Branched-chain fatty acids (BCFA) are primarily SFA with one methyl branch or more on the carbon chain. BCFA are categorised as mono-, di- or multi-methyl BCFA. In monomethyl BCFA, the predominant branching is near the terminal end of the carbon chain. Fatty acids (FA) terminating with an isopropyl or isobutyl group are referred to as iso- or anteiso-BCFA, respectively (Fig. 1). BCFA modulate the biophysical properties of membranes in a manner similar to that of cis double bonds: both types of BCFA interfere with the ability of SFA to pack tightly to form rigid, high-melting point extended structures, and thus reduce the phase transition temperature of membrane phospholipids( Reference Kaneda 1 ).
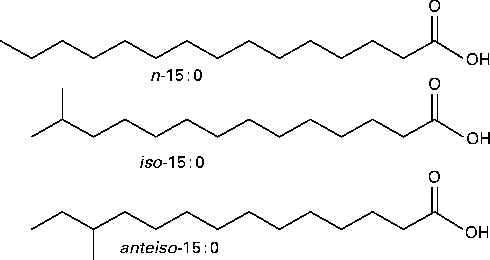
Fig. 1 Structures and naming of representative branched-chain fatty acids (BCFA). n (normal)-Fatty acids have no branching. iso-BCFA have a methyl branch on the penultimate carbon and anteiso-BCFA have a methyl branch on the antepenultimate carbon.
BCFA are major components of bacterial membranes across many genera and species. They are particularly prominent in Bacillus, constituting 95 % of the FA in many species of Bacillus and Lactobacillus ( Reference Kaneda 1 ). In some Bifidobacterium strains, a single BCFA concentration can be as high as 24 wt%( Reference Veerkamp 2 ). When BCFA concentrations are present in large amounts in bacteria, they may influence the phenotype in potentially important ways. For instance, when they are present in the environment, BCFA are readily taken up by the pathogen Pseudomonas aeruginosa and dramatically reduce motility and virulence( Reference Inoue, Shingaki and Fukui 3 ).
BCFA are rare in internal human tissues; however, they are present in high concentrations in the skin and vernix caseosa, the unique waxy white substance coating the skin of term newborns, with their concentrations being about 29 wt%( Reference Ran-Ressler, Devapatla and Lawrence 4 ), including BCFA with branches in positions other than the iso and anteiso positions, and also dimethyl BCFA. We have reported previously( Reference Ran-Ressler, Devapatla and Lawrence 4 ) that BCFA are constituents of the healthy term newborn infant's gut and that the human alimentary canal selectively metabolises BCFA, suggesting that they play a specific role in the gut. Consistent with this hypothesis, our recent study in neonatal rats showed that substitution of 20 wt% of fat as BCFA altered the gastrointestinal microbial ecology towards organisms that use BCFA and reduced the incidence of necrotising enterocolitis( Reference Ran-Ressler, Khailova and Arganbright 5 ). Others( Reference Yang, Liu and Chen 6 , Reference Wongtangtintharn, Oku and Iwasaki 7 ) have shown that BCFA induce apoptosis in human breast cancer cells, and inhibit tumour growth in cultured cells and in a mouse model. These data all point to previously neglected nutritional properties of BCFA that may be important for the development and maintenance of the microbiota, enterocyte health, skin and possibly other functions.
There are scant data on the intake of BCFA in the human diet. We have recently reported the profile and concentrations of BCFA in a representative sampling of retail milk in the USA. Using these data along with the reports of others for BCFA consumption from beef, we estimated the intake of BCFA from cheese and beef food products in the American diet to be about 400 mg/d. However, these calculations were based on retail milk and did not take into account the changes in BCFA profiles or the increase or loss in total BCFA concentrations due to, for instance, fermentation or processing methods( Reference Ran-Ressler, Sim and O'Donnell-Megaro 8 ). In the present study, we present the analysis of BCFA in foods prominently featured in the American diet, and estimate from the measurements the nutritional contribution of BCFA from these various foods in the American diet using the USDA's Economic Research Service intake data.
