Introduction
Predicting the phenology of insect developmental stages is critical in many aspects of applied entomology (Chuine and Régnière Reference Chuine and Régnière2017). In pest management, many control and monitoring methods are life stage specific, thus requiring accurate predictions of development in order to deploy surveillance or control measures in an efficient manner (Pruess Reference Pruess1983). Phenology is also a key component in modelling survival and adult dispersal, two critical processes in forecasting pest population dynamics (Sturtevant et al. Reference Sturtevant, Achtemeier, Charney, Anderson, Cooke and Townsend2013). More recently, the need to assess the potential impacts of climate change on the geographic distribution and population dynamics of pest populations has given new impetus to pest phenology research, either directly, in relation to both the survival and the ability of an insect to complete a life cycle before the return of unfavourable conditions, or indirectly, in relation to host phenology and the potential for desynchronisation with a seasonal food source (Bale et al. Reference Bale, Masters, Hodkinson, Awmack, Bezemer and Brown2002).
Insects are ectotherms: temperature affects their development rate and consequently their phenology. Measuring the time required for an insect to complete a specific life stage, such as egg hatching or moulting, within a defined temperature regime is frequently used to quantify the effect of temperature on development. Often, the data are then used to formalise and parametrise temperature-dependent phenology models (e.g., Régnière et al. Reference Régnière, Powell, Bentz and Nealis2012a).
A number of challenges arise when designing an experimental protocol to estimate the effects of temperature on development. In the initial phase of the experimental design, a choice must be made with regard to the type of data that will be collected. Development rate can be measured in the form of either stage-specific development times of marked individuals (i.e., individual-based sampling) or stage frequency recorded by sampling a population at various times during development (i.e., cohort-based sampling). Individual-based sampling is both time and resource intensive but provides better estimates of variance (i.e., correlations in the duration of consecutive stages) and mortality than cohort-based sampling (e.g., Goodsman et al. Reference Goodsman, Aukema, McDowell, Middleton and Xu2017). An additional challenge common to both types of sampling is the need for data at or near the thermal limits of the organism to assure accurate models of temperature-dependent development (Régnière et al. Reference Régnière, Powell, Bentz and Nealis2012a). However, slow development and high mortality are common issues when insects are reared near their physiological thermal limits; therefore, these experiments often produce limited data unless specific rearing and sampling protocols are used (Weber et al. Reference Weber, Volney and Spence1999, Table 3). High mortality during rearing has also prevented the study of temperature effects on larval development through multiple generations, a question of increasing importance in the context of climate change (Donelson et al. Reference Donelson, Salinas, Munday and Shama2018).
Spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), is the most severe defoliator of conifers in North America’s boreal forests (Blais Reference Blais1983). Spruce budworm is considered a univoltine species, with the majority of larval feeding occurring from May through July (Fig. 1). This species experiences irregular population outbreaks every 30–40 years (Candau and Fleming Reference Candau and Fleming1998), during which life stage–specific control methods are often applied to prevent widespread host tree mortality.
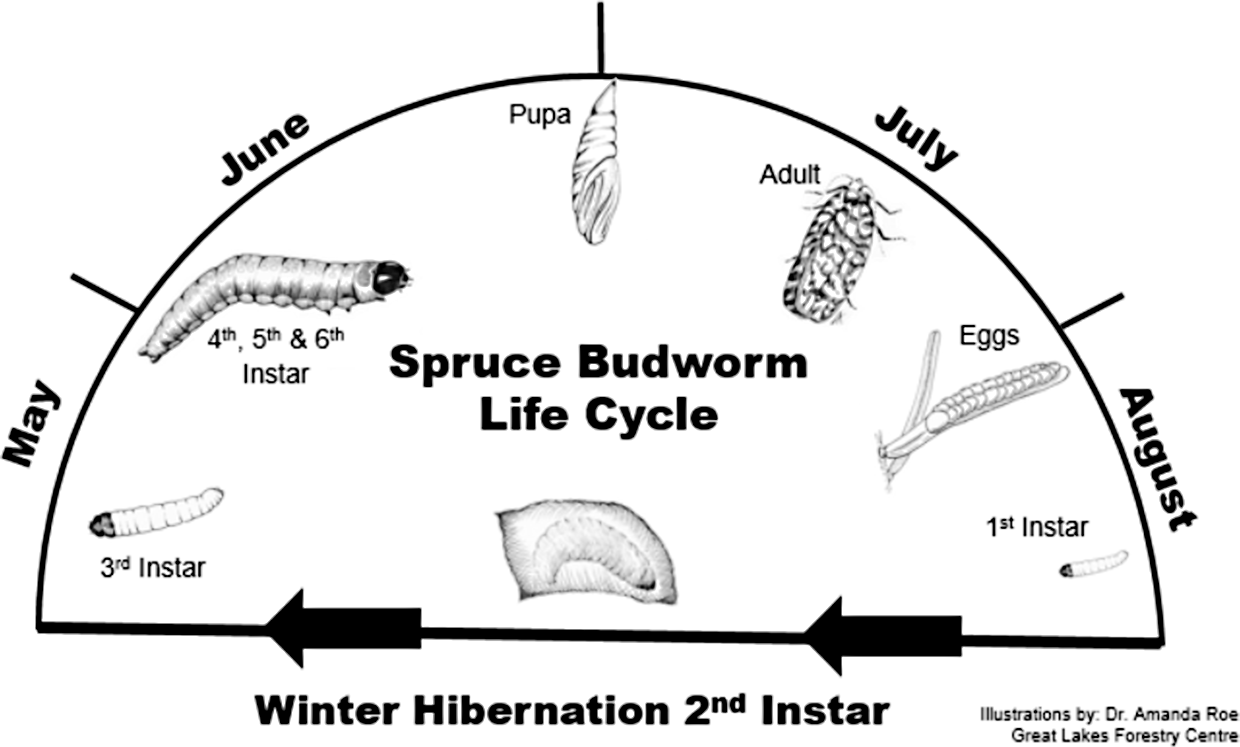
Fig. 1. Life cycle of the spruce budworm Choristoneura fumiferana (Clemens).
Previous authors have measured spruce budworm development rates in rearing experiments at constant temperatures ranging from 9.3 °C (Weber et al. Reference Weber, Volney and Spence1999, p. 227, Table 2) to 32 °C (Régnière Reference Régnière1987, Fig. 2). The high mortality that occurs when spruce budworm larvae are reared at constant temperatures outside this range has, to date, prevented a direct measurement of development. Therefore, development rates closer to the insect’s lower and upper developmental thresholds have been estimated only graphically or numerically (Bean and Wilson Reference Bean and Wilson1964; Eidt and Cameron Reference Eidt and Cameron1972; Régnière Reference Régnière1987). Successfully rearing postdiapause larvae near their thermal thresholds has been difficult, in part because the established rearing protocols for spruce budworm were designed to maintain laboratory colonies at an optimal temperature (e.g., Stehr Reference Stehr1954; Grisdale Reference Grisdale1970; Thomas Reference Thomas1984; Ebling and Dedes Reference Ebling and Dedes2015) not to measure development rates. Specific rearing protocols and modelling tools recently have been developed to collect and analyse development rate data near thermal thresholds (Régnière et al. Reference Régnière, Powell, Bentz and Nealis2012a; McManis et al. Reference McManis, Powell and Bentz2018).
Table 1. Summary of experimental conditions used to determine development rates of spruce budworm larvae reared at seven constant temperatures. Predicted stage-specific development time (after models in Régnière et al. Reference Régnière, St-Amant and Duval2012b) was used in the temperature transfer treatments to determine the duration of exposure to treatment temperatures before the larvae were placed at 20 ± 1 °C to continue development.
