INTRODUCTION
Every year more than 800 million cases of respiratory and gastrointestinal infections occur in the United States [1]. Viruses are a major cause of these illnesses. Rhinovirus is the leading cause of the common cold. Rotavirus is a leading cause of gastroenteritis in children, while noroviruses are usually associated with gastroenteritis in adults. These viruses are known to be readily transmitted by contact with contaminated surfaces and self-inoculation by hand contact with the nose, mouth or eyes [Reference Gwaltney and Hendley2, Reference Ward3]. Hand hygiene involving hand washing or the use of hand sanitizers is believed to play a significant role in reducing infection risks of respiratory and enteric infections [Reference Liu4]. We recently conducted a study on the impact of the spread of viruses from a contaminated hand to fomites and to other family members' hands in the household [Reference Hubner5]. In that study it was found that use of a hand sanitizer in addition to routine hand washing resulted in the reduction of a tracer virus by 97–99·9% on fomites and the hands of household members when the sanitizer was used three times over an 8-h period. The results on the occurrence of virus on family members' hands from that study were used in a quantitative microbial risk assessment model to determine the possible reduction in probability of infection from respiratory and enteric viruses with the use of the hand sanitizer.
METHODS
The objective of this study was to determine the risk of infection from viruses using an alcohol-based hand sanitizer (Purell Advanced Hand Sanitizer, GOJO Industries, USA) three times over an 8-h period. An alcohol gel hand sanitizer known to be effective against rhinoviruses, rotavirus, norovirus and coliphage MS-2 was used in this study [Reference Sattar6–Reference Teunis8]. The viruses selected for modelling were rhinoviruses, rotavirus and norovirus. Because there is uncertainty in using the available dose-response data for norovirus the dose-response for rotavirus developed in adults was used for modelling both viruses. The dose-response data available for norovirus was developed using a non-infectivity assay for the virus and there were questions by the authors about the presence of aggregates in the preparation given to the human volunteers [Reference Teunis8].
Pre-intervention consisted of the family members' routine hand washing with soap and water. Post-intervention involved both routine hand washing with soap and water, and the use of a hand sanitizer at least three times during 8 h. Total concentration of MS-2 coliphage inoculated to the hands of the subjects was 1 × 108 plaque-forming units (p.f.u.) to ensure detection during laboratory analysis.
Dose-response modelling
For rhinovirus and rotavirus dose-response models, datasets from human exposure experiments conducted by Hendley et al. and Ward et al. were used [Reference Ward3, Reference Hendley, Edmondson and Gwaltney9]. For each of the viruses, different statistical distribution models were assessed to determine best fit using maximum likelihood estimation techniques. It was determined that the beta-Poisson model best fits the dose-response data-sets for both rhinovirus and rotavirus. The general form of the beta-Poisson distribution is given by equation (1), in which P(response) is the probability of response or infection, a and b are the optimization coefficients that follow a beta distribution, and D is the concentration or dose of the pathogen under investigation [Reference Haas, Rose and Gerba10].
The Solver routine in Microsoft Excel program was used in this study to optimize the beta-Poisson model by varying a and b coefficients in equation (1) with the objective being maximization of the χ 2 goodness of fit between the observed and the expected probability of risk of infection.
Exposure model
By knowing the count of viruses on the hand post- and pre-intervention, the amount of virus ingested or coming into contact with the eye or nose can be calculated from equation (2).
