Introduction
Buruli ulcer (BU) is a disfiguring and disabling skin infection that belongs to the group of neglected tropical diseases (NTDs). The disease mostly affects marginalized rural populations in developing countries owing to their remoteness and difficulty of access to good health care, in addition to other socioeconomic and socio-environmental factors.
In Côte d’Ivoire, where the disease’s endemic foci are scattered and confined to specific areas, the proportion of the population affected by BU can reach 16% in some communities (WHO, 2000). Approximately 2000 cases are being reported annually since the national prevalence survey was initiated in 1997 (Kanga & Kacou, Reference Kanga and Kacou2001; Kanga et al., Reference Kanga, Kacou, Kouamé, Kassi, Kaloga and Yao2006), positioning Côte d’Ivoire at the very top of endemic African countries in terms of annual number of cases. In 1998, the World Health Organization hosted an international conference in Yamoussoukro, Côte d’Ivoire, where the representatives of more than 20 countries signed a declaration of their engagement to fight BU. One of the major strategies was the setting up of national control programmes. In Côte d’Ivoire, the national BU programme has succeeded in reducing the number of cases in some health districts by educating and sensitizing the population, yet rural communities remain badly affected by the disease.
Buruli ulcer disease is caused by Mycobacterium ulcerans, which belongs to the group of environmental non-tuberculosis mycobacteria found in soil, dust and slow-moving water bodies. Sufferers are often subjected to severe stigmatization and discrimination, as underlined by previous research (Stienstra et al., Reference Stienstra, Van Der Graaft, Asamoa and Van Der Werft2002). Superstitious beliefs are often the basis of this stigmatization, and this can lead to avoidance, neglect or suboptimal management of the disease in affected individuals (Adamba & Owusu, Reference Adamba and Owusu2011). Although antibiotic treatment and surgery are effective means of treating early-stage BU, sufferers often report at stages where substantial damage has already been done. The reasons for this are not fully understood but seem to include a strong societal factor (Kibadi et al., Reference Kibadi, Boelaert, Kayinua, Minuku, Muyembe-Tamfum, Portaels and Lefèvre2009).
The location of M. ulcerans in the natural environment and its mode of transmission have not yet been fully characterized. However, fish, molluscs, beetles and water bugs have been identified as potential hosts (Portaels et al., Reference Portaels, Chemlal, Elsen, Johnson, Hayman and Hibble2001), while water bugs and carnivorous and aggressive insects have recently been proposed as vectors of the bacillus (Portaels et al., Reference Portaels, Elsen, Guimaraes-Peres, Fonteyne and Meyers1999, Reference Portaels, Chemlal, Elsen, Johnson, Hayman and Hibble2001). The main alternative hypotheses to vector-based transmission is direct transmission via skin lesions, implying that M. ulcerans, which is present in water bodies, directly infects open skin lesion, for example via bites, cuts and open wounds (Merritt et al., Reference Merritt, Walker, Small, Wallace, Johnson, Benbow and Boakye2010). Some authors have suggested that this mode of transmission depends on the proximity of humans to infected environmental sources or animals (Van Ingen et al., Reference Van Ingen, Boeree, Kosters, Wieland, Tortoli, Dekhuijzen and Van Soolingen2009). It is thought that people living near wetlands generally become contaminated through water-based activities such as rice farming, fishing and swimming (Barker, Reference Barker1973; Oluwasanmi et al., Reference Oluwasanmi, Solankee, Olurin, Itayemi, Alabi and Lucas1976; Marston et al., Reference Marston, Diallo, Horsburgh, Diomande, Saki and Kanga1995; Aiga et al., Reference Aiga, Amano, Cairncross, Adomako, Nanas and Coleman2004).