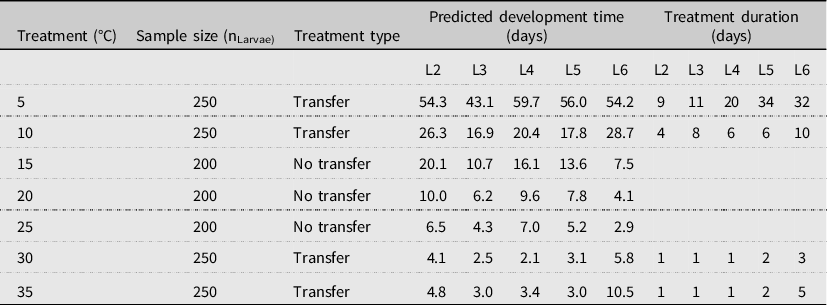
L#, larval instar stage
Table 2. Parameters of the Régnière et al. (Reference Régnière, St-Amant and Duval2012b) development model (equation 1) for each spruce budworm larval instar stage (L#).
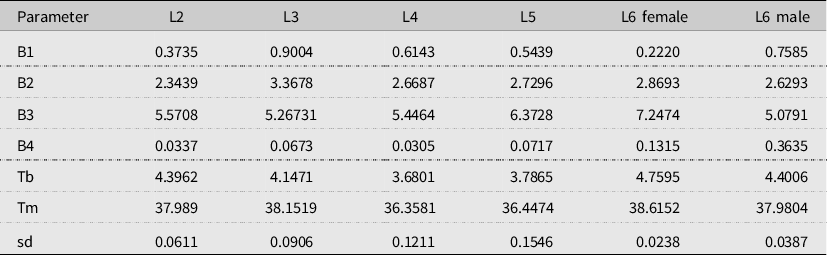
sd, standard deviation; Tb, thermal minimum; Tm, thermal maximum.
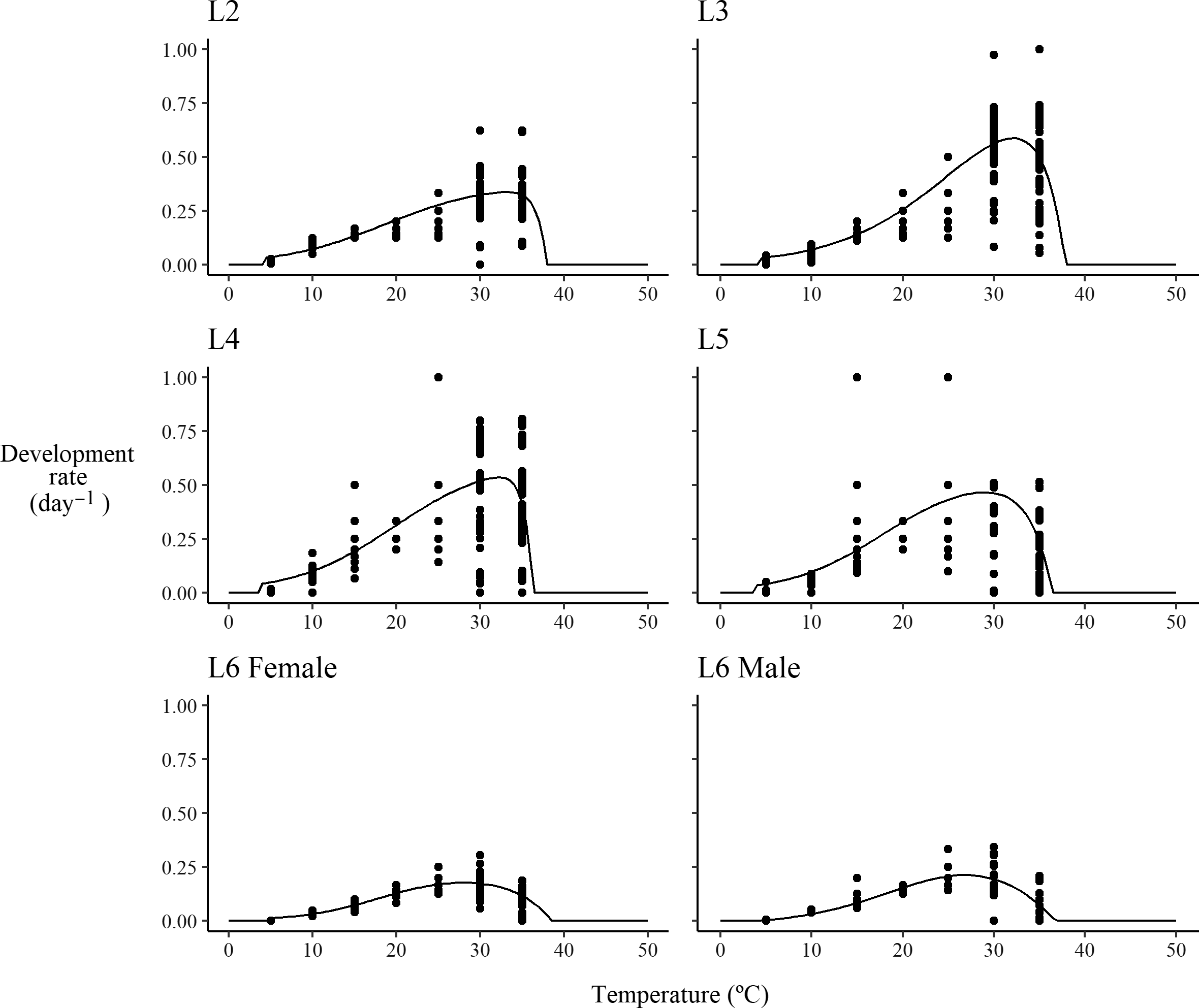
Fig. 2. Modelled and observed stage-specific development rates of six-instar spruce budworm reared at constant temperature. L#, larval instar stage.
Herein, we describe an experimental rearing protocol and compare sampling methods to estimate spruce budworm postdiapause development rates across a range of constant temperatures that approach the insect’s upper and lower thermal thresholds. We show that our rearing method produced estimates of development rates in spruce budworm close to its thermal limits. We use these data to fit stage-specific thermal development models. We also compare results obtained from two different sampling protocols and describe variations in the number of larval stages.
Methods
In Canada, spruce budworm lay eggs in July and August on spruce (Picea) or fir (Abies) trees. These eggs hatch, and the first-instar larvae disperse within the crown to locate an overwintering site. They then spin hibernacula, moult into second-instar larvae, and enter an obligate winter diapause. In the spring, the second-instar larvae emerge from their hibernacula and initiate feeding on year-old foliage until budburst, webbing together needles to create a protective feeding structure. After budburst, feeding continues on the developing foliage through six larval instars (second through sixth instars) until late June when the larvae pupate, emerging as adult moths a few weeks later (Fig. 1).
Laboratory rearing techniques for spruce budworm were developed in the 1940s (McMorran Reference McMorran1965; Ebling and Dedes Reference Ebling and Dedes2015; Perrault et al. Reference Perrault, Wardlaw, Candau, Irwin, Demidovich, MacQuarrie and Roe2021). These techniques have been used with only minor modifications to produce self-reproducing laboratory colonies that have existed since the 1960s. (For a historical summary, see Roe et al. Reference Roe, Demidovich and Dedes2017.)