 $$D = \; \displaystyle{{C_{{\rm hand}}} \over {A_{{\rm hand}}}} \; \times \; \mathop \sum \limits_{i = 1}^m \left( {\,f_{2,i} \; \times \; A_i \; \times \; N_i \; \times \; T_i} \right),$$
$$D = \; \displaystyle{{C_{{\rm hand}}} \over {A_{{\rm hand}}}} \; \times \; \mathop \sum \limits_{i = 1}^m \left( {\,f_{2,i} \; \times \; A_i \; \times \; N_i \; \times \; T_i} \right),$$
where D is the dose or total count of viable (infectious) virus that is ingested or exposed to the mouth, eye or nose by a person in time T; C hand is the concentration of viable (infectious) virus measured on hand per cm2; A hand is the area of two hands (cm2); f 2,i is hand-to-orifice ‘i’ transfer efficiency of virus (fraction; dimensionless); i is orifice i through which viruses can be ingested such as mouth, nose or eye (1, 2, 3, 4, …, m); m is the total number of orifices; A i is the surface area of the hand that touches orifice ‘i’ (cm2); N i is the number of times a person touches his/her orifice ‘i’ (per minute); T i is the time duration of exposure (minutes).
Parameters for exposure model
To determine hand-to-nose, hand-to-eye and hand-to-mouth contacts per minute, data from Nicas & Best were utilized to generate the distributions shown in Figure 1 (a–c, respectively) [Reference Nicas and Best11]. Hand-to-nose contact distribution has an average of 0·0848/min [95% confidence interval (CI) 0·0546–0·1148]; hand-to-eye contact distribution has an average of 0·0411/min (95% CI 0·0247–0·0586); and hand-to-mouth contact distribution has an average of 0·1332/min (95% CI 0·0694–0·2283).

Fig. 1. Generated distributions for face contact frequency using bootstrapping. (a) Hand-to-mouth contacts/min; (b) hand-to-eye contacts/min; (c) hand-to-nose contacts/min.
To determine the area of hand, nose, eye and mouth, data from Snyder et al. were utilized to generate the distributions shown in Figure 2 [Reference Haas, Rose and Gerba10]. Data obtained from U.S. EPA and from Snyder et al. were used to determine the distribution of area of hands (A hand) [Reference Haas, Rose and Gerba10, 12, Reference Snyder13]. Bootstrapping was used to determine the distribution of areas of hands shown in Figure 2a . The value t* represents hand area (cm2) and the y-axis represent the probability density function of the distribution [Reference Snyder13–Reference Zumel and Mount17]. The area of hand distribution is not a normal distribution with an average area of 658 cm2 (95% CI 533·1–881·6). The area of nose has an average of 5·12 cm2 (95% CI 4·608–5·654); area of eye has an average of 1·56 cm2 (95% CI 1·523–1·602); and area of mouth has an average of 6·89 cm2 (95% CI 6·63–7·13).

Fig. 2. Generated distributions for the area (cm2) of the (a) nose; (b) eye; (c) mouth; (d) hand, using bootstrapping techniques.
A point estimate of 0·339 for transfer efficiency of virus from hand to nose, hand to eye and hand to mouth was used in the model based on Rusin et al. since they used MS-2 to develop the data [Reference Rusin, Maxwell and Gerba18]. This is the only data available in the literature. Parameters derived from the bootstrapping are shown in Table 1.
RESULTS
Rhinovirus probability of infection
Rhinovirus is transmitted by contact with the nose and the eyes; hence plugging the parameters presented in Table 1 into equation (2) yields equation (3) shown below, in which C hand is the concentration of rhinovirus found on the subjects' hands.
