Dates are considered a staple food item in the Middle East and North Africa, and are also imported in Europe, the UK and USA( Reference Baliga, Baliga and Kandathil 1 ). Dates contain relatively high levels of polyphenols and insoluble fibre( Reference Eid, Al-Awadi and Vauzour 2 ), both of which have been postulated to possess anti-cancer activity, especially in the gastrointestinal (GI) tract( Reference Murphy, Norat and Ferrari 3 ). Other plant foods have been reported to selectively and positively influence the growth of the gut microbial ecology( Reference Tuohy, Conterno and Gasperotti 4 ), leading to specific changes in the composition and/or activity of the GI microbiota( Reference Gibson, Scott and Rastall 5 ) and potentially an enhancement of immune responses, increased vitamin synthesis in the gut, and even a reduction in cholesterol and TAG( Reference Roberfroid, Gibson and Hoyles 6 ). Recently, a new approach to testing prebiotic potentials and/or functional abilities of plant foods on modulating the gut microbiota in human trials have highlighted that foods rich in dietary fibre( Reference Tuohy, Kolida and Lustenberger 7 ), polyphenols( Reference Tzounis, Rodriguez-Mateos and Vulevic 8 ) and/or both( Reference Costabile, Klinder and Fava 9 ) may exert such actions. For example, cocoa( Reference Tzounis, Rodriguez-Mateos and Vulevic 8 ), wine( Reference Queipo-Ortuño, Boto-Ordóñez and Murri 10 ), blueberry( Reference Vendrame, Guglielmetti and Riso 11 ), whole grain cereals( Reference Costabile, Klinder and Fava 9 ), maize-derived whole grains( Reference Carvalho-Wells, Helmolz and Nodet 12 ), inulin extracted from artichoke( Reference Costabile, Kolida and Klinder 13 ) and apples( Reference Shinohara, Ohashi and Kawasumi 14 ) have all been shown to induce beneficial changes in gut bacterial growth.
The saccharolytic metabolism of carbohydrates by gut microbiota results in the production of SCFA( Reference Rastall, Gibson and Gill 15 ), whereas exposure to (poly)phenols leads to the generation of phenolic metabolites( Reference Del Rio, Rodriguez-Mateos and Spencer 16 ), some of which have been postulated to enhance colon health and reduce colorectal cancer (CRC)( Reference Lee, Munerol and Pollard 17 ). Furthermore, an enhancement of beneficial bacterial growth has been shown to improve bowel function, thus preventing constipation and reducing other factors associated with the promotion of carcinogenesis( Reference Den Hond, Geypens and Ghoos 18 ) and intestinal epithelial damage, such as secondary bile acids, heterocyclic amines, N-nitrosocompounds, cresols and the production of ammonia, resulting from the bacterial metabolism of proteins( Reference Ahmed, Segal and Hassan 19 ). Previous studies have shown strong associations between fibres and/or prebiotic intake and a reduction in many of these bowel cancer biomarkers( Reference Zhang, Aldosari and Vidyasagar 20 ). As both polyphenols and fibres escape significant metabolism in the upper GI tract, they possess the potential to reach the large intestine where they may modify the gut ecology and limit the formation of carcinogenic by-products of bacterial and host metabolism, thus leading to a reduction in CRC risk( Reference Bingham, Day and Luben 21 ).
Previously, we have shown that whole date fruit, and polyphenols extracted from them, selectively stimulates Bifidobacterium spp. and Bacteroides, along with the total bacterial counts in faecal batch culture models, increasing SCFA production concurrently. In addition, promising changes in DNA damage were observed through the anti-proliferative actions of both polyphenol extracts and whole date extracts( Reference Eid, Enani and Walton 22 ). In the present study we aimed to confirm such actions in humans by investigating the potential for regular date consumption to alter the gut microbial ecology, to influence bowel function, to affect levels of various gastro-metabolic end products and to influence blood markers such as cholesterol, TAG and glucose. Furthermore, we also assessed whether the intake of dates reduces the pro-carcinogenic and/or genotoxic potential of human faecal water.
