‘Rapid tranquillisation’ refers to giving medication with the intention of calming highly aroused or agitated individuals in the context of mental disorders, who have not calmed sufficiently with non-pharmacological approaches. It is commonly the initial stage in the treatment of a first or recurrent episode of severe mental illness, and is followed by treatment to reduce other symptoms, leading to remission and prevention of relapse. It therefore offers an opportunity to begin or renew a therapeutic engagement. It is also called acute tranquillisation, understanding that it is not always quickly effective and it may take many days for the underlying condition to be controlled.
The disorders involved are usually affective disorders or schizophrenia (Pilowsky Reference Pilowsky, Ring and Shine1992), often complicated by substance misuse and personality disorder. Delirium and dementia may also cause agitation but are not considered in this article.
Agitation and arousal are of concern because they can lead to aggression (the threat of violence) and violence (the exercise of physical force so as to cause injury or damage to oneself, others or property).
The aims of rapid tranquillisation are summarised in Box 1.
Box 1 Aims of rapid tranquillisation
• Calm the person within 30 min; if necessary, sedate
• Maintain improvement for at least 2 h
• Avoid harm to patient, staff and others
History
A traditional way of coping with the violence of the mentally ill was by physical restraint. Then, in the early 19th century in England, came the demonstration that calm, friendly concern for the individual and simple psychological management made much of restraint unnecessary. With the advent of drug treatments, bromides, chloral, hyoscine, paraldehyde, morphine, and later barbiturates, became the compellers of peace – in effect, by heavy sedation, or partial anaesthetisation or in some cases by inducing a toxic-confusional state (Shorter Reference Shorter1997).
Current services
Modern psychotropics with more subtle effects calm without necessarily making the person unconscious or even unduly drowsy, and psychological handling remains an important component of management (McLaren Reference McLaren, Browne and Taylor1990).
Since 1985, many in-patient services have created psychiatric intensive care units (PICUs) to which patients can be moved, before a ward with links to the community prepares them for life again outside hospital. However, the PICUs have found it increasingly necessary to use restraint and seclusion once again. The timely use of effective tranquillisation should reduce this: PRN (pro re nata or as-required) medication should be used to de-escalate or prevent agitation that may lead to aggression and the need for rapid tranquillisation.
Neurochemical pathways in arousal and agitation
Arousal
The most common neurotransmitters in the brain are glutamate (excitatory) and gamma-aminobutyric acid (GABA) (inhibitory). These are very widely distributed and indeed represent the overwhelming majority of brain neurons. In addition, there are small sets of neurons utilising other transmitters, some of which send axons to wide areas of the brain. These include noradrenaline (NA), dopamine (D), histamine (H), acetylcholine (ACh) and serotonin or 5-hydroxytryptamine (5-HT). Other transmitters known to be involved in arousal are orexin (which is blocked by the drug suvorexant) and adenosine (which is blocked by caffeine).
In the process of wakening, a signal from the serotonergic suprachiasmatic nucleus (‘the biological clock’) activates orexin neurons in the hypothalamus, which cause firing of sets of histamine neurons (in the tuberomamillary nucleus), noradrenaline neurons (including those of the nucleus locus ceruleus) and acetylcholine neurons of the pontine reticular formation. This corresponds to the awake, alert or aroused state (Sutcliffe Reference Sutcliffe and de Lecea2002; Szabadi Reference Szabadi and Guglietta2015). A more frontal acetylcholine pathway from the nucleus basalis of Meynert to the cerebral cortex is involved in attention, concentration and memory (Liu Reference Liu, Chang and Pearce2015).
By contrast, the deep sleeping state (slow wave sleep) involves diminished release of orexin, histamine, noradrenaline and acetylcholine. Rapid eye movement (REM) sleep (dreaming) involves activation of nicotinic acetylcholine receptors via the pontine reticular formation (Sutcliffe Reference Sutcliffe and de Lecea2002; Szabadi Reference Szabadi and Guglietta2015).
Another feature of arousal is activation of dopamine neurons, which have the role of drawing attention to significant sensations or experiences, causing alertness (Schultz Reference Schultz, Dayan and Montague1997) and signalling motivational salience, which may be incentive or aversive (Berridge Reference Berridge and Robinson1998).
Agitation
Anxiety and agitation are commonly attributed to excessive arousal through increased noradrenergic (Berridge Reference Berridge, Schmeichel and España2012) and reduced serotonergic function (Graeff Reference Graeff, Netto and Zangrossi1998). However, according to the dopamine hypothesis, schizophrenia (Howes Reference Howes and Kapur2009) and mania (Cookson Reference Cookson2013; Goodwin Reference Goodwin, Haddad and Ferrier2016; Jauhar Reference Jauhar, Nour and Veronese2017) result from excessive dopamine release in particular pathways. It has been found that dopamine release in basal ganglia regions is increased in first-episode patients with psychotic mania as well as in those with schizophrenia (Jauhar Reference Jauhar, Nour and Veronese2017). Moreover, the increase was greater in mania. This suggests that doses required to control severe mania might be higher than those needed to treat acute schizophrenia (thought to be 15 mg/day with haloperidol).
Assessing the patient
It is helpful in planning treatment to know the patient's medication history and whether agitation is occurring in the context of physical illness, mania, paranoid psychosis, delirium or drug intoxication. It is important, despite the urgency, to read previous notes and listen to what the patient says: this may sometimes be strongly personalised and threatening but nonetheless revealing. An electrocardiogram (ECG) is a prudent investigation for all psychiatric admissions, or a note of its refusal.
