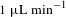1 Introduction
An x-ray free electron laser (XFEL) is a high power x-ray source which produces laser light with a wavelength of the order of angstrom. The first two XFEL facilities in the world, the Linac Coherent Light Source (LCLS) and SPring-8 Angstrom Compact free electron LAser (SACLA), provide femtosecond x-ray pulses with peak powers of the order of tens of GW[Reference Emma, Akre, Arthur, Bionta, Bostedt, Bozek, Brachmann, Bucksbaum, Coffee, Decker, Ding, Dowell, Edstrom, Fisher, Frisch, Gilevich, Hastings, Hays, Hering, Huang, Iverson, Loos, Messerschmidt, Miahnahri, Moeller, Nuhn, Pile, Ratner, Rzepiela, Schultz, Smith, Stefan, Tompkins, Turner, Welch, White, Wu, Yocky and Galayda1, Reference Ishikawa, Aoyagi, Asaka, Asano, Azumi, Bizen, Ego, Fukami, Fukui, Furukawa, Goto, Hanaki, Hara, Hasegawa, Hatsui, Higashiya, Hirono, Hosoda, Ishii, Inagaki, Inubushi, Itoga, Joti, Kago, Kameshima, Kimura, Kirihara, Kiyomichi, Kobayashi, Kondo, Kudo, Maesaka, Maréchal, Masuda, Matsubara, Matsumoto, Matsushita, Matsui, Nagasono, Nariyama, Ohashi, Ohata, Ohshima, Ono, Otake, Saji, Sakurai, Sato, Sawada, Seike, Shirasawa, Sugimoto, Suzuki, Takahashi, Takebe, Takeshita, Tamasaku, Tono, Wu, Yabashi, Yamaga, Yamashita, Yanagida, Zhang, Shintake, Kitamura and Kumagai2]. By focusing the XFEL pulse to
![]() ${\sim}1~\unicode[STIX]{x03BC}\text{m}\;({\sim}0.05~\unicode[STIX]{x03BC}\text{m})$
, the intensity reaches as high as
${\sim}1~\unicode[STIX]{x03BC}\text{m}\;({\sim}0.05~\unicode[STIX]{x03BC}\text{m})$
, the intensity reaches as high as
![]() ${\sim}10^{18}$
(
${\sim}10^{18}$
(
![]() ${\sim}10^{20}$
)
${\sim}10^{20}$
)
![]() $\text{W cm}^{-2}$
[Reference Yumoto, Mimura, Koyama, Matsuyama, Tono, Togashi, Inubushi, Sato, Tanaka, Kimura, Yokoyama, Kim, Sano, Hachisu, Yabashi, Ohashi, Ohmori, Ishikawa and Yamauchi3, Reference Mimura, Yumoto, Matsuyama, Koyama, Tono, Inubushi, Togashi, Sato, Kim, Fukui, Sano, Yabashi, Ohashi, Ishikawa and Yamauchi4]. These characteristics offer research opportunities in various fields of science such as structural biology[Reference Chapman, Fromme, Barty, White, Kirian, Aquila, Hunter, Schulz, DePonte, Weierstall, Doak, Maia, Martin, Schlichting, Lomb, Coppola, Shoeman, Epp, Hartmann, Rolles, Rudenko, Foucar, Kimmel, Weidenspointner, Holl, Liang, Barthelmess, Caleman, Boutet, Bogan, Krzywinski, Bostedt, Bajt, Gumprecht, Rudek, Erk, Schmidt, Hömke, Reich, Pietschner, Strüder, Hauser, Gorke, Ullrich, Herrmann, Schaller, Schopper, Soltau, Kühnel, Messerschmidt, Bozek, Hau-Riege, Frank, Hampton, Sierra, Starodub, Williams, Hajdu, Timneanu, Seibert, Andreasson, Rocker, Jönsson, Svenda, Stern, Nass, Andritschke, Schröter, Krasniqi, Bott, Schmidt, Wang, Grotjohann, Holton, Barends, Neutze, Marchesini, Fromme, Schorb, Rupp, Adolph, Gorkhover, Andersson, Hirsemann, Potdevin, Graafsma, Nilsson and Spence5–Reference Suga, Akita, Hirata, Ueno, Murakami, Nakajima, Shimizu, Yamashita, Yamamoto, Ago and Shen9], nonlinear x-ray optics[Reference Tamasaku, Shigemasa, Inubushi, Katayama, Sawada, Yumoto, Ohashi, Mimura, Yabashi, Yamauchi and Ishikawa10, Reference Yoneda, Inubushi, Nagamine, Michine, Ohashi, Yumoto, Yamauchi, Mimura, Kitamura, Katayama, Ishikawa, Yabashi and Yabashi11], ultrafast physics and chemistry[Reference Zhang, Alonso-Mori, Bergmann, Bressler, Chollet, Galler, Gawelda, Hadt, Hartsock, Kroll, Kjær, Kubiček, Lemke, Liang, Meyer, Nielsen, Purser, Robinson, Solomon, Sun, Sokaras, van Driel, Vankó, Weng, Zhu and Gaffney12–Reference Dean, Cao, Liu, Wall, Zhu, Mankowsky, Thampy, Chen, Vale, Casa, Kim, Said, Juhas, Alonso-Mori, Glownia, Robert, Robinson, Sikorski, Song, Kozina, Lemke, Patthey, Owada, Katayama, Yabashi, Tanaka, Togashi, Liu, Serrao, Kim, Huber, Chang, McMorrow, Först and Hill15], and high energy density science[Reference Vinko, Ciricosta, Cho, Engelhorn, Chung, Brown, Burian, Chalupský, Falcone, Graves, Hájková, Higginbotham, Juha, Krzywinski, Lee, Messerschmidt, Murphy, Ping, Scherz, Schlotter, Toleikis, Turner, Vysin, Wang, Wu, Zastrau, Zhu, Lee, Heimann, Nagler and Wark16].