Postdiapause emergence
Postdiapause second-instar spruce budworm larvae were obtained from two colonies maintained in the Insect Production and Quarantine Laboratory at the Great Lakes Forestry Centre, Sault Ste. Marie, Ontario, Canada (Stock codes: Glfc:IPQL:Cfum11 and Glfc:IPQL:Cfum14; Roe et al. Reference Roe, Demidovich and Dedes2017). Larvae within their hibernacula completed 18–24 weeks of diapause at 2 °C, 70 ± 20% relative humidity, and in total darkness. We triggered postdiapause emergence by placing the larvae in a controlled environment chamber (Hotpack model 792; Hotpack Ltd., Philadelphia, Pennsylvania, United States of America) at 20 ± 3 °C, 70 ± 20% relative humidity, and 14:10-hour light:dark photoperiod in a clear glass or plastic dish with a tight seal to prevent the emerging larvae from escaping. After 4–7 days, emerging second-instar larvae were placed in individual cups of artificial diet (McMorran Reference McMorran1965) using a camel-hair paintbrush and immediately put in a different controlled environment chamber (Conviron models 124L and EF7; Controlled Environments Ltd., Winnipeg, Manitoba, Canada) set to one of the temperature treatments (see below). We recorded the date and time each insect entered their controlled environment chamber.
Rearing protocol to produce diapausing second-instar larvae
Upon eclosion, adults were transferred to a mating bag for copulation. A mating bag is a 40-L clear plastic bag that contains strips of wax paper (Cut-Rite, Reynolds Consumer Products, Mississauga, Ontario, Canada) used to house male and female budworm moths to allow them to mate and the females to produce eggs. The wax paper is an oviposition substrate used to mimic the waxiness of spruce needles. With this method, the adults and oviposition substrate are added to the mating bag, and the bag receives a light misting of autoclaved water, is inflated with filtered air, and sealed. Adult female moths mate with male moths inside the bag and lay their eggs on the oviposition substrate. The substrates are then transferred to hatching pans for larval emergence. Hatching pans are sterilised, 30 × 46-cm metal roasting pans equipped with a top and bottom gauze and Parafilm (Amcor, Zurich, Switzerland) substrate. The bottom substrate is a 13 × 23-cm sheet of gauze attached to a 15 × 25-cm sheet of Parafilm with a rolling pin. The top substrate is the same size of gauze rolled onto a 41 × 51-cm sheet of Parafilm. The bottom substrate is placed in the centre of the pan and sealed smoothly to the pan surface. The top substrate is placed with the gauze facing inwards at the centre, and the Parafilm edges are pulled down and taped to the sides of the pan. This creates a seal over the opening of the pan to prevent loss of larvae. Hatched first-instar larvae that emerge onto the oviposition substrate move into both patches of gauze and use them as a substrate to spin their hibernacula and moult to second-instar stages. After three days, a cover made of opaque cardboard with a cut-out corresponding to the size and location of the top gauze is placed over the pan. The light coming through the window attracts and concentrates phototactic first-instar larvae that have not yet established hibernacula in the bottom cheesecloth into the top one.
At 20 °C, it takes 13 days for the first-instar larvae to hatch, disperse to the gauze, establish their hibernacula, and moult to the second-instar stage. After this period, the top and bottom substrates are cut out, and second-instar larvae are counted, using a fixed-focus magnifier following Ebling and Dedes (Reference Ebling and Dedes2015). The second-instar larvae within their hibernacula are then placed in cool conditions (2 ± 3 °C, 70 ± 20% relative humidity, 0:24 light:dark photoperiod) to diapause, for a minimum of 18 weeks to a maximum of 36 weeks (McMorran Reference McMorran1973; van Frankenhuyzen et al. Reference van Frankenhuyzen, Ebling, Dedes and Pitt2007).
Temperature treatments (individual-based sampling method)
We used seven temperatures (5–35 °C, in 5 °C increments) to test the individual-based sampling method. These temperatures span the expected thermal range of spruce budworm development (Régnière Reference Régnière1990). Two hundred insects were reared at each of the midrange temperatures (i.e., 15 °C, 20 °C, and 25 °C). For the treatment temperatures near the thermal limits (i.e., 5 °C, 10 °C, 30 °C, and 35 °C), we used the temperature transfer method (Régnière et al. Reference Régnière, Powell, Bentz and Nealis2012a) and reared 250 individual larvae per temperature treatment. The temperature transfer method assumes that if the rearing temperature (e.g., 5 °C or 10 °C) is at or below the insect’s lower thermal limit, development occurs only when the insect is moved to the optimal temperature (i.e., 20 °C). This assumption can be tested by comparing the development times of insects in the temperature transfer treatments to those reared at a constant 20 °C. If these times are the same length, then insects in the temperature transfer treatments are developing only at the optimal temperature, and development is paused when they are at their rearing temperature. Any deviation in these times, however small, suggests that development is occurring at the lower temperature.
All insects were reared at their treatment temperature under a constant humidity and light regime (70 ± 20% relative humidity and 14:10 light:dark photoperiod). Insects in the temperature transfer treatments were reared for a set period of time (see below) and then transferred to the optimal spruce budworm rearing temperature (20 ± 1 °C) under the same photoperiod and humidity conditions until development was complete for that stage (e.g., moulting from second- to third-instar stage). After moulting, the larvae were returned to the original treatment temperature for the next predetermined length of time (Table 1). We used an existing spruce budworm development model (Régnière et al. Reference Régnière, St-Amant and Duval2012b) and a series of trials to maximise the time at treatment temperatures while also minimising mortality. As a result, the time spent at each treatment temperature varied, depending on the temperature, from 15% to 67% of the estimated development time (Table 1). We repeated this process for each larval stage until pupation or death. We increased the number of larvae used in the near-threshold temperature treatments to compensate for the higher expected mortality.
F0 generation
We monitored individual larvae daily, recording the timing of moults, pupation, and mortality for each individual. Moults were positively confirmed by the presence of a shed head capsule, which we removed after each moult. The sex of the sixth-instar larvae was determined by the presence of visible gonads. We also monitored the temperature and humidity within each chamber using thermocouples connected to data loggers (HOBO mx110, Onset Computer Corporation, Bourne, Massachusetts, United States of America) that provided a measurement independent from that of the environmental chambers’ own controls. Each insect was placed on fresh diet every two weeks. We minimised the number of times larvae were manipulated to reduce impacts on feeding and growth (van Frankenhuyzen and Espinasse Reference van Frankenhuyzen and Espinasse2010). Upon pupation, we confirmed the sex of each individual by counting the number of segments on the abdomen and placed them at 20 ± 1 °C to improve eclosion success and synchrony. Males and females were placed in separate emergence boxes in preparation for mating.
Testing the cohort-based sampling method
We tested the practicality and appropriateness of the cohort-based method in assessing spruce budworm development by applying it to estimate development rate for one temperature (20 °C) under the same environmental conditions used in the individual-based method (i.e., 14:10 light:dark photoperiod and 70 ± 20% relative humidity). Cohort-based sampling is destructive (sampled cohorts are used only once), so we created 30 cohorts each of 90 spruce budworms reared in individual diet cups and randomly sampled one cohort per day for 30 days. We used 30 cohorts, based on the estimated time of 30 days required for spruce budworm to develop from the second-instar stage to pupation (Régnière et al. Reference Régnière, St-Amant and Duval2012b). Each time a cohort was sampled, the instar of each individual was determined by counting the number of shed head capsules in its rearing cup. Each larva was then placed in a vial of 75% alcohol, where we could later confirm their age (instar) by measuring the width of the head capsule.
Head capsule measurements
Initial inspection of larval development in the temperature rearing experiments indicated that some individuals appeared to go through only five instars to complete development. These individuals produced only four head capsules instead of the expected five (i.e., second to sixth instars). Further inspection showed that the largest head capsules produced by these larvae were consistent in size and colour with that of a sixth-instar spruce budworm larva, but the penultimate head capsule produced by these larvae was intermediate in size between that of a fourth-instar and a fifth-instar spruce budworm. This observation was concerning because we required a high degree of consistency in the determination of larval instar in the development rate data set. We therefore elected to further investigate these observations to ensure that the “missing’ instar was not the result of errors made in our daily checks. In this way, we generated an independent data set of spruce budworm head capsule measurements to allow us to classify shed spruce budworm capsules to the correct larval instar.