Drug treatment
Rapid tranquillisation
Whatever the cause, if violent or disturbed behaviour continues or is threatened, it may need to be controlled rapidly. The aim of rapid tranquillisation is to calm or sedate the patient sufficiently to minimise the risk posed to the patient and to others. Sometimes it addresses also the underlying illness, particularly mania, which can improve with non-sedative antipsychotics within minutes or hours (Cookson Reference Cookson2008). In schizophrenia, the delusions, hallucinations and thought disorder tend to improve over weeks with antipsychotics (Johnstone Reference Johnstone, Frith and Crow1978), but improvement in agitation can be seen much sooner (Agid Reference Agid, Kapur and Arenovich2003).
Table 1 lists the drugs most often used in rapid tranquillisation and their mechanisms.
TABLE 1 Drugs used in rapid (acute) tranquillisation and mechanisms of action
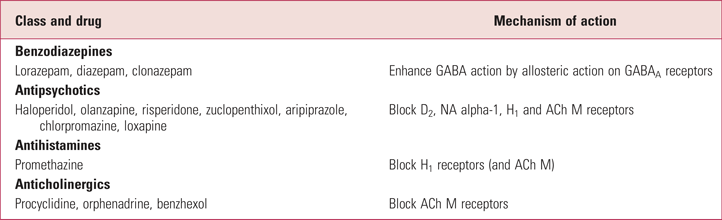
ACh, acetylcholine; D, dopamine; GABA, gamma-aminobutyric acid; H, histamine; M, muscarinic; NA, noradrenaline.
Neurochemical basis of drug treatments for agitation
Glutamate transmission has not yet proved an effective target in rapid tranquillisation, although ketamine, which blocks N-methyl-d-aspartate-sensitive glutamate receptors (NMDAR), is a general anaesthetic agent and has a rapid effect, reducing suicidal ideation and improving mood in severe depression within 1 h, which midazolam does not (Wilkinson Reference Wilkinson, Ballard and Bloch2018). When surgery is needed, for example after a severe injury, rapid tranquillisation may include a general anaesthetic agent such as ketamine administered in a specialist setting with intubation and ventilation. Highly aroused states do, however, benefit from medication that increases inhibitory (GABA) function in the brain or reduces histamine transmission. Thus, benzodiazepines and antihistamines can produce sedation, but are not expected to improve the underlying mental state in psychosis. Likewise, drugs that reduce noradrenaline transmission by inhibiting the locus ceruleus (such as clonidine) cause sedation. Blockade of noradrenergic alpha-1 receptors has also been linked to sedation with antipsychotic drugs (Peroutka Reference Peroutka, U'Prichard and Greenberg1977). The locus ceruleus projects to the medial septal area and cortex through alpha-1 and beta receptors to cause arousal (Berridge Reference Berridge, Schmeichel and España2012). Noradrenergic alpha-1 receptors are of three subtypes, alpha-1B being involved in the central nervous system (Koshimizu Reference Koshimizu, Yamauchi and Hirasawa2002). In the human iris, alpha-1 receptors are blocked by therapeutic doses of haloperidol (Szabadi Reference Szabadi, Gaszner and Bradshaw1981). This action could contribute to the beneficial effects of haloperidol, zuclopenthixol, olanzapine and chlorpromazine – sedative antipsychotics.
Table 2 shows the relative potencies of antipsychotics and promethazine in blocking receptors and in causing QT prolongation by blocking the IKr cardiac potassium channels also known as hERG channels (see ‘Antipsychotics, cardiac conduction and sudden death’ below).
TABLE 2 Binding profiles,a and QT changes at therapeutic doses, for antipsychotics and promethazine
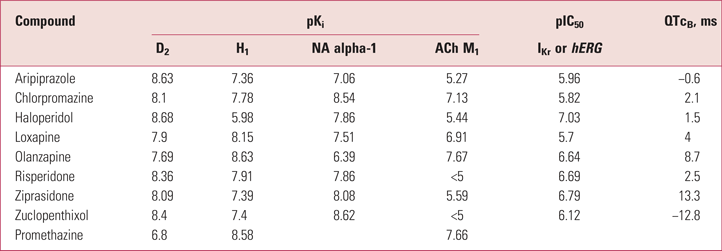
a. pKi and pIC50 denote the drug's binding affinity for the associated receptor: higher numbers signify greater potency; a difference of 1 in pKi value represents a ten-fold difference in binding affinity.
ACh, acetylcholine; D, dopamine; GABA, gamma-aminobutyric acid; H, histamine; M, muscarinic; NA, noradrenaline; QTcB, QT interval with Bazett correction.
Sources: Kubo et al (Reference Kubo, Shirakawa and Kuno1987); Silvestre et al (Reference Silvestre, O'Neill and Prous2014); Cassella et al (Reference Cassella, Spyker and Yeung2015).
To reduce agitation further it is it is necessary to address the underlying condition (e.g. schizophrenia, mania) by reducing dopamine function. This is achieved by antipsychotic or antimanic agents.
It has long been recognised that antipsychotic drugs act by blocking receptors for dopamine (Carlsson Reference Carlsson and Lindqvist1963). Following the animal laboratory work of Schultz et al (Reference Schultz, Dayan and Montague1997) and Berridge & Robinson (Reference Berridge and Robinson1998) showing that dopamine pathways signal incentive salience, Kapur (Reference Kapur2003) proposed that in schizophrenia excess dopamine causes excessive aversive motivational salience to be attached to sensory input and that antipsychotics work by reducing all salience; blockade of dopamine thereby prevents new delusions from being formed, but further time is needed for existing delusions to be ‘unlearnt’.