Experimental procedures
Sampling
Food samples were purchased from local supermarkets in Ithaca, NY, USA. Because previous reports of BCFA content focus on dairy and meat products, we chose a cross-section from these food groups and additionally included fermented foods. Particular foods were chosen based on the consideration of the prevalence of consumption within respective food groups according to the USDA's Economic Research Service, Loss-Adjusted Food Availability data( 9 ).
The following food products from the dairy food group were analysed: whole milk cheese (bovine milk: cheddar cheese, low-moisture mozzarella cheese, provolone cheese, Swiss cheese, cottage cheese, ricotta cheese, cream cheese; two samples of ovine (sheep) milk-derived cheese (blue, romano); one sample of goat-derived cheese); plain and Greek yogurt; ice cream. We also analysed dairy-based food products from the added fat food group such as sour cream, light cream and butter. However, these foods were merged with the dairy food group to simplify the data analysis.
Foods were chosen based on the prevalence of consumption within the dairy and protein food groups according to the USDA's Economic Research Service, Loss-Adjusted Food Availability data( 9 ). From the protein food group, we analysed ground beef, ground turkey, ham, bacon, pork sausages, chicken thighs, chicken breasts, eggs and almonds. In addition to commercial ground beef, ground beef from a private small farm in northern Pennsylvania, in which beef cattle are exclusively pastured, was also included in the analysis. The most consumed cuts of beef and pork were chosen for analysis( Reference Davis and Lin 10 ). Almonds, canned tuna and fresh salmon were also analysed as part of the protein food group.
Fermented food products such as sauerkraut and miso (soya), tofu, kimchi and tempeh were included in the analysis because the addition of bacteria/fungi during the fermentation process of these food products may influence the presence of BCFA. Chocolate bars were also chosen to be included in the analysis as a fat and dairy-containing snack. Samples with a high content of moisture such as yogurt were placed in a centrifugal evaporator (Savant SpeedVac; Thermo Fisher Scientific), for at least 24 h before the analysis. The samples were stored at − 80°C until processed.
Fatty acid analysis
Samples (100–150 mg) from each food were extracted and methylated according to a modified one-step hydrolysis procedure, as described previously( Reference Garces and Mancha 11 , Reference Zhou, Nijland and Miller 12 ). Heneicosanoic acid (21 : 0) was used as the internal standard (Sigma Chemical Company Chemical). Fatty acid methyl esters (FAME) were identified and analysed quantitatively, as discussed in detail previously( Reference Ran-Ressler, Sim and O'Donnell-Megaro 8 ). Briefly, a BPX-70 capillary column (25 m × 0·22 mm × 0·25 μm; SGE) with the H2 carrier gas was installed in a HP 5890 gas chromatograph with a flame ionisation detector. A FAME mixture of equal weight (68A; Nu-Chek Prep, Inc.) was used to calculate response factors and six BCFA were used as authentic reference standards (iso-14 : 0, anteiso-15 : 0, iso-16 : 0, anteiso-17 : 0, iso-18 : 0 and iso-20 : 0; Larodan Fine Chemicals AB). Concentrations of all FA are expressed as wt%.
The identities of FAME were determined by electron ionisation MS, chemical ionisation and electron ionisation–tandem MS, as described previously( Reference Ran-Ressler, Lawrence and Brenna 13 ), using a Varian Star 3400 GC coupled to a Varian Saturn 2000 ion trap MS, with their identities being confirmed by GC retention times. For our protocols with GC-flame ionisation detector analysis, we estimated the limit of quantification to be about 0·15 ng FAME per 1 μl injection. For the present study, we prepared approximately 6 μg FAME mixture per 1 μl injection. These concentrations led to the limit of quantification for individual FAME of about 0·003 % (w/w) of total FAME.
Results and discussion
Branched-chain fatty acid content in food samples from a US market
In the USA, per capita consumption of foods contained in the dairy and protein food groups is 216 and 208 g/d( 9 ), respectively. Here, we analysed food products from both of those groups representing 160 g of 216 g (74 %) of the per capita daily consumption of dairy food products, and 193 g of 208 g (92 %) of the per capita daily consumption of protein food products. The present analysis of the dairy-based added fat food category accounted for 10 g of 14 g (sour cream, cream cheese and light cream). We present the results for these dairy-based added fat food groups together with the dairy food group.
