Numerous studies have shown an increased risk of developing substance use disorders and nicotine dependence in patients with attention-deficit hyperactivity disorder (ADHD). A meta-analysis showed that a childhood diagnosis of ADHD increased the risk of developing substance use disorders and nicotine use. Reference Charach, Yeung, Climans and Lillie1 Although some studies suggest that the increased risk of developing substance use disorders in ADHD is completely dependent on the presence of comorbid conduct disorder and/or oppositional defiant disorder, Reference Flory and Lynam2,Reference Harty, Ivanov, Newcorn and Halperin3 other studies found that ADHD remains a risk factor after adjustment for these disorders. Reference Biederman, Wilens, Mick, Faraone, Weber and Curtis4-Reference Groenman, Oosterlaan, Rommelse, Franke, Roeyers and Oades6 The risks described are substantial and emphasise the need for early intervention to prevent these negative outcomes of a childhood diagnosis of ADHD. Stimulant therapy is the first-choice medication treatment in patients with ADHD. Reference Graham, Banaschewski, Buitelaar, Coghill, Danckaerts and Dittmann7 Since stimulants have the potential to be addictive drugs, concerns have been raised regarding the effects of stimulant treatment on the later development of substance use disorders in ADHD. Reference Goldman, Genel, Bezman, Slanetz and Assoc8 These concerns are mainly based on the sensitisation hypothesis. This theory states that exposure to stimulants alters the dopamine system in such a way that an increased sensitivity is established to the reinforcing effects of previously experienced drugs. This, in turn, may result in an increased risk of developing substance use disorders and nicotine dependence. Interestingly, most evidence for this hypothesis comes from animal studies. Reference Steketee and Kalivas9 So far, the harmful effect predicted by the sensitisation hypothesis on the development of substance use disorders has only been reported by a single study in humans. Reference Lambert and Hartsough10 It should be noted that the results of that study may have been confounded by a larger number of participants with comorbid conduct disorder in the stimulant-exposed group compared with the stimulant-naive group. An alternative hypothesis to the sensitisation hypothesis posits that stimulant treatment protects against substance use disorders and nicotine dependence by decreasing the core symptoms of ADHD (such as impulsivity and poor planning) and associated problems (such as poor self-esteem, school failure, academic or occupational failure) that lead to drug, alcohol and nicotine use. Reference Wilens11 This hypothesis is supported by several studies (for example Katusic et al, Reference Katusic, Barbaresi, Colligan, Weaver, Leibson and Jacobsen12 Wilens et al Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg13 ) and a meta-analysis Reference Wilens11 that showed protective effects of stimulant treatment on the later development of nicotine use and substance use disorders. Interestingly, some studies, that evaluated participants at a higher mean age, did not find any effect of stimulant treatment on the development of substance use disorders and nicotine dependence. Reference Faraone, Biederman, Wilens and Adamson14-Reference Barkley, Fischer, Smallish and Fletcher16 Meta-analytic evidence suggests that the protective effect of stimulant treatment is indeed much larger in adolescence (odds ratio (OR) = 5.8), than in early adulthood (OR = 1.4). Reference Wilens11 Several other factors might influence the effects of stimulant treatment on substance use disorders. One study found that stimulant therapy only influences the development of substance misuse in boys, but not girls. Reference Katusic, Barbaresi, Colligan, Weaver, Leibson and Jacobsen12 However, a different study also found this effect in girls. Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg17 Furthermore, an earlier age of stimulant initiation Reference Mannuzza, Klein, Truong, Moulton, Roizen and Howell18 and a longer duration of stimulant use Reference Barkley, Fischer, Smallish and Fletcher16 have been reported to have a protective effect on the development of substance use disorders; however, other studies did not replicate these findings. Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg13,Reference Molina, Hinshaw, Eugene Arnold, Swanson, Pelham and Hechtman19
To our knowledge this is the first prospective, longitudinal study of European origin investigating the effect of stimulant medication on the development of substance use disorder and nicotine dependence in ADHD. We also sought to assess the effects of specific characteristics and moderators of stimulant treatment (for example age at treatment initiation, duration of treatment, cumulative dose) on the development of substance use disorders and nicotine dependence. We describe a 4-year follow-up of a large sample of well-defined probands with combined type ADHD, their affected siblings and healthy controls.