$\text{W cm}^{-2}$
[Reference Yumoto, Mimura, Koyama, Matsuyama, Tono, Togashi, Inubushi, Sato, Tanaka, Kimura, Yokoyama, Kim, Sano, Hachisu, Yabashi, Ohashi, Ohmori, Ishikawa and Yamauchi3, Reference Mimura, Yumoto, Matsuyama, Koyama, Tono, Inubushi, Togashi, Sato, Kim, Fukui, Sano, Yabashi, Ohashi, Ishikawa and Yamauchi4]. These characteristics offer research opportunities in various fields of science such as structural biology[Reference Chapman, Fromme, Barty, White, Kirian, Aquila, Hunter, Schulz, DePonte, Weierstall, Doak, Maia, Martin, Schlichting, Lomb, Coppola, Shoeman, Epp, Hartmann, Rolles, Rudenko, Foucar, Kimmel, Weidenspointner, Holl, Liang, Barthelmess, Caleman, Boutet, Bogan, Krzywinski, Bostedt, Bajt, Gumprecht, Rudek, Erk, Schmidt, Hömke, Reich, Pietschner, Strüder, Hauser, Gorke, Ullrich, Herrmann, Schaller, Schopper, Soltau, Kühnel, Messerschmidt, Bozek, Hau-Riege, Frank, Hampton, Sierra, Starodub, Williams, Hajdu, Timneanu, Seibert, Andreasson, Rocker, Jönsson, Svenda, Stern, Nass, Andritschke, Schröter, Krasniqi, Bott, Schmidt, Wang, Grotjohann, Holton, Barends, Neutze, Marchesini, Fromme, Schorb, Rupp, Adolph, Gorkhover, Andersson, Hirsemann, Potdevin, Graafsma, Nilsson and Spence5–Reference Suga, Akita, Hirata, Ueno, Murakami, Nakajima, Shimizu, Yamashita, Yamamoto, Ago and Shen9], nonlinear x-ray optics[Reference Tamasaku, Shigemasa, Inubushi, Katayama, Sawada, Yumoto, Ohashi, Mimura, Yabashi, Yamauchi and Ishikawa10, Reference Yoneda, Inubushi, Nagamine, Michine, Ohashi, Yumoto, Yamauchi, Mimura, Kitamura, Katayama, Ishikawa, Yabashi and Yabashi11], ultrafast physics and chemistry[Reference Zhang, Alonso-Mori, Bergmann, Bressler, Chollet, Galler, Gawelda, Hadt, Hartsock, Kroll, Kjær, Kubiček, Lemke, Liang, Meyer, Nielsen, Purser, Robinson, Solomon, Sun, Sokaras, van Driel, Vankó, Weng, Zhu and Gaffney12–Reference Dean, Cao, Liu, Wall, Zhu, Mankowsky, Thampy, Chen, Vale, Casa, Kim, Said, Juhas, Alonso-Mori, Glownia, Robert, Robinson, Sikorski, Song, Kozina, Lemke, Patthey, Owada, Katayama, Yabashi, Tanaka, Togashi, Liu, Serrao, Kim, Huber, Chang, McMorrow, Först and Hill15], and high energy density science[Reference Vinko, Ciricosta, Cho, Engelhorn, Chung, Brown, Burian, Chalupský, Falcone, Graves, Hájková, Higginbotham, Juha, Krzywinski, Lee, Messerschmidt, Murphy, Ping, Scherz, Schlotter, Toleikis, Turner, Vysin, Wang, Wu, Zastrau, Zhu, Lee, Heimann, Nagler and Wark16].
One of the most important targets of XFEL applications is damage-free structure analysis in a ‘diffract-before-destroy’ scheme[Reference Neutze, Wouts, van der Spoel, Weckert and Hajdu17, Reference Barty, Caleman, Aquila, Timneanu, Lomb, White, Andreasson, Arnlund, Bajt, Barends, Barthelmess, Bogan, Bostedt, Bozek, Coffee, Coppola, Davidsson, DePonte, Doak, Ekeberg, Elser, Epp, Erk, Fleckenstein, Foucar, Fromme, Graafsma, Gumprecht, Hajdu, Hampton, Hartmann, Hartmann, Hauser, Hirsemann, Holl, Hunter, Johansson, Kassemeyer, Kimmel, Kirian, Liang, Maia, Malmerberg, Marchesini, Martin, Nass, Neutze, Reich, Rolles, Rudek, Rudenko, Scott, Schlichting, Schulz, Seibert, Shoeman, Sierra, Soltau, Spence, Stellato, Stern, Strüder, Ullrich, Wang, Weidenspointner, Weierstall, Wunderer and Chapman18], in which diffraction events in the sample can be terminated before structural change is initiated[Reference Inoue, Inubushi, Sato, Tono, Katayama, Kameshima, Ogawa, Togashi, Owada, Amemiya, Tanaka, Hara and Yabashi19]. A diffraction pattern of the sample can be recorded even under the irradiation of an XFEL pulse with an intensity higher than the damage threshold. However, as the sample is to be destroyed after the irradiation, a new sample has to be delivered before the next x-ray pulse comes to the interaction point. For this purpose, fluid sample injectors have been developed.