Tables 1–3 present in detail the data on BCFA distribution in dairy, protein and fermented food products, respectively. BCFA were detected in dairy and beef food products. Small amounts of BCFA were also detected in canned tuna and in the fermented food products miso and sauerkraut. BCFA were found to be below the detection limit in the following food products: ground turkey; ham; bacon; pork sausages; chicken thighs; chicken breasts; eggs; almonds; tofu; tempeh; fresh salmon.
Table 1 Total and individual branched-chain fatty acid (BCFA) concentrations in various foods within the dairy food category

Table 2 Total and individual branched-chain fatty acid (BCFA) concentrations in beef and tuna*
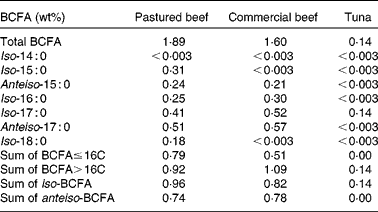
* Other foods in the protein food group tested were ground turkey, chicken breasts, chicken thighs, pork sausages, ham, bacon, fresh salmon, eggs and almonds; all had < 0·003 wt% BCFA.
Table 3 Total and individual branched-chain fatty acid (BCFA) concentrations in sauerkraut and miso*
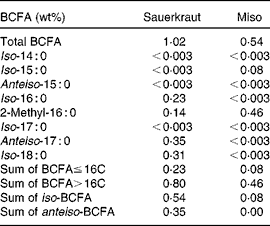
* Other fermented foods tested were tofu, tempeh and kimchi; all had < 0·003 wt% BCFA.
As shown in Table 1, total BCFA concentrations in the various dairy products ranged between 1·37 wt% for light cream cheese and 2·73 wt% for sheep cheese. Sheep and goat milk have long been known for their higher BCFA concentrations, especially when fed a barley-rich diet( Reference Duncan and Garton 14 ). The relatively higher levels of BCFA in sheep cheese and goat cheese show that BCFA concentration in dairy products is influenced by which ruminant species' milk is being used. In addition, differences in cheese composition can be affected by many factors, such as cow breed, feeding practice and microbial activity in milk and cheese, especially during ripening( Reference Bonanno, Tornambe and Bellina 15 ). The original milk containing lower concentrations of BCFA that was used in the production of these cows' milk-based food products is the most likely explanation for differences in BCFA content among these dairy products, particularly BCFA concentrations were found to be lower in low-moisture mozzarella cheese than in provolone cheese (1·4 v. 1·9 wt%), despite sharing a similar production process.
Fig. 2 shows BCFA distribution in retail cows' milk( Reference Ran-Ressler, Sim and O'Donnell-Megaro 8 ), and in dairy products as a group that derive the bulk of their BCFA from cows' milk. Similar to fluid milk( Reference Ran-Ressler, Sim and O'Donnell-Megaro 8 ), dairy products included both iso and anteiso types of BCFA with chain lengths ranging from 14 to 17 carbons, with very little iso-18 : 0. In addition, odd-chain anteiso-BCFA comprise at least half of the total BCFA concentrations in cows' milk-based dairy products. The BCFA profile of the present study is in line with BCFA distribution published previously for European dairy products( Reference Thurnhofer, Lehnert and Vetter 16 , Reference Hauff and Vetter 17 ).
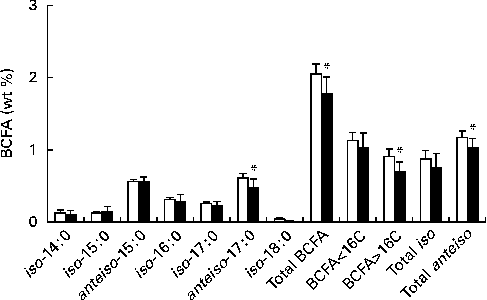
Fig. 2 Description of individual branched-chain fatty acids (BCFA), total BCFA and chain-length distribution of BCFA in fluid milk (□) v. dairy products (■). Fluid milk data were obtained from our previous publication( Reference Ran-Ressler, Sim and O'Donnell-Megaro 8 ). Values are means, with their standard deviations represented by vertical bars. * Mean values were significantly lower than those of the aggregated parameters in retail milk (P< 0·05).