Methods
Participants
A total of twenty-two healthy volunteers (aged between 18 and 55 years; eleven males and eleven females) were recruited from Reading, Berkshire, UK. Inclusion criteria included a medical questionnaire, anthropometric and biochemical measurements including blood pressure (<140/90 mmHg), cholesterol (<5·0 mmol/l), fasting blood glucose (<5·5 mmol/l), Hb (>115 g/l: females; >140 g/l: males) and BMI (20–25 kg/m2). Exclusion criteria included abnormal blood biochemistry based on standard clinical cut-offs, smokers, pregnant women and those with a history of abnormal gut health. Sample size calculations using a significance level of 5 % (one-sided), and within patient sd 0·3, indicated that, to detect a log change in bifidobacteria of 0·31 at a power of 90 %, twenty-two individuals were required( Reference Walton, Rastall and Martini 23 ). Eligible volunteers were informed of their inclusion in the trial by letter, which included their blood analysis results, and were asked to attend the Hugh Sinclair Unit of Human Nutrition, Food and Nutritional Sciences Department, at the University of Reading, where the trial was conducted. Volunteers were requested not to consume probiotics, prebiotics and/or dates for at least 4 weeks prior to the trial, and antibiotics and laxatives for 6 months prior to the start. Medication taken during the trial was recorded. Participants were requested not to deviate from their regular habitual diet for the duration of the study.
Interventions
Ajwa dates at the Tamr stage of ripening were obtained commercially from Bateel. The control intervention was composed of maltodextrin and dextrose, purchased from Myprotein, and was weighed and packaged at the Department of Food and Nutritional Sciences, University of Reading. Volunteers were advised to store both the control (maltodextrin–dextrose, 37·1 g) and the date intervention (seven dates, approximately 50 g) at room temperature. Both the control and the date intervention were isoenergetic and contained similar levels of mainly sugars (Table 1).
Table 1 Chemical composition of control and date fruit interventions

Study design
The Ethical Research Committee of the University of Reading approved this study. This study is registered in Clinical Trials.gov as NCT02288611. The study was designed and powered as a randomised, controlled, single-masked, cross-over intervention trial. A total of twenty-two volunteers were recruited, with one volunteer dropping out during the second treatment period (n 21). Participants were randomly assigned to one of two groups – dates (50 g) or control (maltodextrin–dextrose (37·1 g)) – for 3 weeks (21 d). Following completion of an arm there was a 2-week (14 d) washout period prior to a volunteer’s crossing over to the alternative arm (Fig. 1). Dates (seven fruits; mean weight 50 g) were provided prepackaged, whereas the control intervention consisted of maltodextrin (3·9 g) and dextrose (33·19 g) powder, mixed and packaged in the University Pilot Plant. On arrival at the clinical unit, volunteers were asked to provide a faecal sample and a fasting blood sample. Faecal and blood sampling was conducted on five separate occasions throughout the duration of the study (Fig. 1). Volunteers were asked to record daily information relating to bowel habits, including stool frequency, stool consistency (according to the Bristol chart), abdominal pain, stomach or intestinal bloating, flatulence and psychological status (subjective mood). Two fasting blood samples (30 ml venous blood) and five faecal samples (15 g) were collected following the consumption of each intervention, along with a standard breakfast. Venous blood was collected in BD Vacutainer tubes (BD). EDTA tubes were used for the analysis of fasting total cholesterol, TAG and HDL-cholesterol, whereas fluoride/oxalate tubes were used for the analysis of glucose. The blood samples were kept on ice prior to centrifugation at 1700 g for 10 min, and plasma collected was stored at –20°C until analysis. Anthropometric measures, including systolic and diastolic blood pressure, weight, waist, BMI and body fat percentage (body composition analyser; Tanita), were recorded during each visit. Throughout the trial the volunteers completed 4-d food intake diaries covering a period from each of the treatment periods (after 3 weeks of control and date intervention). To assess compliance, volunteers were required to report their adherence to the trial protocol by recording date intake and by the return of unused control/date packets.

Fig. 1 Study design of a randomised, controlled cross-over trial on twenty-two healthy volunteers receiving a control and/or date fruit intervention for a period of 3 weeks each, including 2-week washout periods between each treatment. At each visit, anthropometric measurements, faecal samples and blood were collected from each volunteer at five different time points (0, 21, 36, 58 and 73 d).
Blood biochemical measures
Total cholesterol, TAG and glucose concentrations were assessed using the Monarch Automatic Analyzer ILAB 600 (Instrumentation Laboratories Ltd) in conjunction with the following kits: IL test TAG, IL test cholesterol, IL test HDL-cholesterol and IL test glucose (hexokinase). In each run, included control samples, which contained high and low concentrations of each biochemical parameter, were assessed using Wako Control Serum I and Wako Control Serum II from Alpha Laboratories Ltd. Results obtained were determined to be appropriate according to the quality control values given in a specific range by the manufacturers.