An independent cohort of overwintered second-instar spruce budworm larvae was obtained from one of the colonies maintained at the Insect Productions and Quarantine Laboratory. These larvae were reared at 20 °C under the conditions previously described. Each insect was reared separately and checked several times per week until pupation. When shed head capsules were observed in the cup, they were collected and then arranged by size on a microscope slide and secured with glue. Each head capsule was first assigned a putative larval-instar stage based on relative size (second instar, third instar, etc.) and then photographed with a Dino-Lite microscope camera (model AM4113ZTS, AnMo Electronics, Hsinchu, Taiwan) and measured with ImageJ software (ImageJ bundled with Java 1.8.0_172; Schneider et al. Reference Schneider, Rasband and Eliceiri2012). Photos and measurement software were calibrated with a stage micrometer (Edmund Optics Inc., Barrington, New Jersey, United States of America). Each head capsule was measured three times at its widest point, and these values were then averaged to obtain the head capsule measurement.
Analysis
We modelled the relationship between temperature and larval development rate using the Régnière et al. (Reference Régnière, St-Amant and Duval2012b) stage-specific individual development model given by:
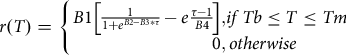 $$r\left( T \right) = {\rm{ }}\left\{ {\matrix{ {B1\left[ {{1 \over {1 + {e^{B2 - B3*\tau }}}} - e{{\tau - 1} \over {B4}}} \right],} { if\,Tb \le T \le Tm} \hskip22pt\cr {\hskip45pt0,}\, {otherwise} \cr } } \right.$$
$$r\left( T \right) = {\rm{ }}\left\{ {\matrix{ {B1\left[ {{1 \over {1 + {e^{B2 - B3*\tau }}}} - e{{\tau - 1} \over {B4}}} \right],} { if\,Tb \le T \le Tm} \hskip22pt\cr {\hskip45pt0,}\, {otherwise} \cr } } \right.$$
where
![]() $$\tau = \left( {T - Tb} \right)/\left( {Tm - Tb} \right)$$
. This model has been used to fit spruce budworm development data in earlier studies (Régnière Reference Régnière1987). The model was fitted for each sex separately at the sixth instar following observations by Eidt and Cameron (Reference Eidt and Cameron1973) that male and female development was identical up to this stage. We estimated the parameters of each larval-stage model by maximum likelihood following McManis et al. (Reference McManis, Powell and Bentz2018). In temperature transfer experiments, the development rate at the treatment temperature, r
treatment, was calculated as r
treatment = (1 – r
optimum × t
optimum)/t
treatment, where r
optimum is the median of the observed development rate in the rearing experiment at optimum temperature (i.e., 20 °C), and t
treatment and t
optimum are the development times predefined at treatment temperature and observed at optimum temperature, respectively.
$$\tau = \left( {T - Tb} \right)/\left( {Tm - Tb} \right)$$
. This model has been used to fit spruce budworm development data in earlier studies (Régnière Reference Régnière1987). The model was fitted for each sex separately at the sixth instar following observations by Eidt and Cameron (Reference Eidt and Cameron1973) that male and female development was identical up to this stage. We estimated the parameters of each larval-stage model by maximum likelihood following McManis et al. (Reference McManis, Powell and Bentz2018). In temperature transfer experiments, the development rate at the treatment temperature, r
treatment, was calculated as r
treatment = (1 – r
optimum × t
optimum)/t
treatment, where r
optimum is the median of the observed development rate in the rearing experiment at optimum temperature (i.e., 20 °C), and t
treatment and t
optimum are the development times predefined at treatment temperature and observed at optimum temperature, respectively.
We further computed median development time to pupation and mortality rate for each postdiapause larval instar for each temperature regime in the individual-rearing experiments. We used the median to account for skewed distributions and allow comparison with earlier models and observations (Régnière Reference Régnière1987). We computed the same measures for larvae reared in the cohort approach and compared development times at 20 °C between the two rearing methods.
To determine the number of larval instars in our spruce budworm populations, we first classified the insects in the independent head capsule experiment into two groups, denoted “5-instar” and “6-instar”. For those spruce budworm in the 5-instar group, we assigned the penultimate larval instar the designation “L4`” to distinguish it from the fourth-instar stage in the 6-instar group.
Spruce budworm are assumed to have six larval instars, but this number can vary from five to seven (Schmidt and Lauer Reference Schmidt and Lauer1977). We confirmed the existence of six larval instars using Dyar’s Rule (Dyar Reference Dyar1890) for larvae in the 6-instar group. Dyar’s Rule states a linear relationship should exist between the log of head capsule size and the putative larval instar. Similarly, we applied Dyar’s Rule to the 5-instar group, with the L4` designation applied to the penultimate instar.
To allow us to classify all spruce budworm to the correct instar, we determined quantitative break points between instars using methods from McClellan and Logan (Reference McClellan and Logan1994). Briefly, we first assigned each larva to an instar, “i”, based on the instar head capsules’ apparent sizes and the order in which they were shed by the larva. For each instar, we then determined the mean (
![]() $${\bar x_i}$$
) and the variance (
$${\bar x_i}$$
) and the variance (
![]() $${s_i}^2$$
) of the head capsule widths and used these measures to estimate parameters using nonlinear least squares for:
$${s_i}^2$$
) of the head capsule widths and used these measures to estimate parameters using nonlinear least squares for:
where
![]() $${a_i}$$
is a scaling parameter dependent on the size of frequency distribution of head capsules,
$${a_i}$$
is a scaling parameter dependent on the size of frequency distribution of head capsules,
![]() $${b_i} = 1/\left( {2{s_i}^2} \right)$$
, and
$${b_i} = 1/\left( {2{s_i}^2} \right)$$
, and
![]() $${c_i} = \;{\bar x_i}$$
(McClellan and Logan Reference McClellan and Logan1994, equation 1). Because overlap likely occurs between the instar distributions, we obtained final parameter estimates for the spruce budworm data by fitting:
$${c_i} = \;{\bar x_i}$$
(McClellan and Logan Reference McClellan and Logan1994, equation 1). Because overlap likely occurs between the instar distributions, we obtained final parameter estimates for the spruce budworm data by fitting:
(McClellan and Logan Reference McClellan and Logan1994, equation 2) after first fitting the present study’s equation (2) for each instar to obtain parameter estimates to initiate the model fitting procedure. We then determined the break points for the instars using McClellan and Logan’s (Reference McClellan and Logan1994) equations 3 and 4.
We attempted to determine instar breaks treating the 5-instar and 6-instar groups in the same analysis (i.e., by fitting the present study’s equation (3) for
![]() $$i = L2, \ldots ,L4,L4`, \ldots L6$$
). However, this proved to be impossible because the model refused to converge. This is likely because the nonlinear modelling approach (McClellan and Logan Reference McClellan and Logan1994) requires relatively distinct head capsule distributions for the larval instars, which was not the case when the two groups in the present study were combined. We therefore determined break points for each group separately by fitting the present study’s equation 2 and equation 3 for the 5-instar and 6-instar groups.
$$i = L2, \ldots ,L4,L4`, \ldots L6$$
). However, this proved to be impossible because the model refused to converge. This is likely because the nonlinear modelling approach (McClellan and Logan Reference McClellan and Logan1994) requires relatively distinct head capsule distributions for the larval instars, which was not the case when the two groups in the present study were combined. We therefore determined break points for each group separately by fitting the present study’s equation 2 and equation 3 for the 5-instar and 6-instar groups.
Once we had confirmed the existence of the two groups in larval spruce budworm reared under laboratory conditions, we then re-examined our rearing records and determined the percentage of individuals in each larval cohort that exhibited each behaviour.