Because introduction of bacteria is an integral part of the production of most dairy products and because bacteria can be a source of BCFA – in addition to the inherent presence of BCFA in fluid milk – we speculated that the total BCFA content and distribution may be different between fluid milk and dairy products, with a tendency or trend to be higher in dairy products. Surprisingly, the collective mean levels of total BCFA, anteiso-17 : 0, the sum of BCFA longer than 16 carbons and the sum of anteiso-BCFA in cows' milk-based dairy products were significantly lower than the mean BCFA levels of these aggregated parameters in retail milk (P< 0·05). These differences are driven by the higher levels of anteiso-17 : 0 in retail milk compared with the collective mean levels of BCFA in cows' milk-based dairy products. The cows' diet can strongly affect the levels of FA and BCFA in milk( Reference Bonanno, Tornambe and Bellina 15 ), and lower levels of BCFA in milk-based dairy products observed in the present study imply that bacterial BCFA may not have the same prominent effect on BCFA levels in dairy products as do diet and production processes. Furthermore, it is possible that milk BCFA used for the production of some of the dairy products contained somewhat lower levels of BCFA than those in average retail milk, masking possible contributions of bacterial BCFA to the final product. Alternatively, the lack of BCFA in bacterial cultures introduced for the production of dairy food products could explain why BCFA concentrations in cows' milk-based dairy products did not increase when compared with those in fluid milk. A literature search for FA composition in known dairy cultures, such as Streptococcus thermophilus and Lactobacillus bulgaricus, did not yield reports on BCFA concentrations in these strains( Reference Veerkamp 2 , Reference Smittle, Gilliland and Speck 18 – Reference Tymczyszyn, Gomez-Zavaglia and Disalvo 20 ), with the exception of one report on the presence of 0·7 wt% anteiso-15 : 0 in Lactobacillus bulgaricus ( Reference Veerkamp 2 ). However, the bacterial culture used in making cheese, for example, is important for production of the texture and flavour expected of various cheeses( Reference Randazzo, De Luca and Todaro 21 ), and thus it is reasonable to assume that different dairy products have different mixtures of bacterial cultures. These cultures can have both BCFA-containing and non-BCFA-containing bacterial strains, and the proportions between them may affect the bacterial contribution of BCFA to different dairy products.
In addition to dairy products, we also analysed the most highly consumed foods from the protein food group according to the USDA's Economic Research Service, Loss-Adjusted Food Availability data( 9 ). Table 2 shows the concentrations of BCFA in food products from the protein food group. Commercial and pastured ground beef had total BCFA concentrations of 1·60 and 1·89 wt%. In a recent survey of retail beef in Canada( Reference Aldai, Dugan and Rolland 22 ), BCFA concentrations ranged between 1·25 and 1·82 wt% depending on the season and the cut, which is consistent with the present results. Similar to cows' milk, BCFA concentrations in beef can be affected by the cattle's diet, the breed( Reference Kraft, Kramer and Schoene 23 , Reference Costa, Lopes and Estevao 24 ) and the cut of the meat within a given breed( Reference Aldai, Dugan and Rolland 22 ). Finally, we noted that production practice such as organic v. conventional dairy products, and wild v. farmed seafood, may all have an influence on the levels of BCFA.
BCFA concentrations were below the detection limit in poultry and pork products, as would be expected if the primary origin of BCFA in meats is from ruminal bacteria. These observations are in line with a previous report on FA concentrations in lard, chicken fat, mutton tallow and beef tallow, using a GC × GC-time of flight mass spectrometer( Reference Chin, Man and Tan 25 ). A wide variety of BCFA were detected in beef and mutton tallow but not in chicken fat, and a small amount of anteiso-17 : 0 was reported in lard.