In the early experiments with XFEL, liquid-jet injectors using a gas dynamic virtual nozzle (GDVN) were applied to protein crystallography[Reference Chapman, Fromme, Barty, White, Kirian, Aquila, Hunter, Schulz, DePonte, Weierstall, Doak, Maia, Martin, Schlichting, Lomb, Coppola, Shoeman, Epp, Hartmann, Rolles, Rudenko, Foucar, Kimmel, Weidenspointner, Holl, Liang, Barthelmess, Caleman, Boutet, Bogan, Krzywinski, Bostedt, Bajt, Gumprecht, Rudek, Erk, Schmidt, Hömke, Reich, Pietschner, Strüder, Hauser, Gorke, Ullrich, Herrmann, Schaller, Schopper, Soltau, Kühnel, Messerschmidt, Bozek, Hau-Riege, Frank, Hampton, Sierra, Starodub, Williams, Hajdu, Timneanu, Seibert, Andreasson, Rocker, Jönsson, Svenda, Stern, Nass, Andritschke, Schröter, Krasniqi, Bott, Schmidt, Wang, Grotjohann, Holton, Barends, Neutze, Marchesini, Fromme, Schorb, Rupp, Adolph, Gorkhover, Andersson, Hirsemann, Potdevin, Graafsma, Nilsson and Spence5, Reference Boutet, Lomb, Williams, Barends, Aquila, Doak, Weierstall, DePonte, Steinbrener, Shoeman, Messerschmidt, Barty, White, Kassemeyer, Kirian, Seibert, Montanez, Kenney, Herbst, Hart, Pines, Haller, Gruner, Philipp, Tate, Hromalik, Koerner, van Bakel, Morse, Ghonsalves, Arnlund, Bogan, Caleman, Fromme, Hampton, Hunter, Johansson, Katona, Kupitz, Liang, Martin, Nass, Redecke, Stellato, Timneanu, Wang, Zatsepin, Schafer, Defever, Neutze, Fromme, Spence, Chapman and Schlichting7, Reference Weierstall, Spence and Doak20–Reference Redecke, Nass, DePonte, White, Rehders, Barty, Stellato, Liang, Barends, Boutet, Williams, Messerschmidt, Seibert, Aquila, Arnlund, Bajt, Barth, Bogan, Caleman, Chao, Doak, Fleckenstein, Frank, Fromme, Galli, Grotjohann, Hunter, Johansson, Kassemeyer, Katona, Kirian, Koopmann, Kupitz, Lomb, Martin, Mogk, Neutze, Shoeman, Steinbrener, Timneanu, Wang, Weierstall, Zatsepin, Spence, Fromme, Schlichting, Duszenko, Betzel and Chapman23]. This type of injector produced a continuous stream of liquid containing
![]() $10^{8}$
–
$10^{8}$
–
![]() $10^{9}~\text{cm}^{-3}$
of protein microcrystals[Reference Weierstall, Spence and Doak20, Reference Weierstall21]. A series of diffraction patterns from the crystal suspension were recorded with femtosecond x-ray pulses in a pulse by pulse manner. This method is called serial femtosecond crystallography (SFX). Because the liquid-jet injectors worked at a typical flow rate of the order
$10^{9}~\text{cm}^{-3}$
of protein microcrystals[Reference Weierstall, Spence and Doak20, Reference Weierstall21]. A series of diffraction patterns from the crystal suspension were recorded with femtosecond x-ray pulses in a pulse by pulse manner. This method is called serial femtosecond crystallography (SFX). Because the liquid-jet injectors worked at a typical flow rate of the order
![]() $10~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
, the early SFX experiments consumed protein crystals in the order of 10–100 mg.
$10~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
, the early SFX experiments consumed protein crystals in the order of 10–100 mg.
To reduce the sample consumption, different injectors have been developed primarily for the application to SFX. A lipidic-cubic-phase (LCP) injector produces a slow flow of highly viscous lipid containing microcrystals at a flow rate of
![]() $10^{-3}$
–
$10^{-3}$
–
![]() $10^{-1}~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
[Reference Weierstall, James, Wang, White, Wang, Liu, Spence, Doak, Nelson, Fromme, Fromme, Grotjohann, Kupitz, Zatsepin, Liu, Basu, Wacker, Han, Katritch, Boutet, Messerschmidt, Williams, Koglin, Seibert, Klinker, Gati, Shoeman, Barty, Chapman, Kirian, Beyerlein, Stevens, Li, Shah, Howe, Caffrey and Cherezov24–Reference Liu, Wacker, Gati, Han, James, Wang, Nelson, Weierstall, Katritch, Barty, Zatsepin, Li, Messerschmidt, Boutet, Williams, Koglin, Seibert, Wang, Shah, Basu, Fromme, Kupitz, Rendek, Grotjohann, Fromme, Kirian, Beyerlein, White, Chapman, Caffrey, Spence, Stevens and Cherezov26]. A typical sample amount required for collecting a full dataset can be as small as 1 mg. Alternative crystal carriers have been utilized not only for insoluble proteins but also for soluble ones[Reference Botha, Nass, Barends, Kabsch, Latz, Dworkowski, Foucar, Panepucci, Wang, Shoeman, Schlichting and Doak25, Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27–Reference Sugahara, Song, Suzuki, Masuda, Inoue, Nakane, Yumoto, Nango, Tanaka, Tono, Joti, Kameshima, Hatsui, Yabashi, Nureki, Numata and Iwata29]. An electrospun injector produces a liquid jet in an electric field to deliver a crystal suspension at a low flow rate of 0.14–
$10^{-1}~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
[Reference Weierstall, James, Wang, White, Wang, Liu, Spence, Doak, Nelson, Fromme, Fromme, Grotjohann, Kupitz, Zatsepin, Liu, Basu, Wacker, Han, Katritch, Boutet, Messerschmidt, Williams, Koglin, Seibert, Klinker, Gati, Shoeman, Barty, Chapman, Kirian, Beyerlein, Stevens, Li, Shah, Howe, Caffrey and Cherezov24–Reference Liu, Wacker, Gati, Han, James, Wang, Nelson, Weierstall, Katritch, Barty, Zatsepin, Li, Messerschmidt, Boutet, Williams, Koglin, Seibert, Wang, Shah, Basu, Fromme, Kupitz, Rendek, Grotjohann, Fromme, Kirian, Beyerlein, White, Chapman, Caffrey, Spence, Stevens and Cherezov26]. A typical sample amount required for collecting a full dataset can be as small as 1 mg. Alternative crystal carriers have been utilized not only for insoluble proteins but also for soluble ones[Reference Botha, Nass, Barends, Kabsch, Latz, Dworkowski, Foucar, Panepucci, Wang, Shoeman, Schlichting and Doak25, Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27–Reference Sugahara, Song, Suzuki, Masuda, Inoue, Nakane, Yumoto, Nango, Tanaka, Tono, Joti, Kameshima, Hatsui, Yabashi, Nureki, Numata and Iwata29]. An electrospun injector produces a liquid jet in an electric field to deliver a crystal suspension at a low flow rate of 0.14–