Faecal sample preparation and analysis
The samples were collected on site on the day of the experiment and processed immediately as follows: a 1:10 (w/v) dilution of faecal samples in anaerobic phosphate buffer (0·1 m; pH 7·4) was homogenised in a stomacher (Seward; Thetford) for 2 min. Then, 1:10 (w/v) faecal slurries were processed for analysis of bacterial populations using florescence in situ hybridisation (FISH) analysis, as previously described( Reference Eid, Enani and Walton 22 , Reference Daims, Stoecker and Wagner 24 ). FISH was conducted using six different bacterial probes, which have been covered previously( Reference Costabile, Klinder and Fava 9 , Reference Eid, Enani and Walton 22 ): Bif 164 for Bifidobacterium, Lab 158 for Lactobacillus/Enterococcus, ATO for the Atopobium–Coriobacterium group, Erec 482 for Clostridium coccoides–Eubacterium rectale, Chis 150 for the Clostridium subgroup Histolyticum, Bac 303 for Bacteroides–Prevotella, Rrec 584 for Roseburia+Eubacterium rectale, RFO probe (a combination of two probes, Rbro 730 for Ruminococcus bromii and Rfla 729 for Ruminococcus flavefaciens) and EUB338-mix for total bacteria. The number of bacterial cells was assessed using fluorescence microscopy (Nikon Eclipse E400; Nikon), fitted with appropriate filters for the DAPI stain (excited at 359 nm and emitting at 461 nm) and Cy3 dye (excited at 550 nm and emitting at 565 nm). A total of fifteen to twenty fields were counted on six-well slides (Tekdon Incorporated).
SCFA
Faecal samples were centrifuged at 13 000 g for 5 min to remove particulate matter, and supernatants were filtered through a 0·2 µm acrodisc filter prior to injection (20 µl) onto the HPLC system (Merck Millipore) equipped with refractive index detection. Separation of compounds was achieved using an ion-exclusion REZEX-ROA organic acid column (Phenomenex) maintained at 85°C. The mobile phase was aqueous H2SO4 (0·0025 mmol/l) with a flow rate of 0·5 ml/min. Quantification of samples was performed using calibration curves constructed using authentic lactic, acetic, propionic, butyric and valeric acids, based on retention time and spectral mapping.
Faecal water genotoxicity and proliferation
Faecal slurries at 1:1 (w/v) were prepared in PBS and homogenised in a stomacher for 2 min. Then, 10 ml aliquots were taken from each sample and centrifuged at 64 000 g for 2 h at 4°C. Supernatants were filtered using 0·22 µm acrodisc filters and stored at –80°C until analysis. Genotoxicity was tested using the Comet assay and HT29 proliferation assays. The Comet assay (single-cell gel electrophoresis) employed was the alkaline Comet assay in which cells were lysed at pH 10 and electrophoresed at pH 13 to detect DNA damage and DNA breaks in faecal water samples( Reference Collins 25 ). Tail intensity was measured as light intensity to determine damage as compared with PBS and H2O2 ( Reference Costabile, Fava and Röytiö 26 ). In all, twenty-two faecal water samples before treatment and after treatment (with control and date fruits) were tested with respect to HT29 cell lines, and then single-cell gel electrophoresis was performed. Slides stained with fluorescent dye ethidium bromide were examined under a microscope to examine the comet shape, wherein the undamaged DNA represents the head of the comet and the damaged DNA represents the tail. In each experiment, cell numbers and viability were assessed using Trypan blue staining. Proliferation was tested using the Sulforhodamine B assay. The colorimetric assay was used to determine the anti-inhibitory effects of treatments by measuring the ‘cellular protein content’ that determines the cell density( Reference Vichai and Kirtikara 27 ), which was explained in a previous work( Reference Eid, Enani and Walton 22 ). Cell density was determined using SRB (500 μl of 0·4 % SRB; 0·5 h) (Sigma Aldrich). Dye incorporation, reflecting cell biomass, was measured at 492 nm, using a GENios microplate reader (Tecan).
