Introduction
With only approximately 100 cases described in the literature since its initial description in the 1950s, a fistulous connection between a pulmonary artery and left atrium is a rare congenital condition that usually presents in adolescence or adulthood.Reference Friedlich, Bing and Blount1,Reference Luo, Ma, Huang, Yan and Zhu2 While surgical repair has historically been the preferred treatment, catheter-based techniques have now become a suitable, safe, and effective alternative in the modern era.Reference Francis, Sivakumar and Kumar3
Three-dimensional (3-D) virtual segmentation and printed models using datasets from CT and MRI allow comprehensive spatial conceptualisation of cardiac anatomy that can assist in pre-procedural planning and peri-procedural guidanceReference Van Der Hoeven, Schalij and Delgado4 The EchoNavigator and VesselNavigator systems (Philips Healthcare, Best, The Netherlands) are software solutions that have more recently been applied to congenital cardiology cases allowing operators the ability to merge echocardiographic and cross-sectional images with fluoroscopic images. The use of multi-modality imaging for pre-procedural planning and procedural guidance has never been described in this lesion.
Case report
A 53-year-old woman presented for investigation of new-onset cyanosis on exertion with a background of surgical repair of a secundum atrial septal defect 8 years previous and obstructive sleep apnoea. Her oxygen saturations were 94% at rest on 1.5L oxygen via nasal cannula and 79–85% with minimal exertion. Prior to her operation, she had no oxygen requirement. A trans-oesophageal echocardiogram and right heart catheterisation were performed prior to surgery which confirmed the diagnosis and demonstrated a Qp:Qs of 1.8. At the time of her surgery, inspection of the fossa ovalis revealed a large secundum atrial septal defect measuring 2 x 3 cm, which was closed with an autologous pericardial patch. There was no evidence of anomalous pulmonary venous drainage.
Her trans-thoracic echocardiogram demonstrated a severely dilated left atrium and residual left-to-right shunting across her interatrial septum by colour Doppler. A cardiac CT and MRI were subsequently performed for anatomical delineation of the shunt that demonstrated a severely dilated right pulmonary artery and a communication between the distal right pulmonary artery and left atrium as the distal right pulmonary artery courses inferior over the atrial mass. (Figure 1) The calculated Qp:Qs on cardiac MRI was 1.14.
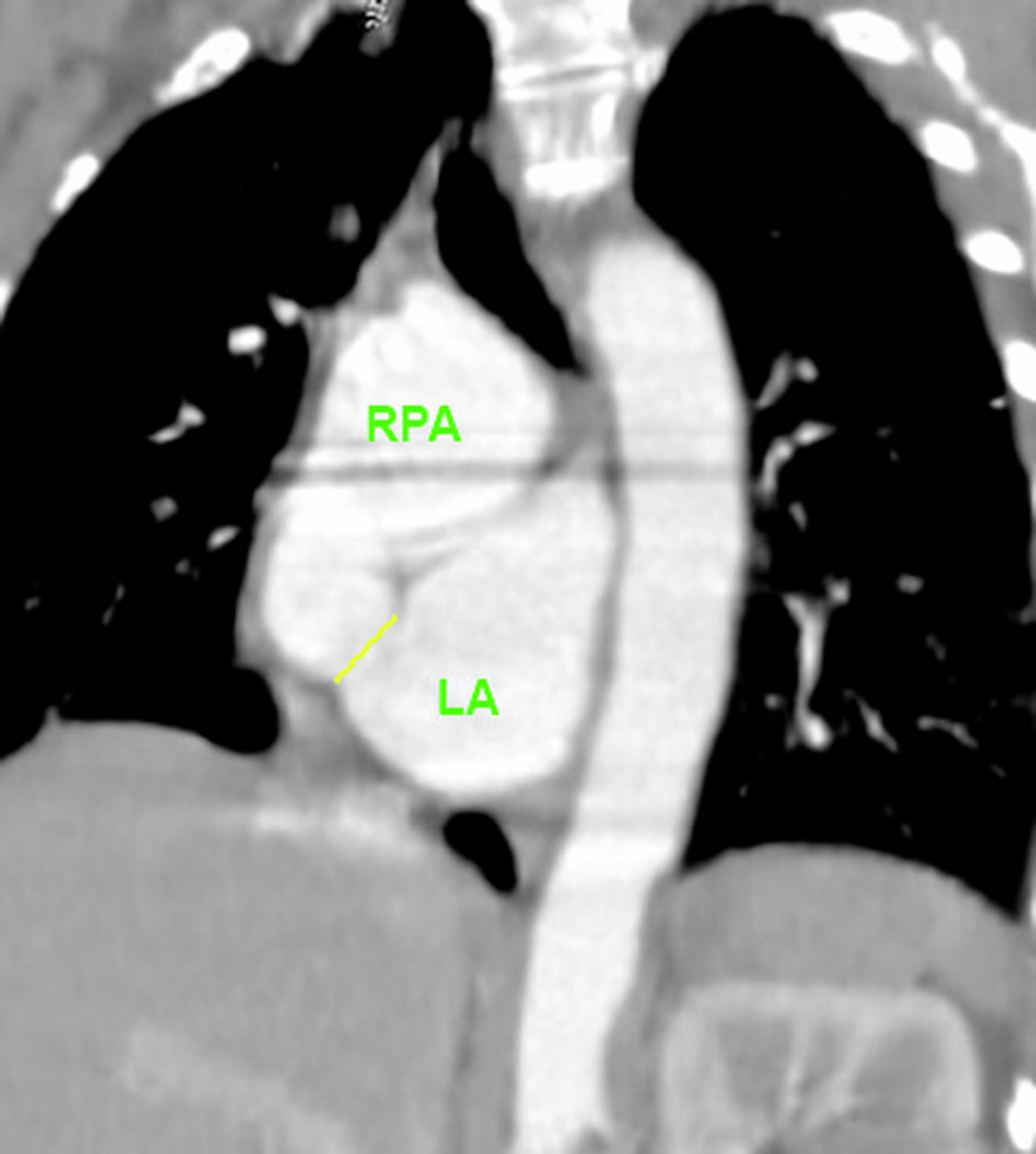
Figure 1. Cardiac CT was used for pre-procedural guidance and delineates the location of the RPA-LA communication (represented by the yellow line). The cardiac CT was then used to create a 3D reconstruction using virtual reality for segmentation. Notably, the 3D reconstruction using the cardiac CT dataset overestimated the size of the communication between the RPA and LA.