Method
Individuals participating in this study were recruited as part of the Belgian (n = 41), Dutch (n = 537) and German (n = 21) International Multicenter ADHD Genetics (IMAGE) study. Reference Brookes, Xu, Chen, Zhou, Neale and Lowe20 Probands with ADHD aged 5-17 years had been recruited from out-patient clinics at the data-collection sites between 2003 and 2006. Participants had to be White and of European descent. Exclusion criteria applying to both probands and siblings included autism, epilepsy, IQ <70, brain disorders and any genetic or medical disorder associated with externalising behaviours that might mimic ADHD. In addition, healthy control participants were recruited from primary and high schools from the same geographical regions as the participating families with ADHD.
In 2008 and 2009 participants were re-invited to participate in the current follow-up study, on average 4.4 years (s.d. = 0.7) after study entry. A total of 505 participants with a baseline diagnosis of ADHD (both probands and affected siblings) and 223 healthy control participants above the age of 12 participated in the follow-up. For 599/728 (82.3%) of these children, information on medication use history were available (i.e. rating of medication use (yes or no) was available). Ethical approval for the study was obtained from the National Institutes of Health registered ethical review boards for each centre. After a complete description of the study, written informed consent was obtained from both parents and children.
Assessment of ADHD, oppositional defiant disorder and conduct disorder at baseline
Baseline measures included the Long Version of Conners' Parent (CPRS-R:L), and Teacher Rating Scale (CTRS-R:L), Reference Conners, Sitarenios, Parker and Epstein21 which were used to quantify ADHD symptoms. Parents and teacher were asked to describe the child's behaviour in a medication-free period when filling out the questionnaire. For a full account of the measures used in IMAGE, see Müller et al. Reference Müller, Asherson, Banaschewski, Buitelaar, Ebstein and Eisenberg22 T-scores ⩾63 on the Conners ADHD subscales (L, M and N) were considered clinical. The CPRS-R:L also assesses symptoms related to oppositional defiant disorder (for example angry and resentful, argues with adults, loses temper, irritable, temper outbursts) on a four-point ordinal scale.
The Parental Account of Childhood Symptoms (PACS) Reference Chen, Taylor and Oades23 interview was administered if scores on the Conners ADHD rating scales were considered clinical. The PACS is a semi-structured, standardised, investigator-based interview developed to provide an objective measure of child behaviour. A trained interviewer administered the PACS to the parents, who were asked for detailed descriptions of the child's typical behaviour in a range of specified situations. Among others, the PACS covers the DSM-IV 24 symptoms of ADHD, conduct disorder and oppositional defiant disorder (for a detailed description of the interview procedure, see Brookes et al Reference Brookes, Xu, Chen, Zhou, Neale and Lowe20 ).
Categorical measures of ADHD, oppositional defiant disorder and conduct disorder were created. Attention-deficit hyperactivity disorder was defined using a standardised algorithm applied to combine symptom counts on the PACS and CTRS-R:L, both providing operational definitions of each of the 18 behavioural ADHD symptoms defined by the DSM-IV. Attention-deficit hyperactivity disorder symptom count was used as a measure of ADHD severity. Situational pervasiveness of ADHD was defined as at least two symptoms being present in two or more different situations as assessed with the PACS interview, as well as the presence of one or more items scored as two or three or more from the ADHD scale of the CTRS-R:L. Oppositional defiant disorder and conduct disorder were defined according to the DSM-IV criteria based on information from the PACS.