Faecal ammonia levels
Faecal ammonia concentrations were determined as previously detailed( Reference Solorzano 28 ), using faecal water samples collected before treatment and after intervention. Ammonia levels in the samples were then detected using a GENios Pro microplate reader set at an absorbance of 570 nm (Tecan). The concentration of ammonia was measured using a standard curve, using ammonium chloride expressed as micromoles per millilitre.
Diet diary analysis
Diet diaries were analysed using Dietplan 6 software, using the Nutrition Data System and USF databases combined with the USDA flavonoid database.
Statistical analysis
For studying the changes in bacterial counts (log10), SCFA (mm), ammonia concentrations (μmol), DNA tail percentage and HT29 growth inhibition between different treatment groups and across time points (before treatment and after control intervention, before treatment and after date intervention, and between control and date interventions), a general linear model, with a two-way repeated measures ANOVA was used. Significant differences between visits were detected using the least significant difference test, and represented by * P<0·05, ** P<0·01 and *** P<0·001. For analysing dietary intakes and bowel frequency during the treatment period, the paired (two-tailed) t test was applied. All statistical analyses were performed with SPSS software, version 18.0 (SPSS Inc.). All data were checked for normality and log transformed when necessary before statistical analyses.
Results
Anthropometry, bowel function and food intake
Consumption of dates for 21 d led to a significant increase in stool frequency (P<0·01; n 21), relative to the control, with stool types recorded between 3 and 4 on the Bristol chart with no signs of diarrhoea. There were no significant differences between the control and date interventions with respect to stool type (Table 2). There were no other significant changes in GI tract function in response to any of the treatments (Table 2). Furthermore, there were no significant differences in any of the biochemical or anthropometric measures (Table 3). Date consumption resulted in a significant increase in polyphenol intake (P<0·01; n 21), whereas none of the other nutrient measures showed a change in response to the interventions (Table 4).
Table 2 Summary of bowel habits and gastrointestinal symptoms recorded after the 3-week control or date fruit intervention (Mean values and standard deviations; n 21)
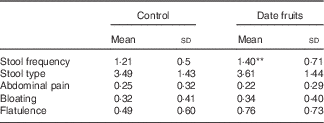
** Mean value was significantly different from that at baseline (P<0·01; paired (two-tailed) t test).
Table 3 Biochemical and anthropometric measurements recorded before and after the 3-week control or date fruit interventionFootnote * (Mean values and standard deviations; n 21)
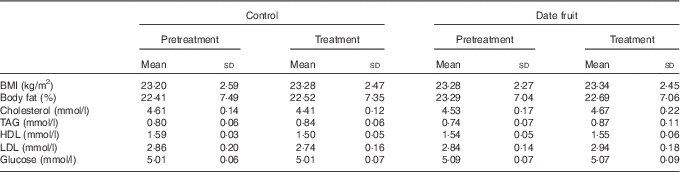
* Mean values after treatment were compared with those at baseline, using two-way repeated-measures ANOVA and the least significant difference test.
Table 4 Summary of dietary intakes using diet diaries and analysed by the diet plan programme recorded after the 3-week control or date fruit intervention (Mean values and standard deviations; n 21)
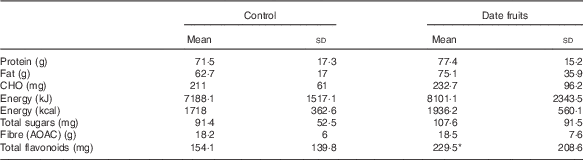
CHO, carbhohydrate; AOAC, Association of Official Agricultural Chemists.
* Mean value was significantly different from that at baseline (P<0·05; paired (two-tailed) t test).
Growth of faecal microbiota and increase in metabolic products
The regular intake of dates for 21 d resulted in no significant alterations in the growth of the faecal microbiota, relative to control (Table 5). Furthermore, lactic acid, acetic acid, valeric acid, butyric acid and propionic acid levels were unaffected by either date or control intake (Table 6). However, the 21-d intake of dates resulted in a significant reduction in faecal ammonia levels in comparison with pretreatment levels (P<0·05; n 21) (Fig. 2).

Fig. 2 Ammonia concentrations in faecal water of twenty-one volunteers in five visits over the human study period. Bacterial metabolites in stool samples measured by the plate reader spectrophotometer expressed as mean values micromoles. Mean values at treatment time points were significantly different from mean values at pretreatment time points (a
P>0·05, * P<0·05, ** P<0·01, *** P<0·001; two-way ANOVA and the least significant difference test). ![]() , Pretreatment;
, Pretreatment; ![]() , treatment.