While biomedical research on BU transmission takes environmental factors into consideration, local knowledge relies to a large extent on local socio-cultural belief systems. According to the conception of societies, the basis of disease appearance goes beyond ecological and biological aspects, and concerns all aspects of social life (Stoetzel, Reference Stoetzel1960). Understanding the social environment and the resulting perceptions of people in areas of prevalence may offer additional indications on where and how BU (or any other infection) has taken place. Each society has different disease perceptions, marked by its socio-cultural and socio-religious background, rules of community life and inter-individual relationships and its natural environment. A given disease may not only be perceived as its observed symptoms or as a physical disorder; it may also be regarded as a misfortune that shapes the lives of individuals. Upon diagnosis, the physical disorder becomes a social determinant influencing an individual’s identity and their situation in society (Bovina, Reference Bovina2006). Epilepsy in the traditional Kiremba community of Burundi, for instance, is sometimes perceived as a disorder in the relationship between the living and the dead, representing the possession by a spirit (Nsengiyumva et al., Reference Nsengiyumva, Nubukpo, Bayisingize, Nzisabira, Preux and Druet-Cabanac2006). In Abidji communities in Côte d’Ivoire, diseases such as infantile diarrhoea are often related to conflict in interpersonal relationships (Kouakou Bah, Reference Kouakou Bah2012).
Social and ecological factors are still widely neglected in research on diseases prevention, transmission and intervention, allowing certain diseases to persist in populations (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). This is particularly true for BU, where previous research approaches have demonstrated an influence of local beliefs on delay in, and access to, appropriate treatment in developing countries (WHO, 2018). An important contributing factor is the preference for biomedical or traditional treatments for BU, which is to a large extent determined by the socio-cultural beliefs of patients and their families (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). The cost, accessibility and failure of treatment are all factors that shape treatment preference (Ackumey et al., Reference Ackumey, Gyapong, Pappoe, Kwakye Maclean and Weiss2012).
Additionally, the social impact of BU and the way the disease is perceived by affected communities are likely to influence the risk of transmission. In some settings, BU is perceived as a mystical infliction caused by sorcery or as a response to infraction, and this could influence the behaviour of people who feel at risk of infection (Stienstra et al., Reference Stienstra, Van Der Graaft, Asamoa and Van Der Werft2002; Renzaho et al., Reference Renzaho, Woods, Ackumey, Harvey and Kotin2007; Mulder et al., Reference Mulder, Boerma, Barogui, Zinsou, Johnson and Gbovi2008). Religious beliefs attribute a demonic origin to BU in some settings (Kibadi et al., Reference Kibadi, Boelaert, Kayinua, Minuku, Muyembe-Tamfum, Portaels and Lefèvre2009) while in others the perceived origin of BU can be simultaneously natural and mystical (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). A lack of information on mode of transmission, disease timeline and treatment outcomes may explain these interpretations linked to witchcraft (Stienstra et al., Reference Stienstra, Van Der Graaft, Asamoa and Van Der Werft2002).
The extent of interactions between humans and the environment is often linked to social and cultural practices of communities and can predispose the former to environmental pathogens. Thus, diseases can be interpreted beyond the physical disorder based on social theories. Analysing different perceptions and social behaviours may help fill the knowledge gaps in understanding different forms of disease expression and transmission. The consideration of these perceptions appears to be largely absent in current health education practices, and it is important to re-evaluate the role of the human social dimension in understanding the mode of transmission of BU and to convey this knowledge to affected populations. Just as local ideas can influence therapeutic choices (Aujoulat et al., Reference Aujoulat, Jonhson, Zinsou, Guedenon and Portaels2003; Mulder et al., Reference Mulder, Boerma, Barogui, Zinsou, Johnson and Gbovi2008), the perception of the mode of transmission might contribute to the population exposition to BU. The perceptions and responses of at-risk communities are of particular importance in BU because rapid diagnosis and immediate subsequent treatment are essential to minimize the social, physical and economic consequences of the disease (Renzaho et al., Reference Renzaho, Woods, Ackumey, Harvey and Kotin2007). The objective of this study was therefore to explore the local aetiology attached to BU disease in endemic areas of Côte d’Ivoire and to analyse perceptions of the disease’s mode of transmission.