Benzodiazepines
If there is uncertainty about the diagnosis, it may be desirable to use benzodiazepines initially and to avoid the use of antipsychotic drugs; this would apply with catatonia where neuroleptic malignant syndrome is a particular risk (Sienaert Reference Sienaert, Dhossche and Vancampfort2014).
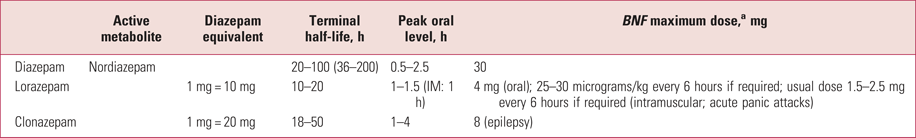
Table 3 shows the different potencies, rates of absorption and onset, and duration of action (terminal half-life) for diazepam, lorazepam and clonazepam in healthy volunteers. The potency of clonazepam is notably higher than that of diazepam, but its duration is shorter, meaning that the equivalence with diazepam is initially 20:1, but diazepam accumulates to a greater extent and the equivalence in long-term use is about 10:1 (Ashton Reference Ashton2002; Taylor Reference Taylor, Barnes and Young2015).
TABLE 3 Pharmacokinetics of three benzodiazepines
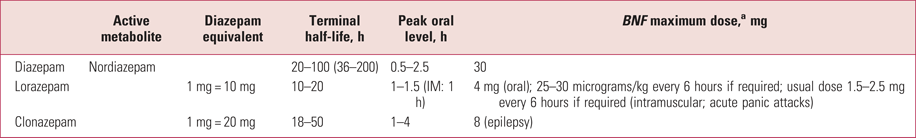
a. BNF maximum doses are taken from Joint Formulary Committee (2018).
IM, intramuscular.
For oral use diazepam or clonazepam may be given, and for intramuscular use lorazepam. They may need to be repeated every 2 hours.
Oral diazepam can be effective as quickly as intramuscular lorazepam. Peak concentrations are reached within 45 min in more than half of patients (Greenblatt Reference Greenblatt, Harmatz and Friedman1989). Intramuscular use of diazepam should be avoided; it is very painful and absorption is slow. Intramuscular lorazepam is absorbed more quickly, with peak levels within 1–1.5 h (Greenblatt Reference Greenblatt, Joyce and Comer1977).
In some cases, medication that is rapidly eliminated is preferred, for instance lorazepam. However, the patient may remain severely disturbed when the medication wears off, in which case the longer-acting diazepam or clonazepam are preferable. The intravenous route offers the most rapid method of sedation, but a high peak concentration of the drug is achieved and may have unwanted side-effects, and the early sedation wears off quickly as the drug is redistributed. Mental health nurses are rarely trained to administer via the intravenous route, which is therefore limited to use by a doctor. Intramuscular injection gives a more gradual rise in blood concentration, but in individuals with low muscle blood flow there is a risk of successive doses accumulating in muscle and then being released to the circulation in unexpectedly high concentrations.
The main risk with benzodiazepines is respiratory depression, which once recognised is readily reversed by flumazenil. Not all patients can be sedated by high doses of benzodiazepines and there is a theoretical risk of behavioural disinhibition, which is poorly documented (Paton Reference Paton2002). False memory is a risk of high-dose benzodiazepines (Pernot-Marino Reference Pernot-Marino, Danion and Hedelin2004).
A Cochrane review of benzodiazepines for agitation in psychosis found only one randomised controlled trial (RCT) comparing a benzodiazepine (lorazepam) with placebo (in mania) (Zaman Reference Zaman, Sampson and Beck2017). This showed global improvement in agitation 2 h after intramuscular injection of lorazepam 2 mg, but no significant change in manic or psychotic symptoms (Meehan Reference Meehan, Zhang and David2001). Another study in mania found greater benefits with lorazepam than placebo from 45 min to 2 h after administration for both agitation and mania ratings (Zimbroff Reference Zimbroff, Marcus and Manos2007).
Antipsychotics
Antipsychotic drugs were formerly known as major tranquillisers and have an important role in calming agitation. Their tranquillising effects do not depend on being sedative. The effects occur within minutes of drug delivery and generally more quickly with parenteral than oral routes. Antipsychotics available for intramuscular use in the UK are haloperidol, olanzapine, aripiprazole and chlorpromazine (McAllister-Williams Reference McAllister-Williams and Ferrier2002). Haloperidol has long been regarded as the first-line drug for parenteral rapid tranquillisation (Wilson Reference Wilson, Pepper and Currier2012). It lacks antihistaminergic and anticholinergic activity, but blocks noradrenergic alpha-1 receptors. The side-effects of haloperidol are particularly the extrapyramidal ones – Parkinsonism, dystonia and akathisia. A Cochrane review on the drug states that ‘Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical’ (Ostinelli Reference Ostinelli, Brooke-Powney and Li2017). It is therefore advisable to consider administering an anticholinergic drug together with haloperidol to prevent dystonia. In the treatment of schizophrenia, there are antipsychotics with greater efficacy than haloperidol (olanzapine, risperidone and amisulpride, in addition to clozapine) (Leucht Reference Leucht, Cipriani and Spineli2013). It is not known whether this superiority applies to rapid tranquillisation. By contrast, no antipsychotic has been shown to have greater efficacy than haloperidol in reducing the symptoms of mania and some (quetiapine, aripiprazole, ziprasidone) have distinctly less efficacy (Cipriani Reference Cipriani, Barbui and Salanti2011).