Prior to the procedure, a 3D virtual model derived from the cardiac CT was created using virtual reality software (Elucis, Realize Medical) for segmentation to aid understanding of the size and location of the communication.
The patient was brought to the cardiac catheterisation laboratory for evaluation of her shunt and transcatheter device closure of the right pulmonary artery-left atrium fistula. A right heart catheterisation was performed in standard fashion which demonstrated an elevated PVR 4.9 Wood units*m2 calculated by Fick (Table 1). A contrast echocardiogram with agitated saline was performed in the superior caval vein with a 6-Fr Pigtail catheter which demonstrated late filling of the left atrium with bubbles within 2 cardiac cycles after right heart opacification. The study was repeated in the right pulmonary artery which demonstrated immediate left atrial filling, confirming the diagnosis (Figure 2 Panel A).

Figure 2. A bubble study was performed in the SVC which demonstrated late filling of the LA with bubbles in a couple of cardiac cycles after right heart opacification via a communication from the RPA (green arrow). (Panel A) Real-time TOE was overlaid onto live fluoroscopy using EchoNavigator software to demonstrate the location of the communication and aid in device deployment. The blue arrow demonstrates the AVP-IV device during and after deployment from the RPA on 2-D and 3-D TOE (Panel B and C).
Table 1. Haemodynamic assessment prior to RPA-LA fistula closure

SVC = superior caval vein, RA = right atrium, RV = right ventricle, EDP = end diastolic pressure, RPA = right pulmonary artery.
Fusion modalities were useful to target and enter the fistula from the right pulmonary artery and exit into the left atrium via the right femoral vein. VesselNavigator was also used to determine appropriate radiographic views with which to best visualise the entrance point of the fistula from the right pulmonary artery (Figure 3). During the procedure, real-time fluoroscopy was merged with trans-oesophageal echocardiogram (EchoNavigator) and fused with three-dimensional CT reconstructions (VesselNavigator) to guide the procedure (Figure 2 Panel B). With a 6-Fr JR Guide catheter and 4-Fr Glide catheter positioned in the distal right pulmonary artery, an 0.035” Glide wire was passed through the communication into the left atrium. The 4-Fr Glide catheter was then passed over the wire into the left atrium and the wire then removed. An 8 mm Amplatzer Vascular Plug IV was placed into the 4-Fr Glide catheter and positioned across the communication using fluoroscopic and TOE guidance. Device position was checked on TOE and then the device deployed. Bubble contrast studies were repeated in the superior caval vein and the right pulmonary artery which demonstrated no residual shunt across the communication from the right pulmonary artery to the left atrium (Figure 2 Panel C).