Methods
Study site
The study was conducted in Taabo and Daloa from 10th February to 14th June 2012, two areas in southern Côte d’Ivoire that are known to be endemic for BU. Additional selection criteria were the type of ecosystem (i.e. rainforest, with or without streams and savannah) and the presence of water bodies. The sub-district of Taabo, in central–south Côte d’Ivoire, is located 160 km north of Abidjan and has experienced several environmental changes, including the construction of a hydroelectric dam that has resulted in massive displacement of the local population. The region of Daloa in Haut-Sassandra district, central–west Côte d’Ivoire, is 406 km north-west of Abidjan. A total of five villages were selected across the two main study sites, namely Ahondo, Léléblé and Sokrogbo in Taabo (savannah woodland with streams) and Gorodi and Zaïbo in Daloa (rainforest with streams). These villages are inhabited by people of diverse origin, with some predominant Ivorian ethnic groups such as the Swamlin and Baoulé in Taabo, and Niaboua and Senoufo in Daloa. Both areas are inhabited by many populations from neighbouring countries such as Burkina Faso and Mali. The criterion for sample selection was the number of households per village, which was decisive in defining the sample size. Among the study villages, one was selected in each region for a more in-depth investigation, namely Ahondo in Taabo and Gorodi in Daloa. Both villages were selected based on the number of cases identified by the National Program for BU control (PNLUB) and on the environmental factors that promote endemicity of BU. Ahondo is close to the Bandama River, which is regularly frequented by the local population for bathing and fishing or crossed to get to the farms. Gorodi is surrounded by the Sassandra River tributaries, which is used for agricultural irrigation and fishing activities.
The study was conducted in two phases: quantitative and qualitative.
Quantitative phase
Quantitative data were collected over a period of a month from 10th February to 10th March and consisted with the administration of 500 household questionnaires to selected individuals in the five villages. The questionnaire was standardized and consisted mostly of closed questions. It elicited information on the demographic characteristics of the participants, household activities, common diseases, knowledge of BU and perception of mode of transmission and attitudes and practices regarding the disease. The questions were designed based on information recorded during an exploratory site visit. The quota sampling method was used for data collection in order to achieve a representative sample of the total population (Agrinier et al., Reference Agrinier, Baumann, Guillemin and Hédelin2010). The allocation of questionnaires to each village was based on their stratification into subsets, with 250 questionnaires for villages with ≥30 BU cases, 200 questionnaires for villages with ≥15–29 BU cases and 50 questionnaires for villages with <15 BU cases. In addition, the number of households was taken into account to adjust the final sample size for each village to 145 for Sokrogbo, 105 for Zaïbo, 93 for Léléblé, 107 for Ahondo and 50 for Gorodi. Collected data were recorded in Epi-data with a double-entry system and subsequently transferred to SPSS18 for analysis.
Qualitative phase
Qualitative data were collected over the period 12th April to 14th June 2012 in the two villages Ahondo and Gorodi using semi-structured interviews and focus group discussions (FGDs). The interviewed participants were community leaders, current and former BU patients, traditional healers, parents of patients and health care workers, and involved women, men, youth and children. A total of 30 interviews were conducted with patients or their parents, former BU patients and traditional healers.
Buruli ulcer patients were selected on the basis of hospital records and consultations in various health centres visited. However, some patients not mentioned in health centre records were directly contacted at home. Interviews were held in patients’ homes, and included questions on their socioeconomic activities, socio-demographic characteristics, relationships with family members and disease experience. Additionally, direct observations were made to reach a better understanding of the physical reality of these communities. Social life was described on the basis of water collection habits, farming work and household activities such as cooking, washing and cleaning. Particular traditional healing practices were observed and the treatment of patients, prescriptions and compliance of patients with treatment/advice were included.
Four FGDs were conducted in each village respecting the criteria of age and sex. Community members of Ahondo and Gorodi were invited to participate in order to assess the perceptions of BU of people living in endemic areas. The FGDs were conducted with women, men and youth. Each discussion session had approximately eight participants. The age of informants ranged from 18 to 45 years. The selection of age range was based on the fact that, in rural African communities, people tend to marry at an early age, sometimes before reaching the legal age of adulthood. Those who were not married were considered children in the study. Children in late childhood (aged between 8 and 10 years) and adolescence (between 13 and 15 years) were included in the FGDs because they represent an important source of information due to the high prevalence of BU in these age groups. The FGDs were carried out in private settings chosen by the participants. Participants were requested to provide information on their activities and knowledge on BU transmission and aetiology. Interviews and FGDs were recorded using a digital voice recorder, transcribed into Microsoft Word and subsequently analysed with the software MAXQDA10. This was made possible with the use of codes and sub-codes created on the basis of information received during the discussions in addition to key indicators derived from the objectives of the study. Various themes used by the informants were extracted from the transcripts to constitute classes of codes that were subsequently grouped according to the major categories for systematic data analysis (Kuckartz, Reference Kuckartz2010). The tools used in the collection of data were an observation check list, a questionnaire, an interview guide and a digital camera for photos.