Combined antipsychotic and benzodiazepine
This combination engages different mechanisms of action in the expectation of mutual augmentation while avoiding complications from higher doses of antipsychotics. The amnesic effect of benzodiazepines is also advantageous when patients are undergoing such potentially distressing and traumatic events as compulsory treatment, restraint and seclusion (Steinert Reference Steinert, Birk and Flammer2013). Even using the intravenous route, there appears to be faster onset of tranquillisation with a combination of diazepam and haloperidol than with diazepam alone (Pilowsky Reference Pilowsky, Ring and Shine1992).
Haloperidol plus lorazepam
Favourite combinations are oral haloperidol (with procyclidine) and diazepam, and intramuscular haloperidol (with procyclidine) and lorazepam. However, few RCTs have examined these. Those that did have shown evidence for faster onset than with either alone (Table 4).
TABLE 4 Randomised controlled trials comparing combined haloperidol and lorazepam with either drug alone

HAL, haloperidol; LZP, lorazepam.
Antipsychotics, cardiac conduction and sudden death
Several factors contribute to an increased risk of sudden cardiac death in highly aroused individuals on psychotropic medication. One that has received most attention is the ability of drugs to enter the potassium channels that open during the cardiac action potential (the IKr or hERG channel: Box 2); this delays repolarisation, leading to prolongation of the QT interval, a factor predisposing to ventricular tachycardia (torsades de pointes) and hence to ventricular fibrillation and death. Prolongation of the QT interval is thus a surrogate marker for drug-related cardiotoxicity, albeit a weak one. There are excellent reviews of the mechanisms and monitoring of sudden cardiac death with antipsychotics by Abdelmawla & Mitchell (Reference Abdelmawla and Mitchell2006a,Reference Abdelmawla and Mitchellb) and by O'Brien & Oyebode (Reference O'Brien and Oyebode2003).
Box 2 IKr and hERG channels
IKr is the outward repolarising current in the cardiac delayed rectifier K+ (potassium ion) channel Kv11.1. This channel is also known as the hERG channel, after the human Ether-a-go-go-related gene, which encodes the pore-forming subunit of the rapid component of Kv11.1. Drugs including antipsychotics can block these channels in high doses.
Interpretation of the QT interval is difficult if the pulse rate is increased, because tachycardia shortens the ECG, including the QT interval. Corrections are applied if the pulse rate is over or under 60 beats per minute. However, the often-used Bazett correction overcorrects in the presence of tachycardia and an alternative (Fridericia's correction) can be used (Vandenberk Reference Vandenberk, Vandael and Robyns2016).
Although several antipsychotics have this effect, haloperidol has attracted most concern, because it has been used in high doses parenterally for rapid tranquillisation.
Changes in BNF recommendations for haloperidol
From 1988 to 2000, the British National Formulary (BNF) recommended a maximum haloperidol intramuscular dose of 30 mg followed by 5 mg up to every hour. However, nurses would not want to enter a seclusion room every hour. Over the past 30 years there have been dramatic changes in the advised doses of haloperidol – most notably since 2000 (Table 5). These changes reflect concerns about potential cardiac side-effects of the drug (in high doses) as well as a lack of evidence for greater efficacy with higher doses in the control of symptoms of schizophrenia. There is now a requirement for an ECG in any patient before administration of haloperidol. Intravenous use of haloperidol is no longer licensed or recommended, owing to a greater adverse effect on cardiac conduction.
TABLE 5 Changes to the advised haloperidol prescribing in the British National Formulary: 2000–2018
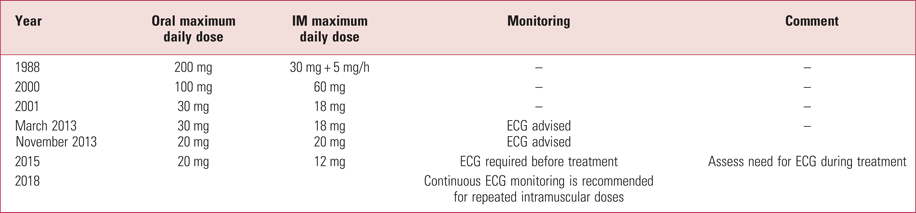
ECG, electrocardiogram; IM, intramuscular.
Views about haloperidol changed following the US Food and Drug Administration's (FDA) assessment of ziprasidone (FDA Psychopharmacologic Drugs Advisory Committee 2000). The manufacturer Pfizer was required to conduct a study (Study 054) of QT changes. The FDA had extensive data from regulatory trials suggesting that at doses up to 15 mg/day the change in QTc with haloperidol was no greater than with placebo. However, laboratory tests on hERG channels showed dose-dependent inhibition of potassium currents by haloperidol, olanzapine, risperidone, thioridazine and ziprasidone (Crumb Reference Crumb, Ekins and Sarazan2006). Study 054 randomised 183 patients to take ziprasidone 160 mg, haloperidol 15 mg, risperidone 16 mg, olanzapine 20 mg, quetiapine 750 mg or thioridazine 300 mg daily for 19 days. Changes in QTc duration (using Bazett's correction) were least for haloperidol, followed by risperidone, olanzapine and quetiapine, but greater for ziprasidone and thioridazine. Pfizer noted a dose-dependent prolongation of QTc with thioridazine, ziprasidone, haloperidol, olanzapine and quetiapine. Data from the same study, using a different correction, QTcb/l (baseline correction), noted that all the drugs other than haloperidol led to an increased pulse rate and that the QTcb/l increased with all, especially thioridazine and ziprasidone (Harrigan Reference Harrigan, Miceli and Anziano2004).
Ziprasidone was subsequently approved as an antipsychotic and available for intramuscular use, including rapid tranquillisation (Brook Reference Brook, Lucey and Gunn2000), in the USA and Europe but not in the UK, where ECG monitoring would have been required.