 $$\eqalign{& D = \; \displaystyle{{C_{{\rm hand}} \; \left( {{\rm number\; of\; rhinoviruses}} \right)} \over {658\cdot94\; {\rm cm}^2}} \cr & \; \times \left( {\matrix{ {0\cdot39\times5\cdot12\; {\rm cm}^2 \times \displaystyle{{0\cdot0848} \over {{\rm minute}}} \times 480\; {\rm min}\, +} \cr {0\cdot39\times1\cdot56\; {\rm cm}^2 \times \displaystyle{{0\cdot0411} \over {{\rm minute}}} \times 480\; {\rm min}} \cr}} \right).} $$
$$\eqalign{& D = \; \displaystyle{{C_{{\rm hand}} \; \left( {{\rm number\; of\; rhinoviruses}} \right)} \over {658\cdot94\; {\rm cm}^2}} \cr & \; \times \left( {\matrix{ {0\cdot39\times5\cdot12\; {\rm cm}^2 \times \displaystyle{{0\cdot0848} \over {{\rm minute}}} \times 480\; {\rm min}\, +} \cr {0\cdot39\times1\cdot56\; {\rm cm}^2 \times \displaystyle{{0\cdot0411} \over {{\rm minute}}} \times 480\; {\rm min}} \cr}} \right).} $$
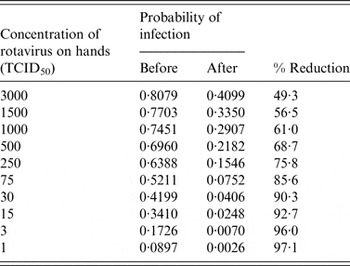
Table 2 shows different scenarios of concentrations of rhinovirus on the hands and the corresponding probability of infection before and after the use of the hand sanitizer three times during an 8-h period. Table 2 also gives the calculated percent risk reduction of infection due to the use of a hand sanitizer. Because of the nature of the dose-response curve for rhinovirus, greater reduction in risk of infection is seen with a lower initial concentration of virus on the hands.
Table 2. Probability of infection as a function of initial rhinovirus concentration on the hand
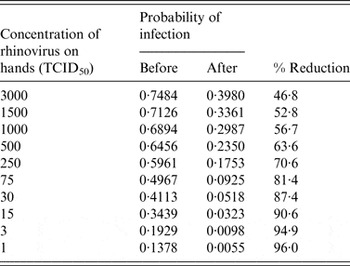
TCID, Tissue culture infective dose.
Figure 3 shows rhinovirus risk of infection pre- and post-intervention through the use of a hand sanitizer three times during 8 h assuming an initial concentration of 1000 infectious rhinoviruses on the hands (from Table 2) for each individual in the studied households. The risk reduction of infection varies with each individual depending upon the initial average reduction of the surrogate on the hands of each individual.

Fig. 3. Rhinovirus risk of infection before and after intervention assuming a concentration of 1000 infectious rhinoviruses on the hands. Each letter represents an individual in the household. Same letter represents a different individual in the same household.
Rotavirus risk of infection
Rotavirus is transmitted by contact with the mouth; hence plugging the parameters presented in Table 1 into equation (2) yields equation (4), in which C hand is the concentration of rotavirus found on the subjects' hands.
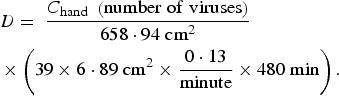 $$\eqalign{& D = \; \displaystyle{{C_{{\rm hand}} \; \left( {{\rm number\; of}\; {\rm viruses}} \right)} \over {658\cdot94\; {\rm cm}^2}} \; \cr & \times \left( {39\times6\cdot89\; {\rm cm}^2 \times \displaystyle{{0\cdot13} \over {{\rm minute}}} \times 480\; {\rm min}} \right).} $$
$$\eqalign{& D = \; \displaystyle{{C_{{\rm hand}} \; \left( {{\rm number\; of}\; {\rm viruses}} \right)} \over {658\cdot94\; {\rm cm}^2}} \; \cr & \times \left( {39\times6\cdot89\; {\rm cm}^2 \times \displaystyle{{0\cdot13} \over {{\rm minute}}} \times 480\; {\rm min}} \right).} $$
Table 3 shows the reduction in percent of infection for rotavirus with different initial concentrations of rotavirus on the hands. Again, because of the nature of the dose-response curve for rotavirus, greater reduction in risk of infection is seen with a lower initial concentration of virus on the hands.
Table 3. Reduction in probability of infection as a function of initial rotavirus concentration on the hand

TCID, Tissue culture infective dose.