Results
Among the populations surveyed, Ivorians were the dominant group (79.0%), followed by Burkinabe (15.6%), Malians (4.6%) and Guineans (0.2%). The main livelihoods of the populations were agriculture and livestock keeping (66.6%), and a few were hairdressers/traders (8.4%) or civil servants (2.4%). The most common classification of women was ‘housewife’ (16.2%). Sokrogbo had the highest proportion of people with completed high school education (24.9%), followed by Zaïbo (21.0%). However, approximately half of the population assessed across all villages had no formal education (Table 1).
Table 1 Percentage distribution of study participants by level of education and area (N=500)

Empirical knowledge of BU disease
Nosographic context of BU disease
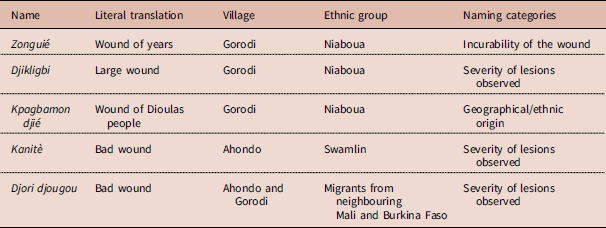
Several terms were used in the group discussions to characterize BU among the Niaboua and Swamlin communities in the villages of Ahondo and Gorodi (Table 2). Participants perceived the disease to be difficult to cure because the ulcerations are larger than normal wounds. Correspondingly, BU is called djikligbi in the Niaboua language, meaning ‘large wound’, or zonguié, literally meaning ‘wound of years’.
Table 2 Common names used for Buruli ulcer by communities of Ahondo and Gorodi
Symptoms of BU disease
The most frequently mentioned symptoms in both areas were pimples (38.9% in Taabo and 28.7% in Daloa) and wounds (29.8% in Taabo and 30.8% in Daloa). Swelling and fever were mentioned with higher frequency in Daloa (16.0% and 12.1%) compared with Taabo (8.8% and 3.6%). Additional symptoms included plaques (7.1 %Taabo and 5.7% in Daloa), itchiness (8.8% in Taabo and 4.2%) and headaches (2.3% in Taabo and 0.3% in Daloa).
Gravity of BU disease
Participants who stated that they knew of BU were asked about their perception of the gravity of the disease (Table 3). In both localities perception of gravity was dominated by a fear of pain (47.8% in Daloa and 37.6% in Taabo), cost of treatment (24.6% in Taabo and 8.7% in Daloa) and shame (19.9% in Daloa and 18.1% in Taabo). A significant difference in the perception of cost of treatment of BU was observed between the two communities, this being higher in Taabo compared with Daloa (p<0.005).
Table 3 Reasons given by participants in Taabo and Daloa for fearing Buruli ulcer

The perception of the gravity of BU disease was also assessed during the FGDs using open-ended questions. Both affected and unaffected participants perceived BU to be a dangerous disease that can lead to death in some cases, and in most cases invalidity/disability:
I am afraid of this disease because it can kill persons.
I don’t even wish my enemy to get BU because it destroys individuals and family members too.
Many persons lost a foot or hand because of BU.
My husband got married to another woman because of this disease, I can’t do anything in the household, it was urgent for him to look for a wife to help for the field work.
(Women and men from Gorodi and Ahondo)
Aetiology of BU disease
Respondents considered BU to have several causes, summarized in Table 4. Dirty water and witchcraft were the most frequently mentioned aetiologies in the two major study areas of Daloa and Taabo. However, significant differences were observed in the perceived causes of BU in the two locations. Among environmental causes of BU, respondents cited dirty water (42.4% in Taabo; 20.4% in Daloa; p<0.005) and insects/animals bites/stings (11.9% in Taabo, 4.8% in Daloa; p<0.009). More than a quarter of the respondents in each location claimed that witchcraft and curses were responsible for BU disease, with witchcraft being the leading cause in Daloa (37.7%) and the second leading cause in Taabo (25.7%) (p=0.004). Despite the different aetiologies of BU mentioned, a notable proportion of the respondents in the two areas did not know the causes of the disease (26.9% in Daloa; 14.3% in Taabo; p<0.005).