To investigate effects of higher doses of ziprasidone, Pfizer randomised 59 patients to receive two intramuscular doses of ziprasidone or haloperidol (7.5 and 10 mg) 4 h apart (Miceli Reference Miceli, Tensfeldt and Shiovitz2010). None of those on haloperidol had QTcb/l greater than 450 ms (500 ms being regarded as a threshold for increased risk of arrhythmia (Taylor Reference Taylor2003)). There was a linear correlation between blood level of haloperidol and lengthening of the QTc interval. The manufacturer of haloperidol, Janssen, used this to predict that oral doses of haloperidol 10 mg twice daily carried a risk of producing a mean increase of just 8.2 ms; and 4 × 5 mg given intramuscularly over 4 h would produce a mean increase of 8.1 ms at peak (Jansen Medical Information, personal communication, 2 June 2014).
The International Conference on HarmonisationFootnote a (ICH) stated that ‘drugs that prolong the mean QT/QTc interval by >20 ms have a substantially increased likelihood of being proarrhythmic’ (ICH Expert Working Group 2005: p. 13).
In November 2003, Janssen changed the summary of product characteristics for haloperidol to decrease the oral dose from 30 to 20 mg/day, and increase the intramuscular dose from 18 to 20 mg/day. Subsequently, Janssen reduced the intramuscular maximum to 12 mg/day.
However, the dose range for haloperidol in mania (or in rapid tranquillisation) has not been defined. Rifkin et al (Reference Rifkin, Doddi and Karajgi1994) found that doses above 10 mg/day and up to 80 mg/day produce a greater rate of remission of mania.
Other antipsychotics
Zuclopenthixol
Zuclopenthixol is more sedative than haloperidol and does not prolong the QT interval (Silvestre Reference Silvestre, O'Neill and Prous2014).
If severe agitation persists and repeated injections are required (because the patient refuses oral medication), zuclopenthixol acetate has an effect lasting for about 3 days. The onset of action takes 2–8 h. Maximum serum concentrations of zuclopenthixol are usually reached 36 h after an injection, so that side-effects may be delayed. With up to four doses, zuclopenthixol acetate may maintain improvement for 2 weeks.
With the final dose, the longer-acting zuclopenthixol decanoate may also be given. It has a duration of 1–2 weeks and peak plasma concentrations are usually reached between 4 and 7 days after injection. Following multiple injections, the apparent elimination half-life is 19 days.
Chlorpromazine
Chlorpromazine is a sedative antipsychotic and may be given orally as tablet or syrup. It may also be given by intramuscular injection; however, this can cause local pain, acute hypotension and occasionally a painful sterile abscess. This route of administration avoids first-pass metabolism, which can be substantial in patients previously exposed to treatment over long periods; high blood levels can be reached unless the dose is lowered for drug-naive patients. It has a higher risk of inducing seizures. For these reasons chlorpromazine should not be the first-line treatment, and some authorities avoid its use.
Newer antipsychotics for intramuscular use
Olanzapine
Olanzapine 10 mg is as effective in reducing agitation in schizophrenia as 7.5 mg haloperidol over the course of 2 h, although a benefit is seen within 15 min with olanzapine and within 30 min with haloperidol (Wright Reference Wright, Birkett and David2001). It is also effective in reducing agitation in mania over 2 h; benefit is significant within 30 min, and there is greater improvement in agitation and mania ratings than after 2 mg lorazepam or placebo (Meehan Reference Meehan, Zhang and David2001). The manufacturer Lilly stopped promoting its intramuscular use in the UK and it is no longer in the BNF – its use is now off-licence.
It should not be administered simultaneously with benzodiazepines (Lilly Reference Lilly2018). A dose-ranging study with intramuscular olanzapine for agitation in schizophrenia found a dose–response relationship in the range of 1–10 mg over 2 h (Breier Reference Breier, Meehan and Birkett2002). An open-label RCT comparing intramuscular olanzapine 10 mg with intramuscular haloperidol 5 mg plus lorazepam 2 mg in acutely agitated patients with schizophrenia or schizoaffective disorder found no difference in improvement in the following 2 h (Huang Reference Huang, Hwang and Chen2015).
Aripiprazole
Aripiprazole given intramuscularly improves moderately severe agitation in schizophrenia over 2 h. A dose of 9.25 mg was no less effective than haloperidol 7.5 mg, the improvement being greater than placebo from 45 min (Tran-Johnson Reference Tran-Johnson, Sack and Marcus2007). For agitation in mania, aripiprazole 15 mg was superior to placebo from 60 min, and aripiprazole 9.75 mg was superior to placebo from 90 min to 2 h, a rather slow effect (Zimbroff Reference Zimbroff, Marcus and Manos2007). A pragmatic RCT found intramuscular olanzapine 10 mg superior to aripiprazole 9.75 mg in controlling agitation within 2 h, being more sedative (Kittipeerachon Reference Kittipeerachon and Chaichan2015).
Loxapine by nasal inhalation
Loxapine blocks histamine H1 and dopamine D2 receptors (Table 2). Inhaled loxapine 5–10 mg produces significant improvement in agitated patients with schizophrenia or mania, from 10 min. The number needed to treat for 40% improvement in Positive and Negative Syndrome Scale – Excited Component (PANSS-EC) score at 2 h after 10 mg loxapine was 4 in schizophrenia and, even more impressively, 3 in mania (Zeller Reference Zeller, Zun and Cassella2017).