Previous reports have indicated the presence of small amounts of BCFA in fish( Reference Hauff and Vetter 17 ). Because the current 2010 Dietary Guidelines for Americans recommend consumption of two servings of 114 g (4 oz) each of seafood per week, we investigated the presence of BCFA in canned tuna and salmon, which, among Americans, are the second- and third-most consumed types of seafood (after shrimp), according to the National Oceanic and Atmospheric Administration statistics( 26 ). In addition, these fish are considered to be coldwater fish. Because BCFA modulate membrane properties at low temperatures( Reference Kaneda 1 , Reference Annous, Becker and Bayles 27 ), we speculated that BCFA may be present in their membranes. The present data show that BCFA levels in salmon were below the detection limit, and very small amounts of iso-17 : 0 (0·14 wt%) were detected in canned tuna. Low concentrations of BCFA in fresh tuna and salmon have been reported by others( Reference Hauff and Vetter 17 ), and the observation of iso-BCFA in canned tuna is in agreement with that of the same authors suggesting that the dominant BCFA in fish are odd-numbered carbon iso-BCFA.
Taken together, the aforementioned results indicate that BCFA are found in high concentrations in ruminant products, but not in poultry products and at zero or low amounts in the fish that we examined. Considering that the foods in the present study were the most commonly consumed foods in the US diet, the present data indicate that the main source of BCFA in the dairy and protein food groups originates from ruminant products. In addition, BCFA levels in cows' milk-based dairy products were similar to or lower than those detected in retail cows' milk.
BCFA levels were also detected in fermented products such as sauerkraut and miso (Table 3). These products undergo fermentation with various fungi and bacteria, both of which may serve as a source of BCFA in these products. Miso is processed by using the fungus Aspergillus oryzae, a variety of bacterial strains such as Enterococcus and Pediococcus, as well as Lactobacillus and Weissella ( Reference Onda, Yanagida and Tsuji 28 ). In the present study, total BCFA concentrations in miso were found to be 0·54 wt%. These included a small amount of iso-15 : 0 (0·08 wt%); the rest (0·46 wt%; Table 3) was contributed by a unique BCFA with a putative assignment of 2-methyl hexadecanoic acid. This rare BCFA was also present at a lower concentration in sauerkraut (0·14 wt%; Table 3). Park et al. ( Reference Park, Kim and Abell 29 ) reported the presence of Weissella in sauerkraut, which may explain the presence of this BCFA in sauerkraut and miso. A study on FA distribution in Weissella strains did not report BCFA( Reference Samelis, Rementzis and Tsakalidou 30 ).
BCFA were, however, not detected in other fermented food products such as kimchi or tempeh. Kimchi, for example, contains many different bacterial genera( Reference Park, Chun and Cha 31 ), and thus it is likely that BCFA present in some strains were diluted to below the detection limit by the overwhelming amounts of bacteria with normal FA. In addition, various processing methods may also influence bacterial ecology in kimchi( Reference Park, Chun and Cha 31 ) and the detection of BCFA in other fermented food products. However, we did not attempt here to do an exhaustive sampling of fermented foods.
Small amounts of BCFA (0·15 wt%) were detected in milk chocolate. The two BCFA detected in chocolate had 17 carbons, iso-17 : 0 (0·07 wt%) and anteiso-17 : 0 (0·08 wt%) (data not shown). The ratio between iso-17 : 0 and anteiso-17 : 0 in chocolate (1:1) is different from their ratio in milk (1:2 or more). This and the absence of anteiso-15 : 0, a predominant BCFA in milk, implies that the milk ingredient in chocolate is not solely responsible for the presence of BCFA in chocolate, and there may be another source of these two BCFA in chocolate.
Branched-chain fatty acid intake of Americans compared with consumption of other bioactive fatty acids
In the present study, the contribution of BCFA to the nutrition of Americans was estimated from measured and estimated intakes of the dairy and protein food products. The intake data were estimated using the USDA's Economic Research Service, Loss-Adjusted Food Availability data( 9 ). The nutritional contribution of BCFA from various dairy and protein food groups analysed in the present study and their data on a per capita consumption basis that is greater than 1 g/d are given in Table 4 . The contribution of the selected foods, presented in Table 4, to the consumption of BCFA by the US population reaches to a level of about 492 mg/capita per d. Americans consume about 317 mg BCFA from dairy products and about 170 mg BCFA from beef. Consumption of chocolate contributes about 6 mg BCFA/d. Concentrations of BCFA were estimated to be 0·6 and 1·9 wt% from the total daily intake of 76·8 g fat and 25·5 g saturated fat, respectively, for American children aged 2 years and older.