Follow-up measures
A parental report of substance use disorders was obtained using the substance use disorder module of the Diagnostic Interview Schedule for Children (DISC-IV-P). Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone25 The DISC-IV-P was administered by telephone interview, and scored with a computer-based algorithm to derive DSM-IV-defined substance use disorder diagnoses. Age at first substance use was assessed in the interview. Participants above the age of 12 completed a number of questionnaires. The Alcohol Use Disorders Identification Test (AUDIT) Reference Saunders, Aasland, Babor, de la Fuente and Grant26 was used to identify self-reported alcohol dependence. Scores on the AUDIT range from 0 to 40. A score of 9 or higher was used to define alcohol abuse, and a score of 13 or more in girls and 15 or more in boys was used as a cut-off to define alcohol dependence. Reference Saunders, Aasland, Babor, de la Fuente and Grant26 The Drug Abuse Screening Test-20 (DAST) Reference Gavin, Ross and Skinner27 was used to assess drug use disorders. Scores on this questionnaire range from 0 to 20. A cut-off of five was used to identify possible drug use disorders. Reference Gavin, Ross and Skinner27 The Fagerström Test for Nicotine Dependence (FTND) Reference Heatherton, Kozlowski, Frecker and Fagerstrom28 was used to assess nicotine dependence. Scores on this questionnaire vary between zero and ten. A cut-off of six was used to identify nicotine dependence. Reference Heatherton, Kozlowski, Frecker and Fagerstrom28 Age at first nicotine use was also assessed in this questionnaire.
To create best estimate diagnoses of substance use disorders, these were considered present if scores on either self- or parent-report measures met criteria as stated above. We created summary diagnostic groups to aggregate diagnostic information across instruments and informants. For alcohol use disorder, the AUDIT and alcohol module of the DISC-IV-P were used, for drug use disorder, the DAST and the marihuana and other drugs module of the DISC-IV-P were used. Alcohol use disorder and drug use disorder were collapsed into one category to form an overall measure of substance use disorders, to increase reliability of the measure and reduce the number of statistical tests. For nicotine dependence the FTND and the tobacco module of the DISC-IV-P were used. Two main dependent variables were used: an overall measure of substance use disorders and one measure of nicotine dependence.
Medication history was assessed using parental report of medication use combined with pharmacy records. Predictors derived from this information are previous and/or current stimulant use (yes/no), current use of stimulants (currently on medication yes/no), age at stimulant treatment initiation, age-adjusted duration of stimulant use (defined as the percentage of time treated with stimulants since the onset of ADHD), and age-adjusted cumulative dosage of stimulants (defined as dosage corrected for number of days since the onset of ADHD).
Statistical analyses
All analyses were conducted using SPSS (IBM SPSS Statistics version 20 for Windows). Differences between groups in gender, age, IQ, ADHD severity, and conduct disorder and/or oppositional defiant disorder were examined using analysis of variance and chi-squared tests. Differences between participants successfully followed up and those lost to follow-up for gender, age ADHD severity and conduct disorder and/or oppositional defiant disorder were examined using t-test and chi-squared tests.
The possible effects of stimulant treatment on the development of drug- and alcohol-related substance use disorders and nicotine dependence were studied using Cox proportional hazard models. The models used age at first substance use as the survival time for the ‘cases’ (classified as having a substance use disorder and/or nicotine dependence) and current age as the time of censoring for the ‘non-cases’. Correction for clustered (family) data was done using robust standard errors. Reference Huber29 Three groups were included in this analysis: participants with a childhood diagnosis of ADHD who were stimulant-naive (n = 30) and participants with a short or inconsistent history of stimulant medication never exceeding 12 months (n = 31, n = 61 no-stimulant treatment group); participants with a childhood diagnoses of ADHD with a history of stimulant medication longer than 12 months (n = 327, stimulant treatment group) and a healthy control group (n = 211).
Differences in the number with substance use disorder and nicotine dependence between the participants from Germany (n = 21), Belgium (n = 41) and The Netherlands (n = 537) were examined using generalised estimated equations (GEE) Reference Norton, Bieler, Ennett and Zarkin30 robust estimators and exchangeable structure for working correlation matrices.
Within-group analyses were performed to evaluate the potential subtle effects of stimulant medication on the development of substance use disorders and nicotine dependence. A logistic regression model was fitted using GEE, Reference Norton, Bieler, Ennett and Zarkin30 robust estimators and exchangeable structure for working correlation matrices. All participants with a childhood diagnosis of ADHD and any history of stimulant medication were included in these analyses (n = 358). Any substance use disorder or nicotine dependence were used as the dependent measure. Our data-analytic approach was similar to that suggested by Hosmer & Lemeshow. Reference Hosmer and Lemeshow31 In short, several steps were taken to identify potential predictors of substance use disorders and nicotine dependence.