Anticholinergics
Although atropinic drugs – atropine and hyoscine (scopolamine) – were used orally and parenterally in the 19th century for their calming effects, they cause confusional states in higher doses. Today, anticholinergics are used only to counteract extrapyramidal (Parkinsonian) side-effects. Procyclidine and, to a greater extent, benzhexol have stimulant properties.
Antihistamines
During the 1940s the pharmaceutical industry developed drugs that block histamine; they were used for allergic conditions, but had sedative side-effects and were tried in psychiatric patients. The French anaesthetist and explorer Henri Laborit included promethazine with pethidine and other agents in what he called a ‘lytic cocktail’ to assist in anaesthesia. It was not until chlorpromazine was included in 1952 that he recognised a dramatic advance and encouraged psychiatric colleagues Delay and Deniker to explore its use in psychosis. They too were impressed by the difference between chlorpromazine and antihistamines for calming schizophrenia and mania.
Histamine was established as a central neurotransmitter in 1984. Blockade of H1 receptors blocks histamine input to cholinergic and noradrenergic nuclei in the brainstem and to principal cells in the cerebral cortex (Haas Reference Haas, Sergeeva and Selbach2008; Panula Reference Panula and Nuutinen2013).
Promethazine is also a potent antimuscarinic drug (Table 2), with a ratio of 8:1 in binding affinity for H1:AChM1. It can be regarded as a sedative anticholinergic (Kubo Reference Kubo, Shirakawa and Kuno1987). It prolongs the QT interval and is ictogenic, but not prone to cause torsades (Owczuk Reference Owczuk, Twardowski and Dylczyk-Sommer2009).
Its duration of action is usually 4–6 h (but can be up to 12 h). The oral bioavailability is only about 25%. The time to maximum serum concentration is 3 h after syrup, 2 h after intramuscular injection (Schwinghammer Reference Schwinghammer, Juhl and Dittert1984). The elimination half-life is about 10 h.
TREC studies
The Tranquilização Rápida-Ensaio Clínico (rapid tranquillisation clinical trial, TREC) conducted four open or single-blind ‘pragmatic’ RCTs in Brazil and India, coordinated by Hof from Brazil and with Adams of the Cochrane Schizophrenia Group in Leeds (Table 6). Consent was requested from relatives if available. These compared the use of promethazine (25 or 50 mg) in combination with haloperidol (5 or 10 mg) with four different comparators (midazolam, lorazepam, haloperidol alone and olanzapine). The combination was being used as a cheap alternative to lorazepam and to atypical antipsychotics.
TABLE 6 Four TREC randomised controlled trials of combined intramuscular haloperidol and promethazine
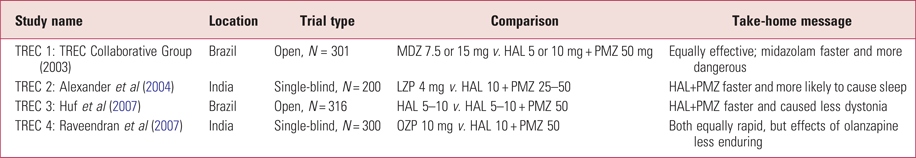
HAL, haloperidol; LZP, lorazepam; MDZ, midazolam; OZP, olanzapine; PMZ, promethazine; TREC, Tranquilização Rápida-Ensaio Clínico (rapid tranquillisation clinical trial).
They demonstrated the relative safety of the combination, with a low incidence of extrapyramidal side-effects, but the speed of tranquillisation (number calmed or asleep within 30 min) was less than with midazolam. Curiously, the sedative effect of the combination was more apparent in India than in Brazil, perhaps because the higher dose of haloperidol was consistently used; if so, the dose–response relationship with haloperidol would be important to note, especially as the maximum BNF daily dose is now 12 mg (Joint Formulary Committee 2018). Olanzapine 10 mg was observed to be as fast acting as the combination.
Other sedatives
The barbiturate amobarbital sodium (sodium amylobarbitone, sodium amytal) (250 mg) given intramuscularly as an adjunct to sedative antipsychotics and benzodiazepines can be very effective for sedation. Careful nursing observation is important because of the risk of respiratory depression, hypotension and chest infections. There is no pharmacological antagonist for barbiturates as there is for benzodiazepines.
Other antimanic drugs
Valproate is recommended (usually in combination with antipsychotics) in guidelines as a first-line treatment for mania (Goodwin Reference Goodwin, Haddad and Ferrier2016), although its use in women of childbearing age requires stringent safeguards (confirmed negative blood test for pregnancy and detention in a PICU or other secure facility).
Intravenous valproate (15 mg/kg) works within 20 min in status epilepticus, but evidence about its time course in mania or in severe agitation is conflicting. Phrolov et al (Reference Phrolov, Applebaum and Levine2004) found no improvement in mania within 2 h after intravenous semisodium valproate (20 mg/kg over 30 min). Asadollahi et al (Reference Asadollahi, Heidan and Hatamabadi2015) found comparable calming effects to haloperidol (5 mg intramuscular) within 30 min in acutely agitated patients with psychosis or mood disorders given intravenous sodium valproate (20 mg/kg over 10 min), but haloperidol was significantly more sedative.
Oral ‘loading doses’ lead to faster improvement than standard dosing (Hirschfeld Reference Hirschfeld, Allen and McEvoy1999). In schizophrenia there is little or no evidence that valproate augments the effects of antipsychotics. However, many patients with severe agitation display features of mixed schizoaffective psychosis, and valproate may be helpful in that context and is widely used. RCT evidence to support the use of valproate in schizophrenia comes from studies in China, either as an adjunct to risperidone in agitated patients with schizophrenia (Ostinelli Reference Ostinelli, Hussein and Ahmed2018) or to clozapine (Zheng Reference Zheng, Xiang and Yang2017).