We tested whether development differed in the cohort-based sampling method from that in the individual-based method by comparing the evolution of the daily median larval stage observed in the former with a distribution of daily median larval stage in the latter. The daily median larval stage in the cohort-based experiment was calculated as the median larval stage in each consecutive cohort. For each day of the individual-based experiment, we simulated the draw of cohorts by drawing 500 bootstrap samples of five individual-based observations (i.e., 3.3% of the total, corresponding to the same fraction of the total population as a cohort). We calculated the median larval stage for each bootstrap sample, thereby obtaining a daily distribution of median larval stage.
All analyses were done in the R statistical computing environment, version 4.0.4, using functions in the base and stats libraries (R Core Team 2021) and bbmle (Bolker and R Development Core Team Reference Bolker2020). Data and code for the analysis of temperature and larval development rate can be obtained by contacting J.-N.C. All other data and code are deposited on Dryad (Wardlaw et al. Reference Wardlaw, Perrault, Roe, Dedes, Irwin, MacQuarrie and Candau2021).
Results and discussion
Temperature treatments (individual-based sampling method)
The maximum development rate of early instars (second to fourth instars) peaked between 30 °C and 35 °C, and the maximum development rate of later instars peaked between 25 °C and 30 °C (Fig. 2). The third-instar stage had the highest development rate, followed, in order, by the fourth-, fifth-, second-, and sixth-instar stages. The lower development threshold temperature varies from 3.7 °C (fourth-instar stage) to 4.8 °C (female sixth-instar stage), whereas the higher-threshold temperature varies from 36.4 °C (fourth-instar stage) to 38.6 °C (female sixth-instar stage; Table 2). These observations were generally consistent with reports by Régnière (Reference Régnière1987), although he found that the fourth-instar stage had a slightly higher maximum rate than the third-instar larval stage. Régnière (Reference Régnière1987) did not estimate the lower and higher thresholds (i.e., they were kept fixed during parametrisation).
Median development time to pupation of postdiapause spruce budworm decreased with increasing temperatures until it reached a minimum of 16 days at 25 °C (Fig. 3). This is consistent with previous reports of median development times of 19 days at 23.9 °C and 17 days at 26.7 °C (Eidt and Cameron Reference Eidt and Cameron1972). At 20 °C, the observed median time to pupation was 25 days, which is also consistent with previous observations of 24 days at 21.1 °C reported by Eidt and Cameron (Reference Eidt and Cameron1972). Development time then begins to increase as temperature increases to 35 °C, which is closer to the thermal maxima of 36.4–38.6 °C, depending on the larval stage (Fig. 2).
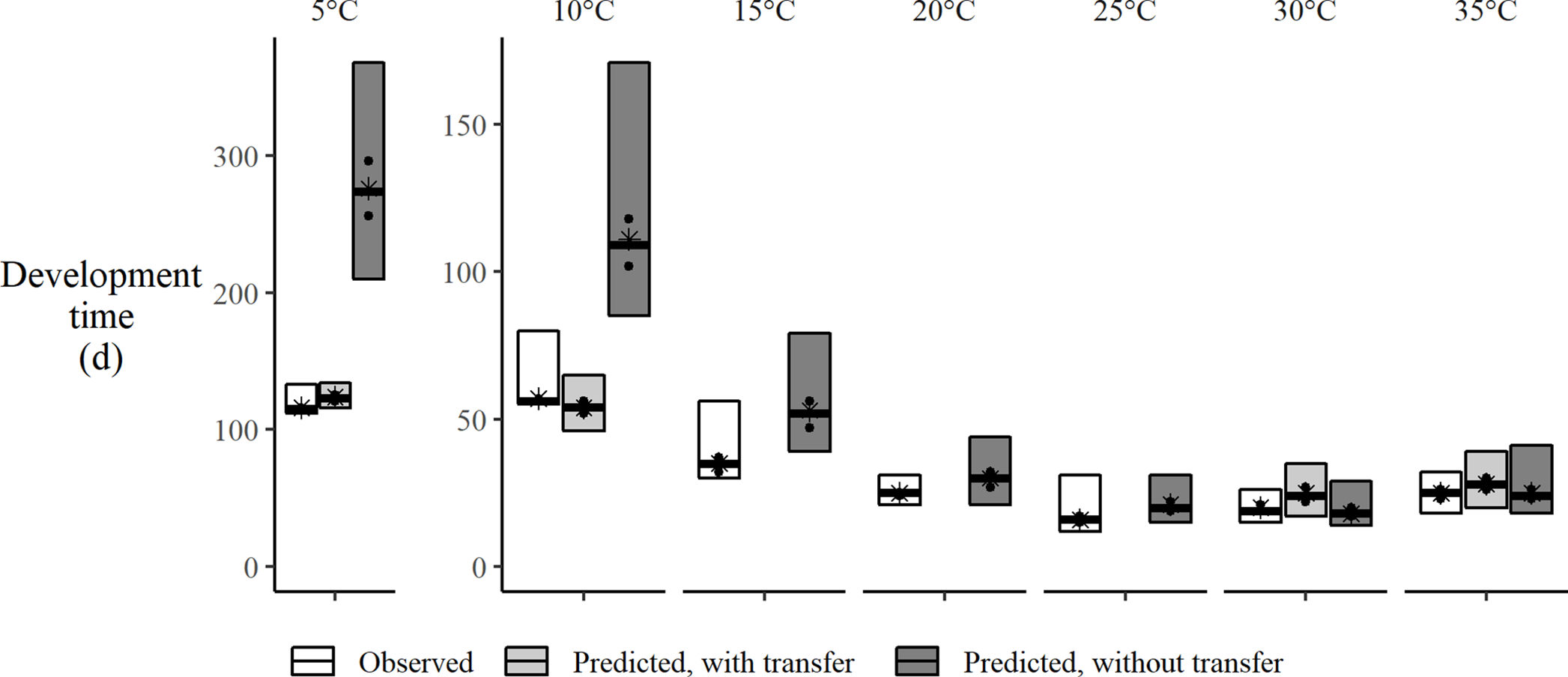
Fig. 3. Observed and predicted time to pupation for spruce budworm reared at constant temperatures. Predicted developmental times from Régnière et al. (Reference Régnière, St-Amant and Duval2012b) with and without temperature transfers at thermal extremes. In temperature transfer treatments, at each development stage, larvae are reared at the treatment temperature for a fixed duration and then transferred to optimal temperature (20 °C) until moulting occurs. Without temperature transfer, larvae are continuously reared at treatment temperature. Boxes show range between maximum and minimum development times, median development time (bar), mean development time (*), and first and third quartile (filled circles). Note the different scale of the y-axes.
Development time to pupation near the lower-threshold temperatures was slower than near the optimal temperatures (Fig. 3). Larvae within these treatments were exposed to treatment temperatures and to optimal rearing temperatures for a proportion of each life stage following the temperature transfer method (Table 1). Under these conditions, development at 5 °C took 115 days, which was faster than the 123 days predicted by the Régnière et al. (Reference Régnière, St-Amant and Duval2012b) model (Fig. 3). Similarly, at 10 °C, the median duration to reach pupation was 56 days, with 34 days spent at 10 °C and 22 days spent at 20 °C (Table 1).
Our data provide the first experimental confirmation that postdiapause development in spruce budworm occurs at temperatures as low as 5 °C. In the constant temperature treatment at 20 °C, we observed that the median time to pupation was 25 days (Fig. 3). We observed that insects required only two days at 20 °C after rearing for 34 days at 10 °C and only 9 days at 20 °C after rearing for 106 days at 5 °C. This suggests that spruce budworm development does occur at these low temperatures once the larvae have emerged from diapause. Previous postdiapause development lower-threshold temperatures were interpolated graphically from developmental models fitted with higher temperatures (Bean and Wilson Reference Bean and Wilson1964; Eidt and Cameron Reference Eidt and Cameron1973).
