy reporters were included, no association between EF and BMI percentile in all three groups of young people (3–5, 6–11 and 12–19 years) was found( Reference Huang, Howarth and Lin 23 ). The relationship between EF and adiposity measures may differ in populations that have different dietary habits and adiposity profiles or both. The positive association between EF and BMI z-score that we observed in adolescents seems reasonable given the strong association between EF and EI (Pearson's r: 0·57), which has also been observed in many adult populations( Reference Titan, Bingham and Welch 2 , Reference Hartline-Grafton, Rose and Johnson 33 – Reference Yannakoulia, Melistas and Solomou 35 ). Although there was a positive but weaker association between EF and EI in children (Pearson's r: 0·33), EF was not associated with BMI z-score. Previous studies have shown that children are generally good energy compensators, although this ability declines with age( Reference Cecil, Palmer and Wrieden 41 ), which might explain the positive association in adolescents but the null association in children that we observed.
In the present study, EF was found to be inversely associated with total cholesterol and LDL-cholesterol concentrations in children, independently of potential confounding factors including BMI z-score. Similar associations have also been observed in adult populations( Reference Titan, Bingham and Welch 2 – Reference Edelstein, Barrett-Connor and Wingard 4 ). This may be due to reduced cholesterol synthesis or enhancement of reverse cholesterol transport( Reference Mann 42 ). We do not know why we found a positive association only in children but not in adolescents, but it may be due to higher mean values of and variations in blood lipid profiles in children or an adverse effect of EF on adiposity measures only observed in adolescents. Further research on this topic, particularly with a prospective design, is warranted. A limited number of previous studies( Reference Titan, Bingham and Welch 2 , Reference Smith, Blizzard and McNaughton 3 , Reference Barba, Troiano and Russo 7 ) have consistently shown null associations between EF and blood pressure. We also found no association between EF and blood pressure, but in the analysis of only children with plausible EI, EF was found to be inversely associated with diastolic blood pressure.
The advantages of the present study include the use of an objective definition of EF based on data obtained from 7 d weighed dietary records and the use of an individualised measure of EER to identify EI misreporters. However, there are also several limitations. One of the limitations is that the cross-sectional nature of the study does not permit the assessment of causality, owing to the uncertain temporality of the association. Only a prospective study taking into account dietary misreporting would result in a better understanding of the associations between EF and metabolic risk factors.
We used BMI and WHtR as proxy measures of body fatness. As BMI reflects not only body fatness, but the relative length of the legs, body frame size and fat-free body mass( Reference Prentice and Jebb 43 ), subjects with a similar BMI (z-score) do not necessarily have the same amount of body fat. A more valid measure of body fat mass (e.g. dual-energy X-ray absorptiometry) may be needed for further investigation. Also, only a single measurement of lipid concentrations was used, which is not optimal for characterising individual lipid profiles, and such random measurement errors are likely to obscure or minimise the effect size of any association.
Another limitation of the present study is that only 61 % of the eligible sample was included, although the response rate was relatively high (80 %). The subjects included in the present analysis (n 1636) differed somewhat from those excluded from the analysis (n 491). The excluded subjects were more likely to be younger and be in a social class classified as manual occupations (all P< 0·05). Furthermore, subjects with blood sample data (n 843) had a higher mean value of EF and EI (all P< 0·05) than those without blood sample data (n 793), although there was no difference in other dietary variables examined. However, a previous analysis has concluded that there is no evidence to suggest a serious non-response bias in the NDNS( Reference Gregory and Lowe 27 ). Additionally, although we adjusted for a variety of potential confounding variables, residual confounding could not be ruled out. In particular, adjustment for physical activity levels may be insufficient in the analysis of children as all subjects aged ≤ 6 years were categorised into the same category because of a lack of information. Also, we could not control for puberty status or parental weight status because of a lack of information, which may cause potential confounding by unknown or unmeasured factors. Furthermore, because only about 5 % of the subjects reported eating less than three meals per d in the present study, the findings in adolescents should not be interpreted as a piece of evidence that eating less frequently (e.g. one or two meals per d) is an effective way to prevent obesity. Moreover, in the present study, an eating occasion was classified as any event that provided ≥ 210 kJ of energy with a minimum time interval >15 min between episodes. Although this definition has been used in several previous studies( Reference Kontogianni, Farmaki and Vidra 18 , Reference Thompson, Ballew and Resnicow 19 , Reference Hartline-Grafton, Rose and Johnson 33 – Reference Ruidavets, Bongard and Bataille 37 ), some arbitrary decision (i.e. energy content and time interval) is inevitable by nature. However, there is currently no consensus about what constitutes an eating occasion. Thus, the present results should be interpreted in this regard, and different findings may be obtained based on different definitions of eating occasions.
Finally, we assessed misreporting of EI against calculated EER using published equations( 39 ). In the absence of measured total energy expenditure, these equations with high R 2 values ( ≥ 0·95)( 39 ) should serve as the best proxy. Nevertheless, the selection of physical activity category was based on self-reports (i.e. 7 d physical activity diaries) in subjects aged ≥ 7 years and fixed in subjects aged ≤ 6 years, which may be susceptible to systematic errors. Additionally, we do not know the sensitivity and specificity of the procedure for identifying EI misreporters used. However, even though some misclassification of subjects according to EI reporting status did occur in the present study, we are confident of our conclusions, because the associations of EF with metabolic risk factors observed in the entire population were similarly observed in AR. Nonetheless, it should be stressed that the role of misreporting was mainly evaluated only in terms of under-reporting because over-reporting occurred in such a low number of cases that no conclusions could be drawn in this regard.
In conclusion, the present cross-sectional study in Britain demonstrated that after adjustment for potential confounding factors, EF was inversely associated with total cholesterol and LDL-cholesterol concentrations in children and positively associated with BMI z-score in adolescents. These findings were not influenced by misreporting of EI, as similar associations were observed not only in the entire population but also in subjects with plausible EI. Further research, particularly with a prospective design, is needed, taking into account energy misreporting, so that firm conclusions can be drawn with regard to the effect of EF on metabolic risk factors in young populations.
Acknowledgements
The present study was supported in part by the JSPS Postdoctoral Fellowships for Research Abroad, Japan Society for the Promotion of Science, Japan (to K. M.). The authors' contributions are as follows: K. M. designed the study, analysed and interpreted the data, and wrote the manuscript; M. B. E. L. helped with the writing of the manuscript. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.