, treatment.
Table 5 Faecal bacterial numbers in faecal samples of twenty-one volunteers in five visits over the human study periodFootnote * Footnote † (Mean values and standard deviations)

* Mean values for the date treatment were compared with those of the control treatment and/or baseline using two-way repeated-measures ANOVA and the least significant difference test.
† Bacterial counts in stool samples determined by fluorescence in situ hybridisation expressed as mean log10 cells/g faeces.
Table 6 SCFA concentrations in faecal samples of twenty-one volunteers in five visits over the human study periodFootnote * Footnote † (Mean values and standard deviations)

* Mean values for the date treatment were compared with those of the of control treatment and/or baseline using two-way repeated-measures ANOVA and the least significant difference test.
† Bacterial metabolites in stool samples measured by HPLC are expressed as mmol.
Cellular DNA damage and HT29 proliferation
Consumption of dates for 21 d led to a significant reduction in the genotoxicity of faecal water, as evidenced by a significant decrease in Comet DNA tail length, compared with that observed after the control intake (P<0·01; n 21) (Fig. 3). Furthermore, faecal water collected following date consumption also showed an inhibition of cancer cell proliferation (6·76 % inhibition) in comparison with faecal water harvested after control intake (Fig. 4), although this change was not statistically significant (P=0·1).

Fig. 3 Faecal water genotoxicity of twenty-one volunteers before and after treatment (control and date fruit consumption) over the human study period. DNA tail intensity was measured by single electrophoresis expressed as a percentage of damage. Mean values at treatment time points were significantly different from mean values at pretreatment time points (a
P>0·05, * P<0·05, ** P<0·01, *** P<0·001; two-way repeated measures ANOVA and the least significant difference test). ![]() , Pretreatment;
, Pretreatment; ![]() , treatment.
, treatment.

Fig. 4 HT29 growth inhibition tested in faecal water of twenty-one volunteers before and after treatment (control and date fruit consumption) over the human study period. Percentage was measured with a spectrophotometer at 570 nm. Changes in mean values at treatment time points are significantly different from mean values at pretreatment time points (a
P>0·05, * P<0·05, ** P<0·01, *** P<0·001; two-way repeated measures ANOVA and the least significant difference test). ![]() , Pretreatment;
, Pretreatment; ![]() , treatment.
, treatment.
Discussion
Epidemiological studies have suggested that the regular intake of fruit and vegetables may help reduce CRC risk( Reference Eid, Walton and Costabile 29 ), although the precise bioactive components responsible for these actions remain unclear( Reference Bingham, Day and Luben 21 ). The microbial fermentation of certain dietary components potentially acting as a bioactive compound, such as dietary fibres( Reference Tuohy, Kolida and Lustenberger 7 ) and polyphenols( Reference Tzounis, Rodriguez-Mateos and Vulevic 8 ) or both( Reference Costabile, Klinder and Fava 9 ), and the resultant effects on the growth of microbiota and the bioactive actions of any metabolic products may represent one mechanism by which such foods enhance colon health. We show that, although the consumption of dates for 21 d does not induce selective changes in the growth of selected bacterial groups (or SCFA concentration), beneficial changes with respect to stool frequency, ammonia concentrations and genotoxicity of faecal water were observed.