Table 4 Participants’ perceived causes of Buruli ulcer

A popular perception was that BU disease was linked to other communities. The native population blamed migrant communities from neighbouring countries as bearers of the causative agent and the disease. They perceived that the appearance of the disease coincided with the arrival of migrants, who have different habits and belief systems. They claimed that the children of migrants always have wounds on their feet which take a long time to be treated. In their view, that could explain the appearance BU cases. One informant stated:
Before it was not here, but now, since the Mossi [migrants from Burkina Faso] people migrated here they brought the disease along. When they arrived the Mossi people had wounds everywhere; it is the Mossi who came with wounds here. (Young man, Gorodi)
Modes of transmission of BU infection
The majority of informants, at all levels of education, asserted that the most likely route of BU contamination was swimming in the same water body as a BU patient or contact with an ulcerated wound (average ~35.4%). Contact with soil contaminated with mycobacteria was mostly mentioned by respondents with a high school level of education (34.9%). However, a significant proportion of the population surveyed (38.1%) did not know the mode of transmission of BU. No significant relationship was found between knowledge of mode of BU transmission and level of education of respondents (Table 5).
Table 5 Participants’ perception of mode of transmission of Buruli ulcer by level of education

a Primary and no education;
b secondary education and above.
Perceptions of means of BU transmission
Infection via insect bites or tsetse flies
Respondents in Ahondo and Gorodi perceived insect and tsetse fly bites to play an important role in the transmission of BU disease. Participants reported that after an insect bite, minor wounds occur and develop into infection. They asserted that people have developed local knowledge on the origin of these insects in their areas. Also, mould appearing on stagnant waste water in the villages and fields was perceived to be linked to infection with BU. Women reported that mouldy places can serve as niches for biting ‘beasts’ that transmit BU to exposed persons in those areas.
Contamination of the environment by BU-infected individuals
Youths and adult females also stated that people who are infected with M. ulcerans can contaminate a healthy environment, believing that BU wounds breed ‘microbes’ that contaminate the wetlands. When people cross rivers or backwaters they expose their feet in this potential risk zone where the ‘microbes’ have been ‘deposited’. This is illustrated by the following statement:
We believe that BU could have the same mode of transmission as the Guinea worm. When an infected person puts his/her feet into the rivers, the deposited microbes can contaminate another person. (Adult man, Ahondo)
Participants recognized that humans are actors in the chain of BU transmission and can contaminate a previously healthy environment. This perception often results in isolation of patients or the disuse of certain water sources by the population, as illustrated by the following statement:
It has become a stream for the Burkinabe; we are not going over there anymore. (Adult woman, Gorodi)
Consumption of contaminated water and fruit
Water was perceived to be an important source of contamination with BU. People from Gorodi described the process as follows. Ingestion of the eggs of microbes contained in swamps and rivers; these then establish in a person’s body; and the infected individual starts showing disease symptoms when the microbes grow to maturity. This is illustrated by the following statement:
When the water from the creek remains two days in a reservoir, beasts appear inside the water. If you drink the water containing those beasts and they enter your blood, it swells and becomes BU. (Adult man, Ahondo)
The consumption of over-ripe fruit such as papaya and mango is also seen as a source of infection due to the presence of maggots. During a group interview with youth in Gorodi, one respondent stated:
There are beasts that enter the core of the harvested mangoes by first passing through the pulp and finally depositing their eggs within the core. (Young man, Gorodi).