![]() $3.1~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
[Reference Sierra, Laksmono, Kern, Tran, Hattne, Alonso-Mori, Lassalle-Kaiser, Glöckner, Hellmich, Schafer, Echols, Gildea, Grosse-Kunstleve, Sellberg, McQueen, Fry, Messerschmidt, Miahnahri, Seibert, Hampton, Starodub, Loh, Sokaras, Weng, Zwart, Glatzel, Milathianaki, White, Adams, Williams, Boutet, Zouni, Messinger, Sauter, Bergmann, Yano, Yachandra and Bogana30]. Recently drop-on-demand injectors have been applied to SFX experiments under the atmospheric pressure[Reference Mafuné, Miyajima, Tono, Takeda, Kohno, Miyauchi, Kobayashi, Joti, Nango, Iwata and Yabashi31, Reference Roessler, Agarwal, Allaire, Alonso-Mori, Andi, Bachega, Bommer, Brewster, Browne, Chatterjee, Cho, Cohen, Cowan, Datwani, Davidson, Defever, Eaton, Ellson, Feng, Ghislain, Glownia, Han, Hattne, Hellmich, Héroux, Ibrahim, Kern, Kuczewski, Lemke, Liu, Majlof, McClintock, Myers, Nelsen, Olechno, Orville, Sauter, Soares, Soltis, Song, Stearns, Tran, Tsai, Uervirojnangkoorn, Wilmot, Yachandra, Yano, Yukl, Zhu and Zouni32]. This type of injector delivers crystal-containing droplets in synchronization with XFEL pulses. In contrast to the continuous-flow injectors, the pulsed operation of the injector can help to reduce the sample consumption.
$3.1~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
[Reference Sierra, Laksmono, Kern, Tran, Hattne, Alonso-Mori, Lassalle-Kaiser, Glöckner, Hellmich, Schafer, Echols, Gildea, Grosse-Kunstleve, Sellberg, McQueen, Fry, Messerschmidt, Miahnahri, Seibert, Hampton, Starodub, Loh, Sokaras, Weng, Zwart, Glatzel, Milathianaki, White, Adams, Williams, Boutet, Zouni, Messinger, Sauter, Bergmann, Yano, Yachandra and Bogana30]. Recently drop-on-demand injectors have been applied to SFX experiments under the atmospheric pressure[Reference Mafuné, Miyajima, Tono, Takeda, Kohno, Miyauchi, Kobayashi, Joti, Nango, Iwata and Yabashi31, Reference Roessler, Agarwal, Allaire, Alonso-Mori, Andi, Bachega, Bommer, Brewster, Browne, Chatterjee, Cho, Cohen, Cowan, Datwani, Davidson, Defever, Eaton, Ellson, Feng, Ghislain, Glownia, Han, Hattne, Hellmich, Héroux, Ibrahim, Kern, Kuczewski, Lemke, Liu, Majlof, McClintock, Myers, Nelsen, Olechno, Orville, Sauter, Soares, Soltis, Song, Stearns, Tran, Tsai, Uervirojnangkoorn, Wilmot, Yachandra, Yano, Yukl, Zhu and Zouni32]. This type of injector delivers crystal-containing droplets in synchronization with XFEL pulses. In contrast to the continuous-flow injectors, the pulsed operation of the injector can help to reduce the sample consumption.
The success of SFX in the initial stage has led to developments of injection methods for chemically activated time-resolved SFX. A mix-and-jet injector enables rapid mixing of a reactant solution and a crystal suspension in a timescale of millisecond for capturing protein motion initiated by chemicals[Reference Wang, Weierstall, Pollack and Spence33, Reference Calvey, Katz, Schaffer and Pollack34].
Also frequently used are injectors which are appropriate for gas-phase samples and homogeneous solutions. An aerosol injector has been employed to deliver isolated particles into a vacuum for coherent diffraction imaging (CDI) with extremely low background signals[Reference Seibert, Ekeberg, Maia, Svenda, Andreasson, Jönsson, Odić, Iwan, Rocker, Westphal, Hantke, DePonte, Barty, Schulz, Gumprecht, Coppola, Aquila, Liang, White, Martin, Caleman, Stern, Abergel, Seltzer, Claverie, Bostedt, Bozek, Boutet, Miahnahri, Messerschmidt, Krzywinski, Williams, Hodgson, Bogan, Hampton, Sierra, Starodub, Andersson, Bajt, Barthelmess, Spence, Fromme, Weierstall, Kirian, Hunter, Doak, Marchesini, Hau-Riege, Frank, Shoeman, Lomb, Epp, Hartmann, Rolles, Rudenko, Schmidt, Foucar, Kimmel, Holl, Rudek, Erk, Hömke, Reich, Pietschner, Weidenspointner, Strüder, Hauser, Gorke, Ullrich, Schlichting, Herrmann, Schaller, Schopper, Soltau, Kühnel, Andritschke, Schröter, Krasniqi, Bott, Schorb, Rupp, Adolph, Gorkhover, Hirsemann, Potdevin, Graafsma, Nilsson, Chapman and Hajdu6, Reference Loh, Hampton, Martin, Starodub, Sierra, Barty, Aquila, Schulz, Lomb, Steinbrener, Shoeman, Kassemeyer, Bostedt, Bozek, Epp, Erk, Hartmann, Rolles, Rudenko, Rudek, Foucar, Kimmel, Weidenspointner, Hauser, Holl, Pedersoli, Liang, Hunter, Gumprecht, Coppola, Wunderer, Graafsma, Maia, Ekeberg, Hantke, Fleckenstein, Hirsemann, Nass, White, Tobias, Farquar, Benner, Hau-Riege, Reich, Hartmann, Soltau, Marchesini, Bajt, Barthelmess, Bucksbaum, Hodgson, Strüder, Ullrich, Frank, Schlichting, Chapman and Bogan35]. Solution samples for x-ray spectroscopy and wide-angle x-ray scattering are often provided by a liquid-jet injector which creates a sheet of liquid[Reference Zhang, Alonso-Mori, Bergmann, Bressler, Chollet, Galler, Gawelda, Hadt, Hartsock, Kroll, Kjær, Kubiček, Lemke, Liang, Meyer, Nielsen, Purser, Robinson, Solomon, Sun, Sokaras, van Driel, Vankó, Weng, Zhu and Gaffney12, Reference Kim, Kim, Nozawa, Sao, Oang, Kim, Ki, Jo, Park, Song, Sato, Ogawa, Togashi, Tono, Yabashi, Ishikawa, Kim, Ryoo, Kim, Ihee and Adachi14].