-
(a) Initially, all possible predictor and possible confounding variables (i.e. current use of stimulants, age at stimulant treatment initiation, age-adjusted duration of stimulant use and age-adjusted cumulative dosage of stimulants, ADHD, oppositional defiant disorder and conduct disorder symptom count at baseline, gender and age at follow-up) were analysed using a univariate approach. Correlations between predictor variables were calculated to assess whether the assumption of multicollinearity (r>0.80) was violated.
-
(b) All predictors with a P<0.20 and variables with known clinical importance were included in a multivariate model.
-
(c) Predictors with P>0.05 were dropped from the model if this positively influenced the overall fit of the model. To assess the fit of the model the quasi-likelihood under independence model criterion (QIC) was used. Reference Pan32 We will refer to this model as the initial main effects' model.
-
(d) We checked whether any meaningful interactions among the main effects improved the fit of the model.
Results
Attrition and demographics characteristics
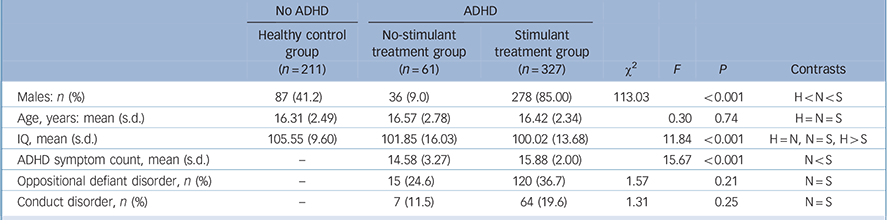
At baseline, among participants with ADHD and controls, there were no significant differences between those successfully followed up and those lost to follow-up on age (t = 0.196, P = 0.845) and gender (χ2 = 3.412, P = 0.065). At baseline, no differences were found among the participants with ADHD followed up and those lost to follow-up on ADHD severity (t = 0.1.533, P = 0.126) and presence of conduct disorder (χ2 = 114, P = 0.735) and oppositional defiant disorder (χ2 = 0.089, P = 0.766). No differences were found in the number with substance use disorder and nicotine dependence between the participants from Germany, Belgium and The Netherlands (respectively Wald χ2 = 3.379, P = 0.337 and Wald χ2 = 3.677, P = 0.299). Table 1 describes demographic and clinical features of the three groups (healthy controls, no-stimulant treatment and stimulant treatment group). The stimulant and no-stimulant groups did not differ in the number of participants who met criteria for oppositional defiant disorder or conduct disorder, none of the healthy control participants were assessed for oppositional defiant disorder or conduct disorder. The three groups did not differ on current age. Controls had a significantly higher IQ than the stimulant ADHD group. Furthermore, the stimulant and no-stimulant groups differed in ADHD severity, in that the no-stimulant group had lower ADHD symptom counts. The ADHD symptom count was assessed over a medication-free period. Finally, the stimulant-treated group were more likely to be male. In the subsequent Cox proportional hazard models we therefore statistically adjusted for gender and current age. Although no difference was found between the ADHD groups in the prevalence of oppositional defiant disorder and conduct disorder, separate models, including the no-stimulant and stimulant treatment groups, were built that corrected for oppositional defiant disorder, conduct disorder and ADHD severity, to completely rule out their effects.
Overall effect of stimulant medication
Table 2 displays prevalence rates and hazard ratios (HRs) for substance use disorders and nicotine dependence for the healthy control, no-stimulant treatment and stimulant groups. The no-stimulant treatment group had a 2.6 times higher risk of developing a substance use disorder when compared with the healthy control group, and had a 2 times higher risk of developing a substance use disorder than the stimulant treatment group. No significant differences were found between the stimulant treatment and the healthy control group (Fig. 1(a)). Both the stimulant treatment (HR = 3.56) and the no-stimulant treatment group (HR = 3.83) had an increased risk of developing nicotine dependence compared with the healthy control group. No differences were found between the no-stimulant treatment and stimulant treatment group in their risk for nicotine dependence (Fig. 1(b)).