Lithium has a slow onset of action, but should be restarted if the patient was already prescribed it, to avoid rebound mania.
Guidelines and policies
The National Institute for Health and Care Excellence (NICE) has published two sets of clinical guidelines on managing violence and aggression: the first, CG25 (National Collaborating Centre for Nursing and Supportive Care 2005), has now been withdrawn and replaced with the second, NG10 (National Collaborating Centre for Mental Health 2015). NICE has access to enormous resources, including systematic reviews published by the Cochrane Collaboration of RCTs on benzodiazepines (Zaman Reference Zaman, Sampson and Beck2017), haloperidol (Ostinelli Reference Ostinelli, Brooke-Powney and Li2017), chlorpromazine (Ahmed Reference Ahmed, Jones and Adams2010), zuclopenthixol acetate (Jayakody Reference Jayakody, Gibson and Kumar2012) and haloperidol plus promethazine (Huf Reference Huf, Alexander and Gandhi2016) for psychosis-induced aggression. However, the lessons from years of use and individual trials can be lost in the process, chlorpromazine and zuclopenthixol acetate, for example, being considered to need more research evidence.
Most National Health Service (NHS) trusts have their own protocols for the management of violence, which are generally compatible with the previous NICE guidance, CG25 (NICE 2005). For rapid tranquillisation many protocols recommend doses according to the BNF, and advice on what to do if these are to be exceeded.
Previous NICE guidance: CG25
The main recommendations in CG25 (NICE 2005) regarding rapid tranquillisation were as follows:
• for those who are not psychotic, initially give lorazepam alone (1.8.4.10)
• in those with psychosis, use a combination of an oral antipsychotic with lorazepam (1.8.4.11)
• where oral medication is not possible, use combined intramuscular antipsychotic and benzodiazepine (e.g. haloperidol and lorazepam) (1.8.4.15).
CG25 also notes that:
• the intramuscular route is preferred over the intravenous route (1.8.4.14)
• with intramuscular haloperidol, an antimuscarinic drug such as procyclidine should be immediately available and given intramuscularly or intravenously (1.8.4.21)
• there was deemed to be insufficient safety evidence for the use of intramuscular combined haloperidol plus promethazine, or for the use of intramuscular midazolam (1.8.4.17), although these could be used as alternatives ‘in very exceptional circumstances’, as could intravenous benzodiazepines or haloperidol (1.8.4.22).
• zuclopenthixol acetate injection may be considered as an option, but is not recommended (1.8.4.26) and is ‘not normally used’ (p. 139).
Further considerations in CG25
It was recognised (1.8.4.27 and 1.8.4.28) that clinicians sometimes use medication for rapid tranquillisation outside the limits indicated in the BNF and acknowledged that, in some circumstances, BNF limits may be exceeded, for example with lorazepam (where the BNF limit is only 4 mg daily). However, it was advised that, where the regulatory authority or manufacturer warns of increased risk of fatality, medication should be used within the marketing authorisation. Where the risk–benefit is unclear, advice may be sought from clinicians not directly involved.
Current NICE guidance: NG10
Limitations of NG10
The current guidelines, NG10, state that all areas of CG25 have been updated and that NG10 replaces it in full (National Collaborating Centre for Mental Health 2015: section 1). However, in NG10 rapid tranquillisation refers only to the use of medication parenterally (8.4.7). The guidance refers to violence, but there is no consideration of the context in which it occurs and no mention of diagnosis.
There is no reference to treatment of schizophrenia, mania or delirium, although ‘staff should be trained to recognise them’ (1.5.5). Separate NICE guidelines do exist for treatment of schizophrenia and bipolar disorder (NICE 2014a,b) though these do not discuss rapid tranquillisation.
Medications that are not mentioned at all include: diazepam, midazolam, clonazepam, chlorpromazine, clopenthixol, aripiprazole, loxapine, anticholinergics, procyclidine, lithium and valproate (the last of which is so useful in acute psychotic mania both alone and as an adjunct to antipsychotics).
There is no text reference to irritability, overactivity or paranoia – symptoms that are frequently the targets for improvement and that assist in diagnosis and choice of medication.
Recommendations on rapid tranquillisation in NG10
The main recommendations on rapid tranquillisation in NG10 are:
• use either intramuscular lorazepam alone or intramuscular haloperidol with promethazine (6.6.3.21)
• if there is no recent satisfactory ECG, avoid intramuscular haloperidol with promethazine and use intramuscular lorazepam (6.6.3.23), and if the response is partial consider a further dose (6.6.3.24)
• if there is no response to intramuscular lorazepam, consider intramuscular haloperidol with promethazine (6.6.3.25), and if there is partial response consider a further dose (6.6.3.26); this implies overruling the BNF advice on the need for an ECG
• if there is no response to intramuscular haloperidol with promethazine, consider intramuscular lorazepam if not already used (6.6.3.27)
• if intramuscular lorazepam has already been used, arrange an urgent team meeting to review and seek a second opinion if needed (no suggestions are offered of what the second opinion might advise).
NG10 also recommends that:
• a senior doctor should review all medication at least once a day (5.7.1.17)
• the multidisciplinary team should review the pharmacological strategy at least once a week (details about this review are provided).
Although quite demanding, these two recommendations do seem sensible, and protective for staff and patients. In particular, the presence of a psychiatric pharmacist at ward rounds can be very helpful.