This paper provides a review on fluid sample injectors at SACLA. The XFEL beamline (BL3) of SACLA produces hard x-ray pulses with peak powers of 6–60 GW, durations shorter than 10 fs (full width at half maximum) and photon energies of 4–20 keV[Reference Ishikawa, Aoyagi, Asaka, Asano, Azumi, Bizen, Ego, Fukami, Fukui, Furukawa, Goto, Hanaki, Hara, Hasegawa, Hatsui, Higashiya, Hirono, Hosoda, Ishii, Inagaki, Inubushi, Itoga, Joti, Kago, Kameshima, Kimura, Kirihara, Kiyomichi, Kobayashi, Kondo, Kudo, Maesaka, Maréchal, Masuda, Matsubara, Matsumoto, Matsushita, Matsui, Nagasono, Nariyama, Ohashi, Ohata, Ohshima, Ono, Otake, Saji, Sakurai, Sato, Sawada, Seike, Shirasawa, Sugimoto, Suzuki, Takahashi, Takebe, Takeshita, Tamasaku, Tono, Wu, Yabashi, Yamaga, Yamashita, Yanagida, Zhang, Shintake, Kitamura and Kumagai2, Reference Tono, Togashi, Inubushi, Sato, Katayama, Ogawa, Ohashi, Kimura, Takahashi, Takeshita, Tomizawa, Goto, Ishikawa and Yabashi36–Reference Yabashi, Tanaka and Ishikawa38]. The fluid sample injectors have been playing central roles in experiments using the ‘diffract-before-destroy’ approach, especially in SFX[Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27, Reference Inoue, Tono, Joti, Kameshima, Ogawa, Shinohara, Amemiya and Yabashi39–Reference Fukuda, Tse, Nakane, Nakatsu, Suzuki, Sugahara, Inoue, Masuda, Yumoto, Matsugaki, Nango, Tono, Joti, Kameshima, Song, Hatsui, Yabashi, Nureki, Murphy, Inoue, Iwata and Mizohata42]. The next section gives short descriptions on experimental instruments to which the fluid injectors are often installed. The specifications and applications of four kinds of injectors are described in the third section. The final section is devoted to summary and future perspectives.
2 Experimental platforms with fluid injectors
2.1 DAPHNIS for SFX
Fluid injectors are installed to experimental systems at the end stations of XFEL beamlines (BL2 and BL3). Diverse Application Platform for Hard x-ray Diffraction in SACLA (DAPHNIS) is a system for serial diffraction/scattering experiments under atmospheric pressure. Its detailed description is found in Ref. [Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43]. Figure 1 shows a standard setup of DAPHNIS, which consists of a sample chamber, microscopes for sample monitoring, and a multi-port charge-coupled-device (MPCCD) detector with eight sensor modules[Reference Kameshima, Ono, Kudo, Ozaki, Kirihara, Kobayashi, Inubushi, Yabashi, Horigome, Holland, Holland, Burt, Murao and Hatsui44]. The chamber is filled with He gas, a partial pressure of which is kept higher than 0.9 atm during measurement. The atmospheric-pressure operation helps stable fluid injection, rapid sample exchange, and temperature control of the sample. The injectors are mounted on motorized stages to be positioned properly to the interaction point. The four kinds of standard injectors at SACLA are fully compatible with DAPHNIS (see Section 3). In addition, DAPHNIS can be easily customized to accept other injection methods such as electrospun liquid microjet and fast mixing liquid jet. This flexibility is useful to users who bring their own injectors into SACLA.

Figure 1. Standard setup of DAPHNIS for serial diffraction/scattering experiments[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43]. The DAPHNIS system consists of a sample chamber, microscopes for sample monitoring, a sample injector, and an MPCCD detector with eight sensor modules. These key components are built on a single table. The sample chamber is filled with a He gas during measurement. The red arrow indicates the XFEL beam direction.