Previously, we have observed that date fruit extracts significantly increase the growth of bifidobacteria and SCFA in vitro ( Reference Eid, Enani and Walton 22 ). However, 21 d of intervention failed to lead to increases in the selective growth of microbiota, despite the actions of dietary fibres( Reference Tuohy, Kolida and Lustenberger 7 ) and polyphenols( Reference Tzounis, Rodriguez-Mateos and Vulevic 8 ) or both( Reference Costabile, Klinder and Fava 9 ). One reason for the absence of bacterial growth alterations in the current study may be the lower content of soluble and insoluble fibres in our intervention (3·4–4·0 g of insoluble fibres and 0·5–1·0 g of soluble fibres) relative to doses tested previously in other whole foods( Reference Costabile, Klinder and Fava 9 ). Furthermore, polyphenol levels were also significantly lower than the levels reported to influence microbial growth in the large intestine with polyphenol-rich drinks( Reference Tzounis, Rodriguez-Mateos and Vulevic 8 ). In addition, variations in host microbial ecology influence changes in bacterial numbers, wherein some volunteers exhibit high counts at baseline of some bacteria, preventing further increases over this timeframe( Reference Kemperman, Bolca and Roger 30 ), in comparison with volunteers at high risk, such as the elderly, malnourished populations and those with obesity and inflammatory conditions( Reference Walton, van den Heuvel and Kosters 31 – Reference Claesson, Jeffery and Conde 34 ). Previous studies had suggested that the intake of 100 g of date fruits for 28 d significantly reduced TAG levels and oxidative stress in human plasma( Reference Rock, Rosenblat and Borochov-Neori 35 ). However, we were unable to detect such changes following our intervention, which is in agreement with similar studies in healthy individuals( Reference Costabile, Klinder and Fava 9 ). In light of this information we extended our investigation by looking at the reasons behind high bacterial counts in certain faecal samples before starting the intervention (Fig. 5(a) and (b)) by analysing the 4-d diet diaries of food intake. Indeed, high habitual intake of dietary fibre (18·5 g/d) in many of our volunteers may explain why faecal samples at baseline encounter higher numbers of the tested bacterial strains (Table 7), in comparison with the seven volunteers consuming low amounts of fibre, an average of 6 g/d (Table 8). In previous human trials of this nature, even though bacterial numbers were significantly changed, SCFA were rarely altered by bacterial fermentation of plant-based foods, as seen previously with whole-grain consumption( Reference Costabile, Klinder and Fava 36 ). The main reason for this seems to be the fact that SCFA are absorbed very quickly( Reference Ahmed, Segal and Hassan 19 ), and thus are difficult to detect. With respect to SCFA concentrations, our data are in agreement with those of previous human trials carried out with inulin extracted from artichoke and maize-based cereals, despite changes in the microbiota( Reference Costabile, Kolida and Klinder 37 , Reference Carvalho-Wells, Helmolz and Nodet 38 ). Little information is found on the role of whole-fruit consumption in changing the gut microbiota, which was mostly seen in apples in vitro ( Reference Chen, Liang and Liu 39 ), and in humans( Reference Shinohara, Ohashi and Kawasumi 14 ), showing a significant alteration in bifidobacteria in a small number of volunteers. Thus, there is still a huge shortage of data with regard to whole fruits and vegetables and their actions on modulating the gut microbiota.

Fig. 5 Comparison between the faecal bacterial numbers of two selected groups with high fibre intake (n 14) and low fibre intake (n 7) before intervention (pretreatment) with control (a) and date fruit (b). Bacterial counts in stool samples as determined by fluorescence in situ hybridisation are expressed as mean log10 cells/g faeces. Changes in mean values on treatment were measured using the paired (one-tailed) t test. * P<0·05, ** P<0·01 (paired (two-tailed) t test). ![]() , Low fibre intake;
, Low fibre intake; ![]() , high fibre intake.
, high fibre intake.
Table 7 Faecal bacterial numbers in faecal samples of volunteers with high fibre intake (n 14) over the human study periodFootnote * Footnote † (Mean values and standard deviations)

* Mean values for the date treatment were compared with those of the control treatment and/or baseline using two-way repeated-measures ANOVA and the least significant difference test.
† Bacterial counts in stool samples determined by fluorescence in situ hybridisation expressed as mean log10 cells/g faeces.
Table 8 Faecal bacterial numbers in faecal samples of volunteers with low fibre intake (n 7) over the human study periodFootnote ‡ (Mean values and standard deviations)

Mean values for the date treatment were significantly different from those for the control treatment and/or baseline: * P<0·05, ** P<0·01, † P>0·05 (borderline significance) (two-way repeated-measures ANOVA and least significant difference test).
‡ Bacterial counts in stool samples determined by fluorescence in situ hybridisation expressed as mean log10 cells/g faeces.