Figure 3. A 3D CT reconstruction was fused with live fluroscopy using VesselNavigator (Phillips, Best, The Netherlands) to guide the procedure.
The patient had an uneventful post-operative course and was discharged the following day. She was commenced on tadalafil in the setting of her elevated PVR and was appropriately managed for her obstructive sleep apnoea with night-time CPAP. Her oxygen saturations improved to 94% in room air.
Discussion
We describe the rare finding of a congenital right pulmonary artery-left atrium fistula in a 53-year-old female following surgical closure of a secundum atrial septal defect. Embryologically, incomplete degeneration of the septum between the arterial and venous plexus of the pulmonary vascular bed can result in formation of a pulmonary arteriovenous fistula. If a pulmonary vein connected to this fistula is absorbed into left atrium, a pulmonary artery–left atrium fistula can result.Reference Chauhan, Agarwal, Gupta, Raja, Geelani and Trehan5 Right pulmonary artery-left atrium fistulae are usually found in association with atrial septal defects, as seen in our case.Reference Chauhan, Agarwal, Gupta, Raja, Geelani and Trehan5
Clinical presentation depends on the size of the communication, amount of right-to-left shunt, the patient’s age at diagnosis and pulmonary vascular resistance. In this case, though the patient was cyanotic with exercise, the communication was small. The MRI finding of a net left-to-right shunt of 1.14:1 was likely a spurious finding given the physiology of this lesion. The co-morbid obstructive sleep apnoea was likely a contributing cause of her cyanosis; however, we could not be sure how much the fistula was contributing. Untreated right pulmonary artery-left atrium fistulae could lead to pulmonary oedema, pulmonary hypertension, and an increased risk of stroke; therefore, early intervention is recommended. In this case, as the defect was small, the decision was made to close the defect and treat her pulmonary hypertension medically with tadalafil as well as address her obstructive sleep apnoea. Although historically surgery has been the primary form of treatment, percutaneous device closure seems a logical choice in the modern era and is now favoured as a safe, less invasive, and effective alternative.Reference Chowdhury, Kothari, Airan, Subramaniam and Venugopal6
Multi-modality imaging using data obtained from non-invasive imaging techniques such as echocardiography, CT, and MRI allows anatomical, morphological, and functional data to be combined, increasing accuracy and efficacy of cardiovascular procedures and clinical outcomes.Reference Van Der Hoeven, Schalij and Delgado4 Historically, three-dimensional printing phantom models have been used for pre-procedural planning. In this case, a virtual 3-D model was created using the dataset from the cardiac CT for preprocedural planning, which allows real-time manipulation of the model and interaction with surrounding structures . Though this method can be useful in pre-procedural planning, it is important to recognise that interpretation of the model is limited by the quality of the dataset. In this case, for example, the cardiac CT overestimated the size of the communication between the right pulmonary artery and left atrium which was small on TOE. Limiting motion artefact as well as higher temporal and spatial resolution reconstruction algorithms can help enhance anatomic detail and tissue differentiation to improve image quality and avoid overestimation of defect size.
Though various transcatheter approaches have been published, this is the first to report the use of multi-modality fusion imaging for procedural guidance in closure of an right pulmonary artery-left atrium fistula.Reference Kumar, Varghese and George7–Reference Ramakrishnan, Shivdas and Kothari9 EchoNavigator and VesselNavigator require teamwork and communication between the proceduralist and the imaging specialists to obtain the best outcomes, improving procedure planification, trajectory choice, and real-time guidance. This defect could be successfully crossed and closed percutaneously with the help of echocardiographic and CT fusion with live fluoroscopy, with the additional benefit of reducing procedure time as well as total contrast and radiation doses which remained low in this case (105 minutes, 0.9 ml/kg, and 59.1 Gycm2 respectively).
Conclusion
We demonstrate the utility of three-dimensional reconstruction and multi-modality fusion imaging in guiding transcatheter closure of a rare right pulmonary artery-left atrium fistula. These techniques likely provide benefit in other rare congenital and structural lesions which may otherwise provide a major angiographic challenge.
Acknowledgements
The authors would like to acknowledge Rachel Ogden RDCS for her contribution in providing imaging support for the procedure.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
We have adhered to a strict confidentiality policy regarding this case report. No identifying information is present within this case. The authors do not have any conflicts of interest to disclose.