Discussion
Several names were used by the study populations to designate BU disease, but in general the disease has a distinct identity within those communities. According to Olivier De Sardan, every disease is named according to particular specifications based on core associations and representations (Olivier De Sardan, Reference Olivier De Sardan1998). In Côte d’Ivoire, BU was originally called the ‘mysterious wound of Daloa’ because the first cases were reported in the Daloa region and also because the causes and manifestations of the disease were difficult to explain from a local viewpoint (Marston et al., Reference Marston, Diallo, Horsburgh, Diomande, Saki and Kanga1995). In the contemporary context the names ‘bad wound’, ‘large wound’ and ‘wound of years’ refer to a wound that is difficult to heal or that is incurable and relates to the social burden that it will cause. However, the expression kpabamon djie, which means ‘wound of Dioula people’, referring to the migrants from the north of Côte d’Ivoire and Burkina Faso, indicates the nature of the relationship between in- and out-groups, indigenous and non-indigenous people and land owners and migrants. ‘Otherness’ is here associated with the disease and other burdens of society. Migrants who are competing with local people for natural resources are being represented as those bringing the disease into the area. This type of discourse is further fuelled in the Ivorian context marked by 10 years of socio-political crisis, often based on community resentment towards foreigners, especially in rural areas where land is often a cause of conflict. The various inter-community conflicts that occurred during the crisis have changed the attitudes of indigenous people towards foreigners and explain the latent conflicts that exist within those communities. The phenomenon linking disease occurrence to the emergence of other (foreign) communities has been described previously, for example in an anthropological study in the Songhay-Zarma communities of Niger and Mali, where a set of diseases including measles, meningitis and cholera were thought to be introduced from the outside, with migrants being the main suspected source of infection (Jaffre, Reference Jaffre1993).
The Ivorian forest zone has hosted several thousand immigrant people from the surrounding Sahel countries (i.e. Mali, Niger and especially Burkina Faso) in search of better living conditions in cocoa plantations over the last few decades. In addition, opportunities for work offered by the construction of several hydropower dams in Taabo and Kossou have led to an influx of migrants into these areas. The emergence of BU in both locations could therefore be interpreted as the consequence of social and ecological disorder caused by the immigration of people with different cultures and belief systems and by environmental transformations. The notions of otherness and foreignness have negative connotations in this cultural context and such perceptions can leave footprints across generations.
The study showed that community members are familiar with BU manifestations. The pimple was found to be the most mentioned BU symptom; however, when actually confronted with this symptom at early-stage BU, people do not usually know what disease they are dealing with. Their life in rural areas exposes them to all kinds of cutaneous trauma. This leads to a carelessness of body care due to the type of daily work they do. This contradiction is in line with the results of a survey conducted in a rural area of the Democratic Republic of Congo (Kibadi et al., Reference Kibadi, Boelaert, Kayinua, Minuku, Muyembe-Tamfum, Portaels and Lefèvre2009), which demonstrated that BU patients perceived the first symptoms of the disease to be mild. Such a perception may be explained by the frequent exposure of rural populations to pimples, unrelated to BU disease, as demonstrated in this study. Only at a later stage, which includes pain or lesion development (commonly after more than 3 weeks), does BU become a more evident explanation. This difficulty in interpreting the early signs of the disease causes important delays in efficient disease management (Johnson et al., Reference Johnson, Makoutode, Hougnihin, Guedenon, Ifebe, Boko and Portaels2004).
A quarter of all respondents from Taabo reported a large financial and social burden linked with BU disease. The fact that there is no health care facility specialized in treating BU near their communities leads to additional costs related to transport, food and loss of productivity if a visit necessitates an extended hospital stay. This leads to the perception that BU is linked to expensive care. The estimation of cost of treatment was based on any expenses that the patients reported to be related to BU disease. Most respondents come from hamlets or villages far away from the next hospital. Populations from Daloa are equally aware of the financial burden; however, they benefit from the ambulatory care offered by a community carer who visits the BU patients in their homes. Consequently, transport-related costs were less mentioned by the surveyed Daloa population compared with those from the Taabo area, and only by those who were hospitalized in specialized treatment centres.
Pain was also a factor associated with the disease. Initially, BU is painless but it can become very painful as the disease progresses. Another factor was a feeling of shame, mainly marked by a strong emotional charge: the embarrassment of having wounds that take a long time to heal and also the negative attention of others. In addition, the offensive smell of the wound usually disturbs family members, especially spouses, with whom one is intimate. One patient said: ‘I prefer not to have sex with my husband because of this disease.’
Even if patients are not discriminated against within communities, large scars caused by the disease often impose a sense of shame or embarrassment on the affected individual, as previously shown by a study conducted in Ghana (Stienstra et al., Reference Stienstra, Van Der Graaft, Asamoa and Van Der Werft2002).