2.2 MAXIC for CDI
Figure 2 shows Multiple Application X-ray Imaging Chamber (MAXIC) which is mostly utilized for CDI in vacuum environment. Detailed descriptions on MAXIC are given in Ref. [Reference Song, Tono, Park, Ebisu, Kim, Shimada, Kim, Gallagher-Jones, Nam, Sato, Togashi, Ogawa, Joti, Kameshima, Ono, Hatsui, Iwata, Yabashi and Ishikawa45]. In a standard configuration for CDI, the chamber is combined with a focusing system which provides a
![]() $1~\unicode[STIX]{x03BC}\text{m}$
spot of x-rays at the sample position[Reference Yumoto, Mimura, Koyama, Matsuyama, Tono, Togashi, Inubushi, Sato, Tanaka, Kimura, Yokoyama, Kim, Sano, Hachisu, Yabashi, Ohashi, Ohmori, Ishikawa and Yamauchi3]. The XFEL beam from the focusing system is collimated with a pair of slits on the path to the sample. Diffraction patterns are recorded with the MPCCD detectors. The in-vacuum operation helps to suppress background signals. The liquid-jet injector with GDVN and aerosol injector are compatible with MAXIC, although they are not frequently employed in CDI at SACLA. In most cases, specimens are fixed on a thin
$1~\unicode[STIX]{x03BC}\text{m}$
spot of x-rays at the sample position[Reference Yumoto, Mimura, Koyama, Matsuyama, Tono, Togashi, Inubushi, Sato, Tanaka, Kimura, Yokoyama, Kim, Sano, Hachisu, Yabashi, Ohashi, Ohmori, Ishikawa and Yamauchi3]. The XFEL beam from the focusing system is collimated with a pair of slits on the path to the sample. Diffraction patterns are recorded with the MPCCD detectors. The in-vacuum operation helps to suppress background signals. The liquid-jet injector with GDVN and aerosol injector are compatible with MAXIC, although they are not frequently employed in CDI at SACLA. In most cases, specimens are fixed on a thin
![]() $\text{Si}_{3}\text{N}_{4}$
membrane, which is mounted on motorized stages and raster-scanned.
$\text{Si}_{3}\text{N}_{4}$
membrane, which is mounted on motorized stages and raster-scanned.

Figure 2. The MAXIC system for in-vacuum diffraction/scattering experiments[Reference Song, Tono, Park, Ebisu, Kim, Shimada, Kim, Gallagher-Jones, Nam, Sato, Togashi, Ogawa, Joti, Kameshima, Ono, Hatsui, Iwata, Yabashi and Ishikawa45]. The vacuum chamber contains a pair of four-jaw slits and motorized stages for injector or fixed targets. In most experiments, a focusing system and MPCCD detectors are employed as well. The red arrow indicates the XFEL beam direction.
3 Sample injectors
3.1 Liquid-jet injector with GDVN
The liquid-jet injector with GDVN produces a thin and fast stream of solution or suspension. The basic structure and working principle of GDVN are found in Refs. [Reference DePonte, Weierstall, Schmidt, Warner, Starodub, Spence and Doak46–Reference DePonte, Mckeown, Weierstall, Doak and Spence48]. Figure 3 shows a microscope image of the tip of GDVN at SACLA. The nozzle has a double capillary structure, in which sample liquid and gas are supplied through the inner and outer capillaries, respectively. The sample liquid is delivered with a hydraulic pump for high performance liquid chromatography (HPLC) pump. The concentric gas flow squeezes the liquid beam to make a thin sample filament, a diameter of which is varied with a flow rate of liquid and a stagnation pressure of He. Table 1 gives diameters of water beam produced by using a center capillary with an inner diameter of
![]() $50~\unicode[STIX]{x03BC}\text{m}$
. The size of inner capillary should be chosen to match sample properties such as viscosity, and particle size and density in suspension. The standard inner diameters are 50, 75, 100 and
$50~\unicode[STIX]{x03BC}\text{m}$
. The size of inner capillary should be chosen to match sample properties such as viscosity, and particle size and density in suspension. The standard inner diameters are 50, 75, 100 and
![]() $150~\unicode[STIX]{x03BC}\text{m}$
.
$150~\unicode[STIX]{x03BC}\text{m}$
.

Figure 3. A microscope image of the tip of GDVN at SACLA. The liquid beam from the inner capillary is squeezed by the gas flow in the outer capillary.
In SFX experiments, the GDVN injector delivers microcrystals dispersed in a buffer solution. Diffraction measurement is usually performed by using DAPHNIS. Figure 4 shows an x-ray diffraction pattern from a lysozyme microcrystal supplied from a suspension with a crystal density of
![]() ${\sim}10^{9}~\text{cm}^{-3}$
and an average crystal size of
${\sim}10^{9}~\text{cm}^{-3}$
and an average crystal size of
![]() ${\sim}1~\unicode[STIX]{x03BC}\text{m}$
. A dataset with 3226 diffraction patterns gave a crystal structure at a resolution of 0.24 nm[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43]. The GDVN injector has also been applied to in-vacuum serial diffraction experiment using MAXIC[Reference Inoue, Tono, Joti, Kameshima, Ogawa, Shinohara, Amemiya and Yabashi39].
${\sim}1~\unicode[STIX]{x03BC}\text{m}$
. A dataset with 3226 diffraction patterns gave a crystal structure at a resolution of 0.24 nm[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43]. The GDVN injector has also been applied to in-vacuum serial diffraction experiment using MAXIC[Reference Inoue, Tono, Joti, Kameshima, Ogawa, Shinohara, Amemiya and Yabashi39].

Figure 4. One of the diffraction patterns of
![]() $1~\unicode[STIX]{x03BC}\text{m}$
lysozyme crystals delivered by using the liquid-jet injector with GDVN. The image was recorded with the MPCCD detector which was placed 50 mm apart from the interaction point. Each of the eight sensor modules of the detector has an effective area of
$1~\unicode[STIX]{x03BC}\text{m}$
lysozyme crystals delivered by using the liquid-jet injector with GDVN. The image was recorded with the MPCCD detector which was placed 50 mm apart from the interaction point. Each of the eight sensor modules of the detector has an effective area of
![]() $25~\text{mm}\times 50~\text{mm}$
.
$25~\text{mm}\times 50~\text{mm}$
.
Table 1. Diameter of a water beam from GDVN a at SACLA[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43].

a An inner diameter of the center capillary is
![]() $50~\unicode[STIX]{x03BC}\text{m}$
.