Table 4 Estimated per capita intake of branched-chain fatty acids (BCFA) from the dairy and protein food groups in the US population

* Data on food consumed are obtained from the USDA( 9 ), and data on fat content taken from the USDA National Nutrient Database for Standard Reference, Release 25 (http://www.ars.usda.gov/Services/docs.htm?docid=8964), and USDA-ARS 2012. Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2009–2010. Available at www.ars.usda.gov/ba/bhnrc/fsrg. Both databases accessed December 2012.
† For estimation of intake, the following were calculated and used: beef – mean BCFA levels in retail and small-farm pastured beef; yogurt – mean BCFA in two major styles (plain yogurt and Greek yogurt); tuna – mean fat concentrations in tuna canned in oil and tuna canned in water.
‡ Per capita consumption data were obtained from the National Research Council( 43 ).
§ Per capita consumption data in the USA were obtained from Seligson et al. ( Reference Seligson, Krummel and Apgar 44 ).
Notable examples of foods excluded from Table 4 are as follows: goat cheese, which contains relatively large amounts of fat (27 %) and BCFA (2·2 wt%) but has no per capita consumption data; miso, whose per capita consumption is not tabulated; sauerkraut, whose per capita consumption is very small ( < 0·4 g/d) and fat contribution is negligible.
The estimated per capita BCFA intake of 492 mg/d from the dairy and protein food groups is greater than the average DHA and EPA consumption of 100 mg/d reported in a survey of 8604 Americans between 1999 and 2000 and in women of childbearing age, based on NHANES III data( Reference Ervin, Wright and Wang 32 , Reference Brenna and Lapillonne 33 ). The estimated per capita intake of BCFA is higher than the recommended intake of DHA and EPA for pregnant and lactating women( Reference Brenna and Lapillonne 33 ). Therefore, the consumption of BCFA from the dairy and protein food products is almost five times the consumption of DHA and EPA in the US population (Fig. 3). As a reference, DHA intake of 200 mg/d, and 300 mg DHA+EPA (combined), is recommended by the FAO and WHO for pregnant and lactating women to support infant development( 34 ).
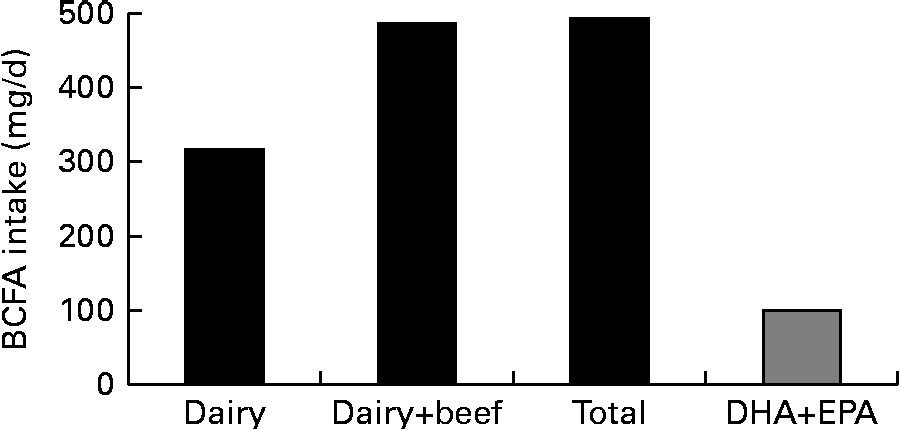
Fig. 3 Daily branched-chain fatty acid (BCFA) intake v. intake of bioactive fatty acids DHA and EPA, calculated from the US Department of Agriculture's intake data. See text for details.