The use of the temperature transfer method allowed us to demonstrate that development can occur at 35 °C. Previous attempts at continuously rearing at this temperature resulted in 100% mortality (Wellington Reference Wellington1949; Régnière Reference Régnière1990), thereby suggesting that exposure to this temperature can last only a short period of time before becoming lethal. Insects reared at 35 °C temperature treatment took longer to complete development than those reared at 30 °C (Table 1; Fig. 3), which was likely due to thermal stress.
Overall mortality rates for each constant temperature treatment ranged from 12% to 44%. For individual instars, mortality rates varied somewhat with temperature, with higher mortality rates observed at the extreme temperatures (i.e., at 5 °C, 10 °C, 30 °C, and 35 °C) and in the 15 °C treatments (Fig. 4). However, even in the extreme temperature treatments, the observed mortality was lower than the approximately 50% mortality observed for existing spruce budworm colonies reared under the standard Insect Production and Quarantine Laboratory colony-rearing conditions (J. Dedes, unpublished data). Rearing in individual cups likely reduced the mortality rates observed in the present study, because overcrowding is a common cause of stress and mortality in laboratory colonies (Grisdale Reference Grisdale1970). The temperature transfer method likely helped reduce the mortality rate within the extreme treatments.
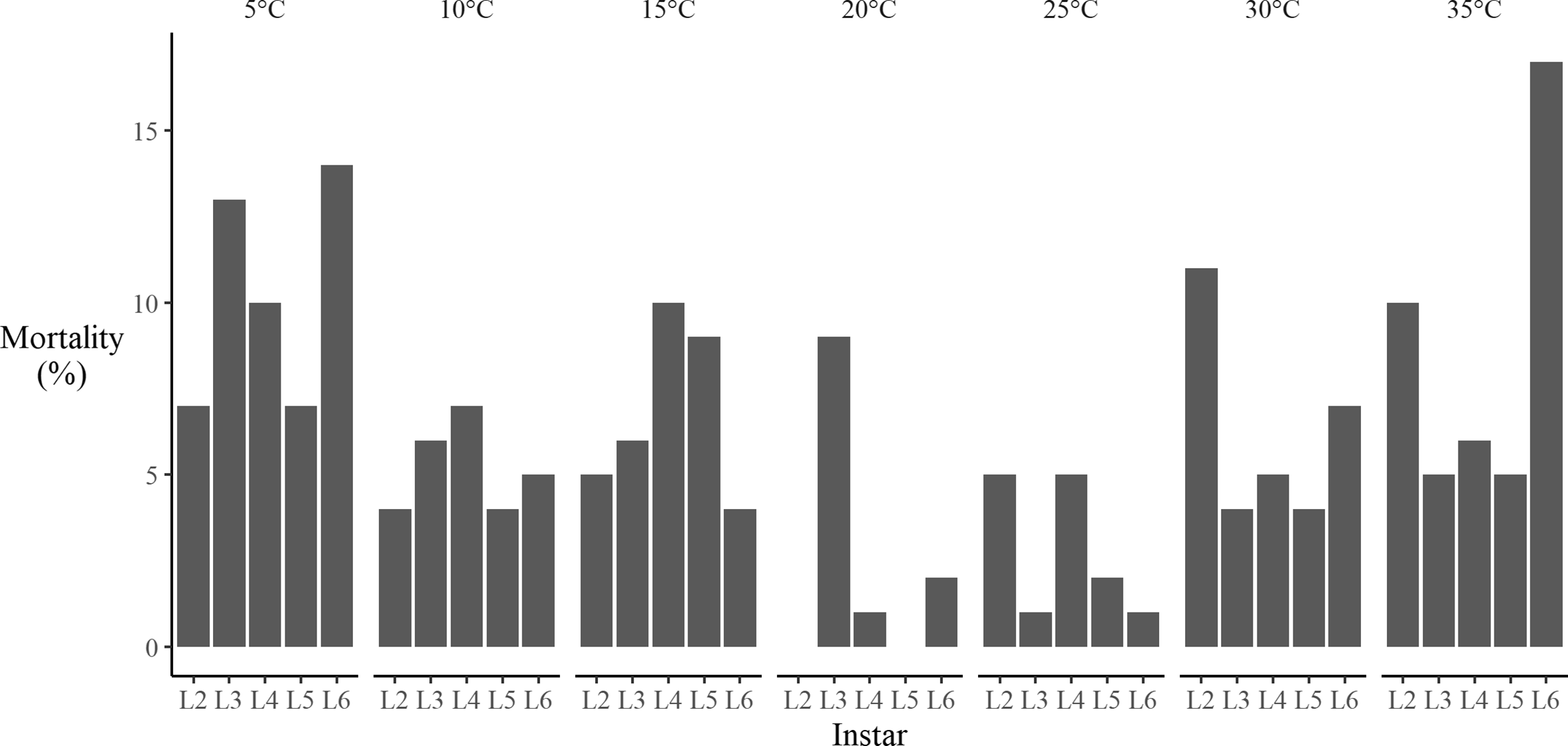
Fig. 4. Observed mortality of larval spruce budworm reared at seven constant temperatures using the individual-based sampling method. L#, larval instar stage.
While overall mortality was low, it was sufficient in the two higher-temperature treatments to eliminate the opportunity to produce an F1 generation. Only five adults eclosed from their pupae in the 35 °C treatment, and attempts to mate these individuals were unsuccessful. Mortality in the 30 °C treatment was lower (31.3%). Of the survivors from the 30 °C treatment, 96% of those that reached the pupal stage eclosed and produced adults that successfully mated, albeit after a longer period than expected (10 days versus five days). The F1 offspring from those matings developed until the second-instar stage and produced hibernacula as expected, but they did not survive the overwintering period. At the lower developmental temperatures, larvae exposed to the 10 °C and 5 °C temperature transfer treatments had high mortality (Fig. 4) but were able to successfully eclose, mate, and produce viable second-instar larvae that survived overwintering. We expect that this inability to successfully produce viable F1 offspring will limit the ability of researchers to explore transgenerational effects of high temperatures on spruce budworm.
Cohort-based sampling method
Stage-specific development measured as the median larval stage in the cohort-based method did not statistically differ (at the 0.05 level) from development in the individual-based method at the same temperature (Fig. 5). Cohort-based sampling is assumed to have an advantage over individual-based sampling in that individual larvae are not disturbed every day during development checks, thereby offering a less-biased estimate of development rates. The similarity in the time to pupation between the two methods suggests that daily checks of larval stage have little effect on budworm development. In contrast, the overall mortality in the cohort-based method (4%) was significantly lower than in the individual-based method (12%; χ 2 = 28.44, P < 0.001). This indicates that the cohort-based method can be useful when low sample sizes are a concern. However, the method does not allow for the capture of development plasticity (see below), because it requires the simultaneous emergence of a large number of individuals to constitute the initial population.