$50~\unicode[STIX]{x03BC}\text{m}$
.
b Gauge pressure.
3.2 Liquid-jet injector with sample circulator
Another type of liquid-jet injector is utilized to keep sample injection with circulating a sample liquid. Figure 5 shows a schematic drawing of the injector system. The liquid is circulated by using a peristaltic pump. The standard nozzle apertures are 100 and
![]() $200~\unicode[STIX]{x03BC}\text{m}$
in diameter. A liquid-beam diameter is almost the same as the aperture size. A typical flow rate is
$200~\unicode[STIX]{x03BC}\text{m}$
in diameter. A liquid-beam diameter is almost the same as the aperture size. A typical flow rate is
![]() $1.5~\text{mL}~\text{min}^{-1}$
(
$1.5~\text{mL}~\text{min}^{-1}$
(
![]() $2.5~\text{mL}~\text{min}^{-1}$
) with the
$2.5~\text{mL}~\text{min}^{-1}$
) with the
![]() $100~\unicode[STIX]{x03BC}\text{m}$
(
$100~\unicode[STIX]{x03BC}\text{m}$
(
![]() $200~\unicode[STIX]{x03BC}\text{m}$
) nozzle. A minimum liquid volume of
$200~\unicode[STIX]{x03BC}\text{m}$
) nozzle. A minimum liquid volume of
![]() ${\sim}5~\text{mL}$
is required for the circulator to keep injection. As in the case of GDVN, a microcrystal suspension is delivered in SFX. Although the
${\sim}5~\text{mL}$
is required for the circulator to keep injection. As in the case of GDVN, a microcrystal suspension is delivered in SFX. Although the
![]() $100~\unicode[STIX]{x03BC}\text{m}$
(
$100~\unicode[STIX]{x03BC}\text{m}$
(
![]() $200~\unicode[STIX]{x03BC}\text{m}$
) liquid beam generally produces high background signals, relatively large crystals can make Bragg-signal intensities high enough for solving crystal structures. For example, a crystal structure of lysozyme was determined at a resolution of 0.24 nm by using
$200~\unicode[STIX]{x03BC}\text{m}$
) liquid beam generally produces high background signals, relatively large crystals can make Bragg-signal intensities high enough for solving crystal structures. For example, a crystal structure of lysozyme was determined at a resolution of 0.24 nm by using
![]() ${\sim}5~\unicode[STIX]{x03BC}\text{m}$
crystals ejected from the
${\sim}5~\unicode[STIX]{x03BC}\text{m}$
crystals ejected from the
![]() $200~\unicode[STIX]{x03BC}\text{m}$
nozzle[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43].
$200~\unicode[STIX]{x03BC}\text{m}$
nozzle[Reference Tono, Nango, Sugahara, Song, Park, Tanaka, Tanaka, Joti, Kameshima, Ono, Hatsui, Mizohata, Suzuki, Shimamura, Tanaka, Iwata and Yabashi43].

Figure 5. A schematic drawing of the liquid-jet injector with a sample circulator. The sample liquid in the reservoir is discharged and circulated by the peristaltic pump.
3.3 Viscous carrier injectors
The liquid-jet injectors generally consume a large amount of sample because of the high flow rate. This issue is especially serious for SFX users who measure precious protein samples. To reduce the sample consumption, viscous carrier injectors have been developed for the application to SFX. Two types are now available at SACLA. The one is basically a syringe pump (Figure 6), which slowly extrudes a viscous fluid containing microcrystals. The plunger is driven with a linear actuator. A standard syringe needle has an inner diameter of
![]() $110~\unicode[STIX]{x03BC}\text{m}$
. A typical flow rate is as low as
$110~\unicode[STIX]{x03BC}\text{m}$
. A typical flow rate is as low as
![]() $0.5~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
. A thinner needle with a
$0.5~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
. A thinner needle with a
![]() $50~\unicode[STIX]{x03BC}\text{m}$
inner diameter can be employed to further suppress the flow rate. Temperature of the syringe is kept constant with a thermoelectric device. The combination of this injector with the grease matrix method can lead to a wide range of application[Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27]. Sugahara et al. determined the crystal structures of lysozyme, glucose isomerase, thaumatin and fatty-acid-binding protein type 3 at resolutions of 0.2 nm or better by using less than 1 mg of the proteins.
$50~\unicode[STIX]{x03BC}\text{m}$
inner diameter can be employed to further suppress the flow rate. Temperature of the syringe is kept constant with a thermoelectric device. The combination of this injector with the grease matrix method can lead to a wide range of application[Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27]. Sugahara et al. determined the crystal structures of lysozyme, glucose isomerase, thaumatin and fatty-acid-binding protein type 3 at resolutions of 0.2 nm or better by using less than 1 mg of the proteins.

Figure 6. (a) The syringe-pump type of injector for a viscous carrier. The plunger of the syringe is pushed down by the push rod mounted on a linear actuator. (b) Enlarged view of the syringe part. Temperature of the syringe is kept constant by using the cooling jacket with a thermoelectric device.
The other type of injector is driven by an HPLC pump. Its details will be described in a separate paper[Reference Nango49]. Currently this injector is routinely used in SFX with a variety of crystal carriers such as LCP, greases[Reference Sugahara, Mizohata, Nango, Suzuki, Tanaka, Masuda, Tanaka, Shimamura, Tanaka, Suno, Ihara, Pan, Kakinouchi, Sugiyama, Murata, Inoue, Tono, Song, Park, Kameshima, Hatsui, Joti, Yabashi and Iwata27], and hyaluronic acid[Reference Sugahara, Song, Suzuki, Masuda, Inoue, Nakane, Yumoto, Nango, Tanaka, Tono, Joti, Kameshima, Hatsui, Yabashi, Nureki, Numata and Iwata29]. The working principle is essentially the same as that of the LCP injector developed by Weierstall et al. [Reference Weierstall, James, Wang, White, Wang, Liu, Spence, Doak, Nelson, Fromme, Fromme, Grotjohann, Kupitz, Zatsepin, Liu, Basu, Wacker, Han, Katritch, Boutet, Messerschmidt, Williams, Koglin, Seibert, Klinker, Gati, Shoeman, Barty, Chapman, Kirian, Beyerlein, Stevens, Li, Shah, Howe, Caffrey and Cherezov24].