The aetiological perceptions of BU by the study population were based on social interactions, contact with the physical environment and the presence of the disease. In Taabo, people incorporated a more accurate medical explanation (e.g. dirty water and insects) in their interpretative model of the origin of the disease than did those in Daloa. This may be explained by the fact that the Taabo area has benefited from the curative and preventive actions of NGOs (e.g. MAP International) in some villages.
A proportion of the study population expressed the view that individuals could be responsible for causing BU. Particularly in the Daloa area (Zaïbo), witchcraft is the main cause of BU, according to the population surveyed. Witchcraft is a common explanation for BU cases in other African countries affected by this disease. In Ghana, various supernatural factors have been mentioned by informants, with witchcraft for bad luck being the most common (59%). In addition, the perception was prevalent that the disease could be ‘sent’ as curse from a relative in case of conflict (Stienstra et al., Reference Stienstra, Van Der Graaft, Asamoa and Van Der Werft2002). In the current study, a similar notion of the involvement of witchcraft was observed. Some respondents thought that a conflict with a family member could be the result of the occurrence of BU. In this case, the disease is believed to be ‘sent’ in order to destroy this person. The accusation takes place during the search of disease causes, and unexplained or unknown phenomena are ascribed to gods, spirits or other supernatural beings.
Buruli ulcer has often been seen as having both natural and mystical causes simultaneously. Such a double causality in the aetiological understanding of BU has been demonstrated by a study in Cameroon where the natural character of the perceived illness can be replaced by a supernatural perception, in which the elements of the natural environment can serve as support for a mystical attack (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). Thus, it appears from various studies on BU that two causal explanations can be made for the same disease (Aujoulat et al., Reference Aujoulat, Jonhson, Zinsou, Guedenon and Portaels2003). The duration of the disease and the outcome of treatment may also influence causal perception, which justifies the plurality of therapeutic choices to which the patients resort, with self-medication usually being the first course of action (Kibadi et al., Reference Kibadi, Boelaert, Kayinua, Minuku, Muyembe-Tamfum, Portaels and Lefèvre2009). Traditional care is still important in the treatment choices (Adjet et al., Reference Adjet, Adou and Konan2016) and some patients use biomedical and traditional treatments simultaneously (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). Others resort to prayer sessions to achieve healing (Kibadi et al., Reference Kibadi, Boelaert, Kayinua, Minuku, Muyembe-Tamfum, Portaels and Lefèvre2009). In the case of BU it is difficult to derive a consensus in the causal explanation of the disease and the therapeutic choices insofar as the entire process of aetiological construction is strongly marked by personal or family experience.
The actual mode of BU transmission is still unclear to date which, to a certain degree, affects the level of knowledge of the disease by local population (Kanga et al., Reference Kanga, Kacou, Kouame, Kassi, Kaloga and Yao2004). The hypothesis on BU transmission is that it is acquired through environmental contact. Indeed, in the current study those with a higher level of education tended to perceive that there was an interpersonal mode of BU transmission through contact with an ulcerated wound. This perception is aligned with the biomedical hypothesis of direct patient-based transmission via wounds or indirectly by an environment that has previously been contaminated by patients (Röltgen & Pluschke, Reference Röltgen and Pluschke2015).
Although several behavioural factors may increase the risk of transmission of M. ulcerans in humans, human-to-human transmission has yet to be proved (Aiga et al., Reference Aiga, Amano, Cairncross, Adomako, Nanas and Coleman2004). Conversely, some epidemiological studies have shown that swimming in a river or stream would be one of the possible modes of transmission (Barker, Reference Barker1973). Even if household surveys demonstrated that affected populations are largely ignorant about the mode of infection, at the same time the current outcomes show that the lack of available information allowed them to establish patterns of infection based on their experiences. Herzlich (Reference Herzlich1973) showed in her work among French communities various opposing concepts around understanding a given disease that are inner versus outer, healthy versus unhealthy, natural versus not natural and individual versus social. Generally, the disease and the factors causing the disease have an exterior origin opposed to the health that concerns the individual (Herzlich, Reference Herzlich1973). The disease is sometimes attributed to evil spirits, divine intervention, supernatural forces or ancestors.