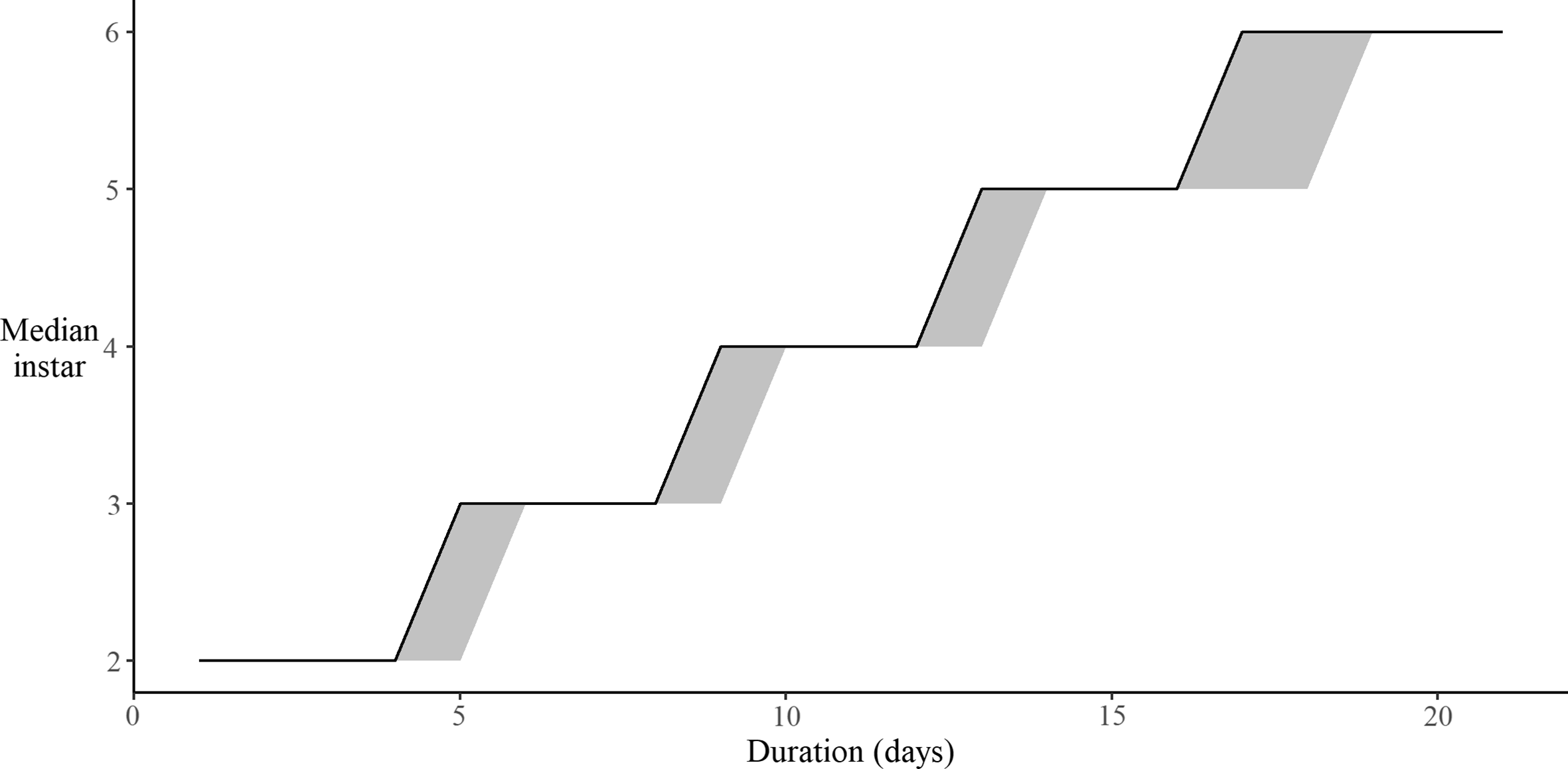
Fig. 5. Median larval stage observed at 20 °C in the cohort-based sampling experiment (solid line), and 2.5 and 97.5 quantiles of the median larval stage observed in 500 bootstrap samples of larval stages observed in the individual-based experiment (shaded area).
Developmental plasticity in spruce budworm larvae reared at constant temperatures
We observed that some larvae developed through only five larval instars. The size distribution of the head capsule of the L4` instar in these larvae is consistent with that predicted by Dyar’s Rule (Fig. 6) and falls almost exactly between that of typical fourth-instar and fifth-instar spruce budworm. We further confirmed that the break points between larval instars determined from the present study’s equation (3) were different between five-instar spruce budworm and those that exhibited the more common six-instar life cycle (Fig. 7). Spruce budworm is described as having six larval instars, and existing development rate models are based on this life history pattern (Hudes and Shoemaker Reference Hudes and Shoemaker1988; Lysyk Reference Lysyk1989; Weber et al. Reference Weber, Volney and Spence1999; Régnière et al. Reference Régnière, St-Amant and Duval2012b; but see Schmidt and Lauer Reference Schmidt and Lauer1977). By measuring the sizes of the shed head capsules present in the diet cups, we determined that 3–42% of larvae in some treatments showed evidence of a five-instar development (Fig. 8).
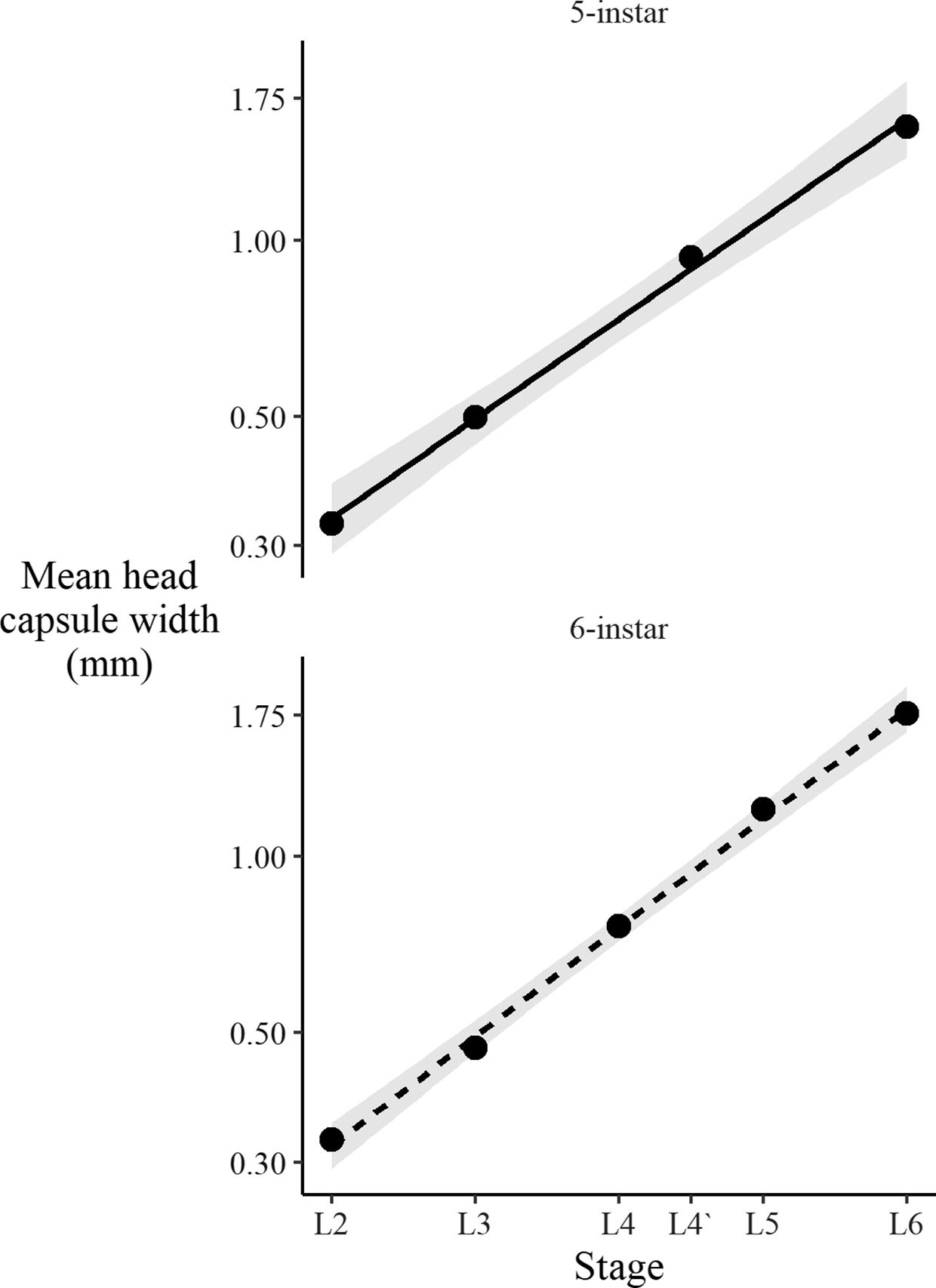
Fig. 6. Mean head capsule widths of successive six-instar (upper panel) and five-instar (lower panel) spruce budworm larvae follow Dyar’s Rule. Note, the y-axis is on a log scale; shaded areas show 95% confidence interval of the linear model fit (lines in each panel); all insects reared at constant 20 °C. L#, larval instar stage; L4`, the penultimate larval instar stage in the “5-instar” group, so designated to distinguish the stage from the fourth-instar stage in the “6-instar” group.