Guideline Development Group found little evidence to support the combination of an antipsychotic with a benzodiazepine, the main recommendation in CG25 (NICE 2005). It found only low- to very-low-quality evidence from between 1 and 3 RCTs and no clear evidence that the intramuscular combination is more or less effective or harmful than intramuscular antipsychotic or intramuscular benzodiazepine alone. This reflects the small number of RCTs that have been performed in rapid tranquillisation.
The trials shown in Table 4 showed faster control of symptoms with the intramuscular antipsychotic plus benzodiazepine combination. For example, a multicentre study involving 98 psychotic, agitated, aggressive patients in five emergency rooms in the USA reported that the combination was superior to lorazepam (2 mg) and to haloperidol (5 mg) alone in psychotic agitation at 1–3 h (Battaglia Reference Battaglia, Moss and Rush1997).
There have been four TREC studies of haloperidol combined with promethazine – however, a potential conflict of interest should be noted for two of the authors of both the TREC and Cochrane reviews that informed NICE 2015 as, in the context of pharmaceutical development, their commitment to the haloperidol and promethazine combination could potentially have affected their interpretation of the literature on rapid tranquillisation.
Audit of rapid tranquillisation in UK
The UK Prescribing Observatory for Mental Health (POMH-UK) audited standards drawn from NG10 (National Collaborating Centre for Mental Health 2015) in 2016, analysing 2172 episodes of acutely disturbed behaviour from 328 clinical teams in 58 mental health trusts (POMH-UK 2017). For further reading and an in-depth look at these findings, please refer to the BAP guidelines (Patel Reference Patel, Sethi and Barnes2018).
The audit revealed that 50% of episodes were managed with oral medication only.
Benzodiazepine (oral or intramuscular) was the most common medication for acute behavioural disturbance.
For those given parenteral medication, the key findings were:
• where an antipsychotic was used, this was most often haloperidol; only two episodes used intravenous medication (haloperidol)
• 38% of those given parenteral haloperidol had no documented ECG in the previous year
• the use of combined haloperidol and promethazine (recommended in CG15) was minimal (3% of episodes)
• the use of combined antipsychotic and benzodiazepine (recommended in NG10) was higher, at 27%
• in 80% of episodes, a regular antipsychotic was also prescribed; in 5% this was a ‘high dose’; the additional antipsychotic used for rapid tranquillisation moved a further 13% of patients into the ‘high-dose’ category on that day.
Conclusions
It is hoped that this article will assist a clinician who wishes to contribute to updating their local trust protocol on rapid tranquillisation. Sedation and reduction of agitation involve different mechanisms.
Although NG10 (National Collaborating Centre for Mental Health 2015) claims to replace CG25 (National Collaborating Centre for Nursing and Supportive Care 2005) in full, it covers the narrow area of parenteral medication and does not address specific diagnoses. It advises seeking a second opinion but offers no advice on what this opinion might consider. The roles of several relevant drugs, including valproate, are not considered. NICE has published separate guidance on treating schizophrenia and mania (NICE 2014a,b) which could inform a second opinion.
A typical prescription for PRN (as-required) medication for rapid tranquillisation would include oral haloperidol with procyclidine (or, and if no ECG is available, an alternative antipsychotic – risperidone, olanzapine – perhaps specifying orodispersible tablets – or zuclopenthixol), and oral diazepam or clonazepam; and for intramuscular use, lorazepam or, if the ECG is satisfactory, haloperidol with procyclidine or promethazine (for sedation and as alternative to procyclidine). An alternative intramuscular antipsychotic (when there is no ECG) would be olanzapine where available (not injected along with benzodiazepine). This would combine the recommendations of CG25 and NG10.
In situations where these approaches are insufficient, a second opinion might review the diagnosis (including substance misuse) and dosages, and the likely compliance with drug administration; it might consider adding valproate, lithium or carbamazepine if there are manic symptoms, and the use of clopenthixol acetate or decanoate. The use of clozapine should also be considered if sustained treatment resistance has been experienced. Beyond that, further patience or the skills of an anaesthetist are required.
Acknowledgments
I am grateful to Dr Jonathan Pimm for help with earlier versions of the manuscript. And I am indebted to all the nurses who are willing to put themselves in harm's way in controlling and restraining patients and administering medication in order to make the environment safer for other patients, staff and carers.
MCQs
Select the single best option for each question stem
1 First-line recommendations for rapid tranquillisation in NICE guideline NG10 include intramuscular use of:
a haloperidol plus benzodiazepine
b haloperidol alone
c haloperidol plus promethazine
d zuclopenthixol acetate
e chlorpromazine.
2 Promethazine blocks the following at therapeutic doses:
a dopamine D2 receptors
b muscarinic acetylcholine M1 receptors
c histamine H2 receptors
d opiate receptors
e calcium channels.
3 Haloperidol blocks the following at therapeutic doses:
a dopamine D1 receptors
b muscarinic acetylcholine M1 receptors
c histamine H1 receptors
d noradrenaline alpha-1 receptors
e sodium channels.
4 As regards benzodiazepines:
a benzodiazepines act by inhibiting GABA receptors
b excess doses are reversed by intravenous midazolam
c intramuscular diazepam is absorbed more quickly than oral diazepam
d diazepam is 20 times as potent as clonazepam
e with repeated doses, clonazepam accumulates to a greater extent than diazepam.
5 A 2017 POMH-UK audit found the most common intervention for rapid tranquillisation to be:
a intramuscular medication
b benzodiazepine alone
c haloperidol plus promethazine
d antipsychotic plus lorazepam
e haloperidol – oral or intramuscular
MCQ answers
1 c 2 b 3 d 4 a 5 b








eLetters
No eLetters have been published for this article.