Even though modulation of gut ecology is involved in CRC aetiology, in human intervention studies the focus is mostly on the ability of dietary ingredients to induce changes in both genotoxicity and toxic metabolites( Reference Roberfroid, Gibson and Hoyles 6 ). Studying the impact of food components on cancer risk is extremely difficult as it is clearly impractical to use cancer as an end point in human intervention trials because of the length of time needed for cancer development. There are few, if any, well-validated biomarkers of cancer available for use as markers of cancer progression( 40 ). On the other hand, there is a range of experimental biomarkers with potential mechanistic links to cancer, which include cell models (Caco-2 and HT29 cell lines), molecular techniques( Reference Saunders 41 ) and Western blotting, as well as the identification and quantification of the activity of certain proteins (ERK, JNK, P-38, Akt)( Reference Corona, Deiana and Incani 42 ), inflammatory enzymes (COX-1 and COX-2)( Reference Deiana, Corona and Incani 43 ) and DNA damage, which is strongly associated with protein metabolic products( Reference Rafter, Bennett and Caderni 44 ). Our previous in vitro work on Ajwa dates have shown that date polyphenols and whole date extracts were effective in inhibiting the growth of colon adenocarcinoma cell growth, something that was reduced when they were initially exposed to the faecal bacteria prior to cell exposure( Reference Eid, Enani and Walton 22 ), which is accompanied with current work. Furthermore, with regard to fruits rich in fibre, human trials have identified anti-cancer actions of cruciferous vegetables, such as Brussels sprouts, watercress and mixed types, resulting in significant reductions in DNA damage and oxidation( Reference Gill, Haldar and Boyd 45 ). With regard to fruits rich in polyphenols, a number of human interventions on berries showed a reducing action on cell proliferation and polyp percentages, in addition to an increase in cell apoptosis in CRC patients( Reference Wang, Sardo and Henry 46 ).
In the current study, colon health was first assessed to show the significant effect of date fruit consumption on increased frequency of bowel movements. This parameter has been seen to be strongly associated with CRC patients( Reference Roberts, Millikan and Galanko 47 ). Previous data strongly link prebiotic intake to significant reductions in constipation, which may be associated with reducing colon cancer risks through an increase in faecal genotoxicity and carcinogen levels( Reference Burkitt 48 ). Indeed, we show that significant reductions in the genotoxicity of faecal water were apparent following date consumption compared with control. DNA damage in colon epithelial cells has been postulated to contribute to the progression of CRC( Reference Pool-Zobela and Leuchtb 49 ), and has been shown to be reduced following the intake of prebiotics and other dietary compounds( Reference Costabile, Fava and Röytiö 26 ), and also in polyp patients following synbiotic treatments( Reference Rafter, Bennett and Caderni 44 ). Following our previous data on inhibiting colon cancer cell proliferation( Reference Eid, Enani and Walton 22 ), utilising similar assays, an increase in cancer growth inhibition was also detected after the consumption of date fruits, but the results were not statistically significant. Most of the cancer-related studies were carried out in vitro, which demonstrated that polyphenols have the potential to inhibit DNA mutations in colon cancer cell lines and to inactivate enzymes such as protein kinases and other pro-oxidant enzymes( Reference Lee, Munerol and Pollard 17 ), which was previously observed with olive oil( Reference Corona, Deiana and Incani 50 ) and in clinical human trials discussed earlier.
Increased risk for CRC has also been linked to the consumption of red or processed meats( 40 ), something thought to be linked to the impact of bacterial metabolic products of protein metabolism (secondary bile acids, ammonia, phenol and cresol) on colon epithelial cells( Reference Ahmed, Segal and Hassan 19 ). Previous trials carried in polypectomised patients indicated the presence of such types of carcinogens( Reference Venturi, Hambly and Glinghammar 51 ) and their impact on genotoxicity( Reference Rafter, Bennett and Caderni 44 ). Date intake in our study leads to a significant reduction in faecal ammonia concentrations, indicating that a daily consumption of seven pieces of date fruit was utilised by the gut microbiota as its energy supply and to reduce toxic metabolites, because of preferred carbohydrate degradation and reduced utilisation of protein products( Reference Nyangale, Mottram and Gibson 52 ). There are other biomarkers related to cancer risk, such as secondary bile acids, which were significantly reduced with prebiotics or probiotics( Reference Larrosa, González-Sarrías and Yáñez-Gascón 53 ), and ammonia, both of which are known risk factors for colon cancer incidence, in association with epidemiology( Reference Burkitt 48 ). Date consumption may have also modulated bacterial enzymatic activity (β-glucoronidase, β-glycosidase and nitroreductase) and caused the suppression of ammonia and other genotoxic compounds, which is worth investigating in future work( Reference Rowland, Rumney and Coutts 54 ).