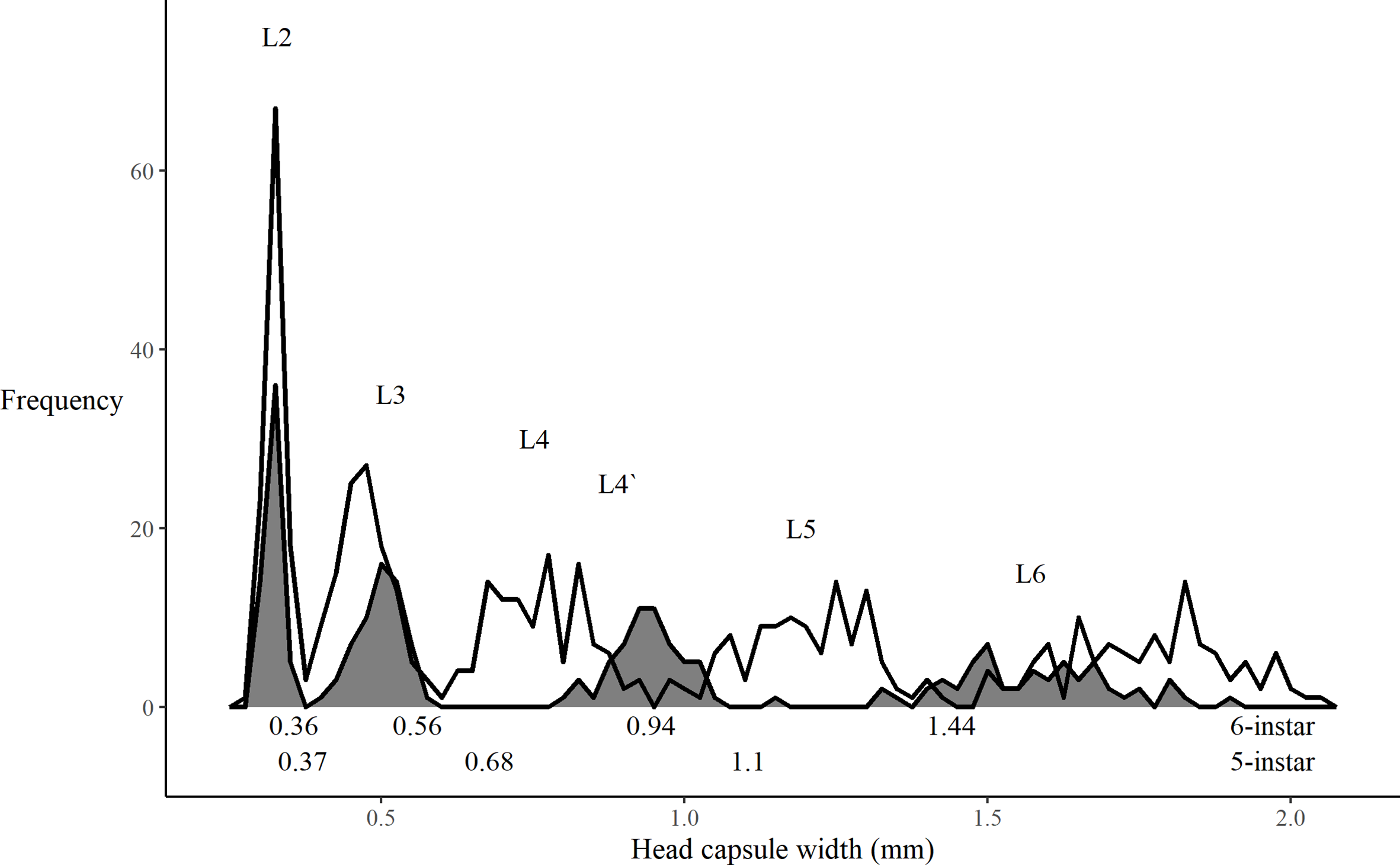
Fig. 7. Frequency distribution of head capsule measurements of six-instar spruce budworm larvae (open) and of larvae showing five-instar development (shaded). Numbers below the plot show the head capsule width “break points” between successive instars as determined from equation (2) (see text) for both groups of larvae. All insects reared at constant 20 °C.

Fig. 8. Observed incidence of five-instar development in cohorts of spruce budworm larvae reared at seven constant temperatures.
Developmental plasticity has been documented in several Choristoneura species (Schmidt and Lauer Reference Schmidt and Lauer1977). The spruce budworm colonies used in this experiment have been reared on artificial diet since the 1960s (Roe et al. Reference Roe, Demidovich and Dedes2017), so we were surprised to find these developmental variants: we had expected this phenotype would have been selected out of the laboratory population (McMorran Reference McMorran1972). This suggests the five-instar phenotype is expressed as a consequence of the artificial rearing conditions or represents natural variation found within wild spruce budworm populations. Similar experiments on wild populations (e.g., Perrault et al. Reference Perrault, Wardlaw, Candau, Irwin, Demidovich, MacQuarrie and Roe2021) may uncover more developmental plasticity than we observed in the present study and may help to characterise the variability expressed in spruce budworm populations.
Rearing ectothermic organisms under controlled conditions at constant temperatures is an essential tool in assessing the response of species to temperature. However, it is increasingly recognised that fluctuating temperature experiments should be used because they better simulate what insects experience and play a key role in insect development, particularly at near-physiological thermal limits (Morash et al. Reference Morash, Neufeld, MacCormack and Currie2018). Measuring the effects of fluctuating temperatures requires the development of more-complex rearing protocols to account for variations in thermal range and time scale and to incorporate temperature transitions near thermal limits. This need to incorporate complexity is reflected in the variety of recent experimental designs (reviewed in Colinet et al. Reference Colinet, Sinclair, Vernon and Renault2015). Many fluctuating temperature designs use constant temperature treatments as controls, suggesting that constant temperature experiments can be a first step in a modern approach to the design of experimental protocols for temperature-dependent development studies.
Acknowledgements
The authors thank the Insect Production and Quarantine Facilities at the Great Lakes Forestry Centre (K. Boissoneau, S. Crispell, S. Dumas, M. Demidovich, T. Ladd, and J. St-Amour), K. Studens, and K. van Frankenhuyzen for help and guidance. The authors especially thank B. Gribelli for keeping the environmental chambers operational. Funding for this project was provided by Natural Resources Canada, The Healthy Forest Partnership, Nova Scotia Department of Lands and Forestry, Québec Ministère des Forêts, de la Faune et des Parcs, Alberta Ministry of Agriculture and Forestry, Saskatchewan Ministry of Environment, Ontario Ministry of Natural Resources and Forestry, and the Spray Efficacy Research Group – International (SERG-I).