Analyses between the stimulant treatment and the no-stimulant treatment group were re-run including oppositional defiant disorder, conduct disorder and ADHD severity at baseline as covariates, to rule out any role of these measures on the observed protective effect of stimulant treatment on the development of substance use disorders. The control group was not included in these analyses because the PACS was not administered if scores on the CPRS-R:L and CTRS-R:L were not considered clinical (for a detailed description of the interview procedure, see Brookes et al Reference Brookes, Xu, Chen, Zhou, Neale and Lowe20 ). Results remained essentially unchanged: the protective effect of stimulant treatment on the development of substance use disorders proved not to be dependent on oppositional defiant disorder, conduct disorder or ADHD severity (no-stimulant treatment v. stimulant treatment: HR = 1.91, 95% CI 1.10-3.36), neither did results concerning nicotine dependence (no-stimulant treatment v. stimulant treatment: HR = 1.12, 95% CI 0.45-2.96).
Table 1 Participant characteristics
| No ADHD | ADHD | ||||||
|---|---|---|---|---|---|---|---|
| Healthy control group (n = 211) |
No-stimulant treatment group (n = 61) |
Stimulant treatment group (n = 327) |
χ2 | F | P | Contrasts | |
| Males: n (%) | 87 (41.2) | 36 (9.0) | 278 (85.00) | 113.03 | <0.001 | H<N<S | |
| Age, years: mean (s.d.) | 16.31 (2.49) | 16.57 (2.78) | 16.42 (2.34) | 0.30 | 0.74 | H = N = S | |
| IQ, mean (s.d.) | 105.55 (9.60) | 101.85 (16.03) | 100.02 (13.68) | 11.84 | <0.001 | H = N, N = S, H>S | |
| ADHD symptom count, mean (s.d.) | - | 14.58 (3.27) | 15.88 (2.00) | 15.67 | <0.001 | N<S | |
| Oppositional defiant disorder, n (%) | - | 15 (24.6) | 120 (36.7) | 1.57 | 0.21 | N = S | |
| Conduct disorder, n (%) | - | 7 (11.5) | 64 (19.6) | 1.31 | 0.25 | N = S | |
ADHD, attention-deficit hyperactivity disorder; N, no-stimulant treatment group; S, stimulant treatment group; H, healthy control group.
Table 2 Prevalence rates of substance use disorders and nicotine dependence in participants with attention-deficit hyperactivity disorder with and without a history of stimulant therapy, and healthy controls
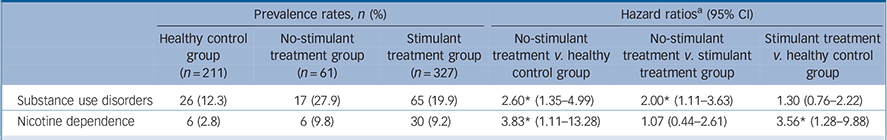
| Prevalence rates, n (%) | Hazard ratiosFootnote a (95% CI) | |||||
|---|---|---|---|---|---|---|
| Healthy control group (n = 211) |
No-stimulant treatment group (n = 61) |
Stimulant treatment group (n = 327) |
No-stimulant treatment v. healthy control group |
No-stimulant treatment v. stimulant treatment group |
Stimulant treatment v. healthy control group |
|
| Substance use disorders | 26 (12.3) | 17 (27.9) | 65 (19.9) | 2.60Footnote * (1.35-4.99) | 2.00Footnote * (1.11-3.63) | 1.30 (0.76-2.22) |
| Nicotine dependence | 6 (2.8) | 6 (9.8) | 30 (9.2) | 3.83Footnote * (1.11-13.28) | 1.07 (0.44-2.61) | 3.56Footnote * (1.28-9.88) |
a. Hazard ratios were calculated using Cox proportional hazard regression. All comparisons were corrected for gender and current age.
* Significant at P<0.05.