Perceptions related to the process of elaboration of mode of transmission were found to be dynamic in both communities assessed in this study. Participants believed that the creation of the Kossou dam in Daloa department in the 1980s and the Taabo dam in Taabo department at the end of the 1970s has resulted in stagnant pools of water downstream and near their communities, which have served as ideal breeding areas for insects. Thus, daily water-related activities such as fishing, swimming and agriculture are linked with a risk of infection by BU due to exposure to those insects. In particular, the village of Gorodi, which is located in the Daloa department, has been slowly surrounded by pools of water where the current major economic activity is fishing. A study by Kanga et al. (Reference Kanga, Kacou, Kouame, Kassi, Kaloga and Yao2004) came to the conclusion that these environmental conditions, whether natural (e.g. floods, swamps, natural lakes) or man-made for energy and agro-pastoral activities (e.g. dams, artificial lakes, irrigated areas, low marsh), have played a major role in the outbreak of BU disease, creating ideal conditions for the proliferation of germs. Laterali (Reference Laterali2005) reported from an investigation in the district of Ayos in Cameroon that people believe that insect bites, water or food contaminated with bacteria cause BU. However, even if insect bites were to be involved in the transmission of BU, some populations still think that these are ‘sent’ to them by sorcery (Grietens et al., Reference Grietens, Toomer, Um Boock, Hausmann-Muela, Peeters and Kanobana2012). Vector-based transmission has indeed gained popularity in recent years with different predatory water bugs, especially belostomatids and naucorids, being the main suspects since the discovery of M. ulcerans in the salivary glands of these insects, which have been shown to transmit the infection to mice (Marsollier et al., Reference Marsollier, Aubry, Saint-Andre, Robert, Legras and Manceau2003).
The study participants believed that contamination of a healthy environment by an infected person might happen with other common diseases such as dracunculiasis (guinea-worm disease) – a parasitic worm infection with a similar manifestation to BU. People affected by dracunculiasis could infect water points by immersing their feet and leaving the parasite behind. Then, when someone drinks water from the water point he/she can develop symptoms after one year. A link to BU was made by study participants because of information received during previous sensitization campaigns on dracunculiasis. Such perceptions on mode of transmission can have an impact on patients’ lives and also lead to stigmatization. Community water points may be abandoned as a consequence.
In conclusion, the different beliefs on the mode of transmission of BU observed in the areas of Taabo and Daloa are aligned with what has been described across communities in Africa. People feel that contact with the environment, mainly stagnant water bodies or/and insects, could be a risk factor for BU transmission. However, there is also a strong socially constructed belief suggesting that BU patients can infect a healthy environment, which can lead to stigmatization or hostility towards the infected person. These aetiological perceptions of BU are aligned with the model described by Kleinman (Reference Kleinman1980), which stipulates several explanatory factors for one disease.
The involvement of social and cultural practices can change the way the public deals with diseases and can hence influence disease management and public health status. Because health problems are often influenced by beliefs, such knowledge helps community members to fill gaps related to scientific uncertainties on BU mode of transmission that are rooted in society. Within different social categories, as well as communities, the mode of transmission of BU is perceived as being related to both environmental and social factors. It is recommended that BU control and prevention programmes should first identify the different perceptions and causal explanatory models of the disease. This will lead to a consensus between patients and practitioners. More importantly, it is essential to consider and involve community-centred care based on each cultural setting.
Acknowledgments
The authors thank all the funding institutions. They would like to express their gratitude to the community members for their participation in this study.
Ethical Approval
Ethical approval for participant recruitment into the study was granted by the Ethics and Research National Committee (CNER) of the Health and AIDS Control Ministry of Côte d’Ivoire (No. 3320/MSLS/CNER-P). Participants signed a written consent form, and in the case of children a consent form was obtained from their parents before enrolment into the study. All participants were confirmed BU cases. Those not recorded in the treatment centre were referred to a nearby approved BU treatment clinic for treatment.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
This study was carried out within the framework of Afrique One under the Africa Institutions Initiative funded fully by the Wellcome Trust (WT087535MA) and the DELTAS Africa Initiative (Afrique One-ASPIRE/DEL-15-008). Afrique One-ASPIRE is funded by a consortium of donors, including the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), the New Partnership for Africa’s Development Planning and Coordinating (NEPAD) Agency, the Wellcome Trust (107753/A/15/Z) and the UK government.