DISCUSSION
MS-2 coliphage was used as a surrogate for rhinovirus, rotavirus and norovirus to quantify the exposure to virus both with and without use of a hand sanitizer three times a day. MS-2 virus is similar in shape and size to these viruses and has been used extensively as a surrogate to study the behaviour of human pathogenic viruses in the environment and assessment of disinfectants [Reference Ward19]. Its distribution on fomites in the household after addition to the hands [Reference Tamimi20] was very similar to that observed by Winther et al. who contaminated households with rhinovirus [Reference Winther21].
Total concentration of MS-2 coliphage inoculated on the hands of the subjects (one member only in each family) was 1 × 108 p.f.u. to ensure detection during laboratory analysis so that reductions of the virus on the hands could be quantified after the intervention. Rotavirus concentrations of 1012 rotavirus particles can be found in the stool of infected persons [Reference Flewett, Tyrrell and Kapikan22]. Data on the occurrence of rotavirus on the hands of infected persons is not available, although it is readily detected on individuals' hands in households in developing countries [Reference Flewett, Tyrrell and Kapikan22]. Norovirus has been detected on the hands of infected individuals ranging from 103·3 to 104·45 genome copies by qPCR in hand rinse samples [Reference Ward3]. The concentration of rhinovirus in nasal mucus has been found to vary with the stage of infection and ranges from 10 to 1 000 000 tissue culture infectious viruses/ml [Reference Sinclair23].
An exposure model was developed based on the work of Nicas & Best to estimate the amount of virus that would reach the nose, eyes and mouth during the 8-h study period [Reference Nicas and Best11]. Using dose-response models for rhinovirus, rotavirus and norovirus, the risk of infection could then be quantified based on the amount of virus on the hands. Because data is not available on the actual concentration of virus that might be on the hands of infected and exposed individuals a range of values of risk of infection were determined for each of the viruses (Tables 2 and 3). Using the data on the reduction in concentrations of MS-2 on the hands post-intervention, the reduction in risk of infection can be determined based on different concentrations of the actual viral pathogens on the hands. It was found that because of the nature of the dose-response models, the less the amount of virus on the hands the greater the reduction in probability of infection. Thus, the risk of infection was reduced by 96% with one virus on the hands vs. 46·8% when 3000 viruses were present on the hands for rhinovirus. The same was true for rotavirus and norovirus. Risk reductions are only estimated for individuals who do not have protective immunity.
The potential for reduction in risk varied both between households and by household members (Fig. 3) based on the amount of MS-2 on their hands pre- and post-intervention. Variation in risk pre-intervention may vary depending on numerous factors including how well and how often family members practice routine hand washing and their activities within the home (i.e. how often they used the toilet, food preparation, playing, cleaning, etc.). Reduction in MS-2 on the hands of individuals post-intervention may be indicative of when they use the hand sanitizer during the day (that was left to their option).
Uncertainty arises from varying concentrations of respiratory and enteric viruses on the hands of infected individuals. Moreover, dose-response data was developed in normal healthy adult individuals and may not be reflective of children, the immunocompromised and the elderly. Differences may also exist between the surrogate and the human pathogenic viruses in their survival on the hands/fomites and transfer efficiency to and from fomites, which could affect the degree of exposure [Reference Ward19, Reference Winther21, Reference Ansari24].
Several epidemiological studies have shown the potential for reduction in transmission of respiratory and enteric infections with the use of hand sanitizers [Reference Lopez25–Reference Prazuck27]. In a review of 16 studies on the impact of reduction of respiratory infections from the use of hand sanitizers Warren-Gash et al. concluded that success was dependent on setting, context and compliance [Reference Warren-Gash, Fragaszy and Hayward28]. The present study also provides evidence for the potential of alcohol-based hand sanitizers to reduce the risk of illness from respiratory and enteric infections in household settings.
The results of the present study suggest that adding the use of an alcohol-based hand sanitizer in the home can result in increased reduction in risk of infection by respiratory and enteric viruses, such as rotavirus, rhinovirus and norovirus, even when routine hand washing is already practised. Future studies should evaluate whether other hand hygiene interventions, such as use of hand sanitizing wipes, would have a similar effect.
DECLARATION OF INTEREST
None.