The current study is considered a preliminary one demonstrating a possible specific property of date fruits in enhancing colon movements and metabolism and in reducing toxicity. Data successfully confirmed significant reduction in the DNA tail percentage of colonocytes and ammonia, and an increase in faecal frequency among twenty-one volunteers after the consumption of date fruit on a daily basis. Because dates contain high amounts of fibre and polyphenol compared with other types of fruit, it can be easily consumed as a snack and has a long shelf life. The current study follows the same concept as discussed by Tuohy et al.( Reference Tuohy, Conterno and Gasperotti 4 ), and is similar to the FLAVURS clinical trial of 2013( Reference Chong, George and Alimbetov 55 ), in that it refers to the consumption of specific types of fruit and vegetables, rather than a generic recommendation, to induce significant changes on the gut microbiota and health. Future approach must consider the fact that bacterial ecology in the gut is altered in populations at higher risk( Reference Tuohy 56 ), and trials are showing promising impacts on immunity and in reducing inflammatory markers( Reference Vulevic, Juric and Tzortzis 57 ). Thus, targeting populations with altered gut ecology and assessing the impact of whole-food consumption at the microbiological, cellular, metabolic and clinical levels must be undertaken to obtain a novel health claim, wherein the gut microbiome can be used as a marker/remedy in clinical practice, which has been seen mostly with obesity and the metabolic syndrome( Reference Fava, Gitau and Griffin 58 ).
More advanced techniques must be applied in future work, such as bacterial enumeration using flow cytometry( Reference Roberfroid, Van Loo and Gibson 59 ), or rRNA gene sequencing, which is excellent in measuring the abundance of rRNA copies( Reference Hold, Pryde and Russell 60 ), when the FISH technique may have limited the detection of different bacterial groups. With regard to SCFA characterisation, GC is a better approach for volatiles, even though the HPLC method used was validated using a column that overcomes volatile compounds( Reference Costabile, Kolida and Klinder 37 , Reference Carvalho-Wells, Helmolz and Nodet 38 ). Indeed, in those trials, no changes in SCFA were seen after the consumption of inulin or maize-based cereals, despite changes in microbial numbers. On the other hand, such a method has shown significant alterations in SCFA in our in vitro work( Reference Eid, Enani and Walton 22 ). One reason could be the type of fermented faecal material analysed in human trials, which affects SCFA amounts and availability and/or the detectors used in HPLC, wherein electrochemical detectors are more precise and accurate( Reference Kotani, Miyaguchi and Kohama 61 ). Epidemiological studies on diet-related health issues for populations living in the Middle East and North Africa do not only rely on the diet, and consuming staple foods, such as date fruits, but genetics may play a role as well. This was seen in the Collaborative Gene–Environment Study, where different populations were stratified according to their diet , genes and other factors( Reference Eeles, Olama and Benlloch 62 ). Future work on the prebiotic potential of whole foods can be optimised with new recommendations by dietitians for reducing DNA damage( Reference Baldrick, Sung and McFadden 63 ), CRC risk( Reference Brown, Rowland and Ternan 64 ) and other chronic illnesses( Reference Tuohy, Fava and Viola 65 ).
Acknowledgements
The authors thank Mr Alfred, Al Bateel shops manager, London, UK, for packaging and transporting the date fruit according to the study design. The authors also thank Miss Raoom Fatani, MSc student, Duygu Yarram and Hannah Mckinnon, BSc students, at the Food and Nutritional Sciences department for helping out with the human study, microbiology and cell work. The authors sincerely thank Mr Sulaiman Al-Odaibi, may he rest in peace, for his support in previous work, which included chemical analysis of dates( Reference Eid, Al-Awadi and Vauzour 2 ), and related in vitro work( Reference Eid, Enani and Walton 22 ), which made this trial possible.
The authors thank the Ministry of Education in Saudi Arabia for the sponsorship of N. E.
N. E.: designing and carrying all experiments, results, data analysis, statistics and writing the paper; H. O.: MSc research assistant, helping human trial period; C. N.: visiting researcher carrying the cell work; G. W.: applicant in the human trial design and guidance microbiology laboratory work (stool processing and FISH analysis); A. C.: applicant in the human trial design and guidance in microbiology laboratory work, specifically in metabolites (SCFA); G. G.: provided N. E. with the opportunity to carry almost all the human trial analysis at the FMSU (Food Microbial Science Unit) at the University of Reading; I. R.: applicant in the human trial design and project second supervisor; J. P. E. S.: principal investigator, project first supervisor and corresponding author. Each author has contributed to the paper drafting.
There were no conflicts of interest.