Figure 7. A schematic diagram of the system of droplet injector. The nozzle with a piezoelectric element is driven by the electric pulse generator. The timing of ejection is adjusted by the delay generator which is synchronized with the XFEL source. The pressure controller keeps an optimum pressure of the sample.
3.4 Droplet injector
The viscous carrier injector has facilitated the delivery of lipophilic samples in lipidic carriers. Although the grease matrix method extended the applicability to soluble-protein crystals, it is more or less an invasive method; i.e., a grease carrier can deteriorate sample quality. Pulsed sample injection is a direct method to deliver non-viscous samples with a reasonably small consumption.
The pulsed droplet injector at SACLA produces a drop of sample liquid at a timing synchronized with the XFEL source[Reference Mafuné, Miyajima, Tono, Takeda, Kohno, Miyauchi, Kobayashi, Joti, Nango, Iwata and Yabashi31]. Figure 7 shows a schematic diagram of the injector system, which basically consists of a nozzle with a piezoelectric element, a sample reservoir, a pressure controller, a delay generator and an electric pulse generator. The piezoelectric element is driven by the electric pulse generator, to which the delay generator provides a timing signal synchronized with the XFEL source. The pressure controller maintains the sample pressure at which the liquid keeps the meniscus at an optimum position of the nozzle tip. The standard nozzle has an aperture of
![]() $80~\unicode[STIX]{x03BC}\text{m}$
in diameter. A typical sample flow rate is
$80~\unicode[STIX]{x03BC}\text{m}$
in diameter. A typical sample flow rate is
![]() $0.5~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
at 30 Hz.
$0.5~\unicode[STIX]{x03BC}\text{L}~\text{min}^{-1}$
at 30 Hz.
The droplet method was successfully applied to SFX. Mafuné et al. determined the structure of lysozyme at a resolution of 0.23 nm from crystals with an average size of
![]() ${\sim}5~\unicode[STIX]{x03BC}\text{m}$
. A complete dataset was obtained in
${\sim}5~\unicode[STIX]{x03BC}\text{m}$
. A complete dataset was obtained in
![]() ${\sim}30$
min with a sample amount less than 0.3 mg.
${\sim}30$
min with a sample amount less than 0.3 mg.
4 Summary and outlook
The fluid sample injectors at SACLA have facilitated experiments in the way of ‘diffract before destroy’ by working with the diverse experimental platforms such as DAPHNIS and MAXIC. They are especially playing a key role in protein SFX using the DAPHNIS system. After the successful demonstrations of the liquid-jet injectors, the viscous carrier injector and the droplet injector expanded the opportunity of SFX to a wide variety of proteins. Now both lipophilic and hydrophilic crystals can be measured with a reasonably small amount.
The increasing demands for SFX lead to the further development of sample delivery methods which will enable data acquisition in a short time with a reduced sample amount. A fixed target method is now capable of delivering crystals at the repetition rates of the existing XFEL sources[Reference Sherrell, Foster, Hudson, Nutter, O’Hea, Nelson, Paré-Labrosse, Oghbaey, Miller and Owen50]. Microfluidic flow cells would be useful for precious protein samples[Reference Heymann, Opathalage, Wierman, Akella, Szebenyi, Gruner and Fraden51, Reference Lyubimov, Murray, Koehl, Araci, Uervirojnangkoorn, Zeldin, Cohen, Soltis, Baxter, Brewster, Sauter, Brunger and Berger52]. Sample injection with a coaxial sheath liquid can help to suppress a sample amount even in the case of continuous-flow liquid jet[Reference Calvey, Katz, Schaffer and Pollack34].
The developments in sample delivery methods add new capabilities to the experimental systems. Time-resolved SFX, for example, is becoming a common technique for studying dynamics of proteins[Reference Barends, Foucar, Ardevol, Nass, Aquila, Botha, Doak, Falahati, Hartmann, Hilpert, Heinz, Hoffmann, Köfinger, Koglin, Kovacsova, Liang, Milathianaki, Lemke, Reinstein, Roome, Shoeman, Williams, Burghardt, Hummer, Boutet and Schlichting53, Reference Pande, Hutchison, Groenhof, Aquila, Robinson, Tenboer, Basu, Boutet, DePonte, Liang, White, Zatsepin, Yefanov, Morozov, Oberthuer, Gati, Subramanian, James, Zhao, Koralek, Brayshaw, Kupitz, Conrad, Roy-Chowdhury, Coe, Metz, Xavier, Grant, Koglin, Ketawala, Fromme, Šrajer, Henning, Spence, Ourmazd, Schwander, Weierstall, Frank, Fromme, Barty, Chapman, Moffat, van Thor and Schmidt54]. In this technique, delivery tools should be compatible with sample activation techniques such as optical pulse irradiation and fast mixing of reactant and sample solutions[Reference Wang, Weierstall, Pollack and Spence33, Reference Calvey, Katz, Schaffer and Pollack34].
Acknowledgements
The author thanks members of the SACLA Science Research Group and the Engineering Team of RIKEN SPring-8 Center for providing the figures and the table in this paper. The experimental data were obtained at BL3 of SACLA with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Numbers 2014B8051, 2015A8047, 2015B8054, 2015B8064 and 2016A8066). The injector developments at SACLA were supported by the X-ray Free-Electron Laser Priority Strategy Program (MEXT); JSPS KAKENHI Grant Number 15K05407.