For children aged 2–11 years, the mean intake of beef is 43·5 g/d( Reference Daniel, Cross and Koebnick 35 ). Children who consume 28 g (1 oz) of cows' milk-based cheese with an average of 25 % fat, and 43·5 g/d of cooked beef with an average of 18 % fat, both with an average BCFA concentration of 1·8 wt%, consume about 267 mg BCFA/d. The addition of one cup of whole milk (156 mg BCFA) increases their BCFA intake to 423 mg/d. For comparison, the mean intakes of DHA and arachidonic acid in Canadian children, aged 4–7 years, have recently been estimated to be 37 and 57 mg/d( Reference Lien and Clandinin 36 ); both DHA and arachidonic acid combined to provide about one-fifth of the daily BCFA consumption from milk, cheese and beef.
Taken together, the aforementioned calculations imply that BCFA are being consumed in substantial amounts by most non-vegans, during different life stages, and their consumption exceeds the consumption of bioactive FA.
The present data indicate that intake of BCFA can exceed the estimated per capita consumption of 492 mg/d by at least 2-fold with common intakes of popular foods. For example, daily consumption of 150 g (5·3 oz) of cooked ground beef (18 % fat, with 1·8 wt% BCFA on average), two cups (473 ml) of whole milk (3·25 % fat, 2 wt% BCFA) and 57 g (2 oz) of cows' milk-based cheese (25 % fat, 1·8 wt% BCFA on average) contains about 1050 mg BCFA. If cows' milk-based cheese was replaced with sheep cheese (31 % fat and 2·7 wt% BCFA), then meal BCFA concentration would exceed 1700 mg or 3-fold the average intake.
The 2010 Dietary Guidelines for Americans( 37 ) recommend that Americans consume low-fat ruminant products in order to lower the consumption of saturated fat and to reduce the risk of metabolic diseases. The present results indicate that a higher intake of BCFA is due to the consumption of animal fat and more specifically to the intake of foods of ruminant origin, with the leading foods in the American diet being dairy and beef food products. A recent meta-analysis of prospective cohort studies has failed to find a significant relationship between dietary saturated fat intake and the risk of CVD( Reference Siri-Tarino, Sun and Hu 38 ). Others( Reference Pereira, Jacobs and Van Horn 39 ) have shown that increased consumption of high-fat dairy products did not significantly increase the OR for obesity, abnormal glucose homeostasis, dyslipidaemia, elevated blood pressure or insulin resistance. In fact, the OR for these disorders were found to be lower with increasing intake of both reduced-fat and high-fat dairy products, and inverse associations were observed for both reduced-fat and high-fat dairy products. Another study has reported a non-significant trend towards lower risk for first myocardial infarction with consumption of high-fat dairy products compared with consumption of low-fat dairy products( Reference Lockheart, Steffen and Rebnord 40 ). Another meta-analysis has found no association between animal fat intake and the risk of colorectal cancer( Reference Alexander, Cushing and Lowe 41 ). Others( Reference Lin, Zhang and Cook 42 ) have shown that intake of saturated fat from an animal source was not significantly associated with the risk of colorectal cancer in women; in that study, consumption of red meat was found to be negatively associated with the risk of colorectal cancer. These studies highlight evolving science on saturated fat intake and disease risks, indicating that the effect of saturated fat or animal fat consumption on metabolic diseases and cancer is complex.
BCFA are normal constituents present in the gut from a very early age and throughout the human life cycle. Previous studies have shown that BCFA are not inert components of the gastrointestinal tract and are metabolised by the enterocytes. BCFA play a beneficial role against inflammation in the premature intestine, alter the microbiota and increase the expression of anti-inflammatory cytokines in an animal model. These studies have shown that BCFA may have a beneficial effect on proper gut functions; thus, their intake in the population becomes relevant. The present study was the first to investigate a wide range of food products consumed by the US population. The present data show that the per capita daily consumption of BCFA is substantial and above the consumption of bioactive n-3 FA such as EPA and DHA. The prominence of BCFA in the US food supply and their bioactivity strongly suggest that the effects of BCFA on health should be studied.
Acknowledgements
The present study was supported by NIH grants T32 HD007331 and R21 HD064604.