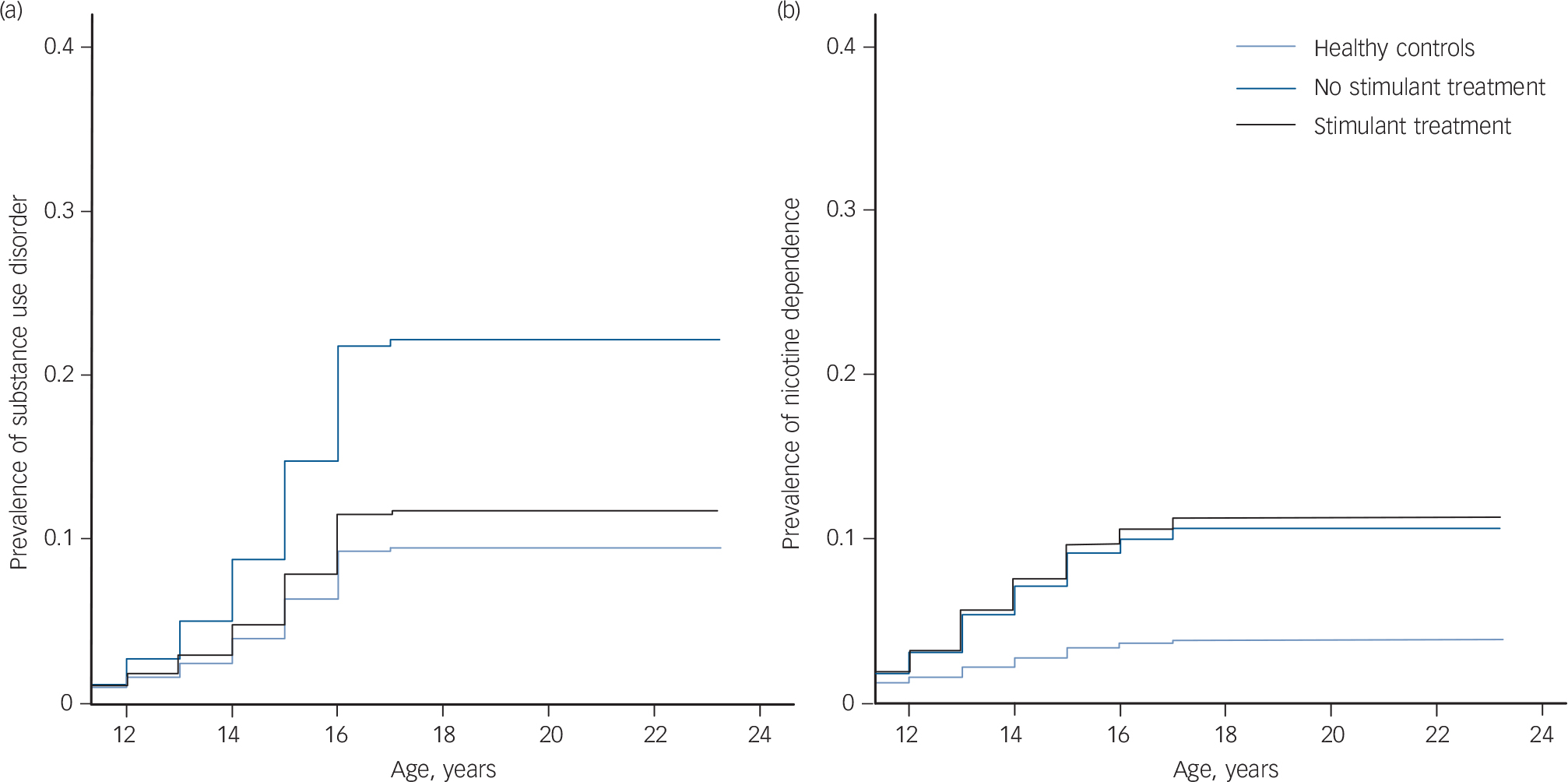
Fig. 1 Cumulative lifetime risk for (a) any substance use disorder and (b) nicotine dependence.
Survival curves were calculated using Cox proportional hazard regression. All comparisons were corrected for gender and current age.
Predictors of substance use disorders and nicotine dependence in the stimulant-treated group
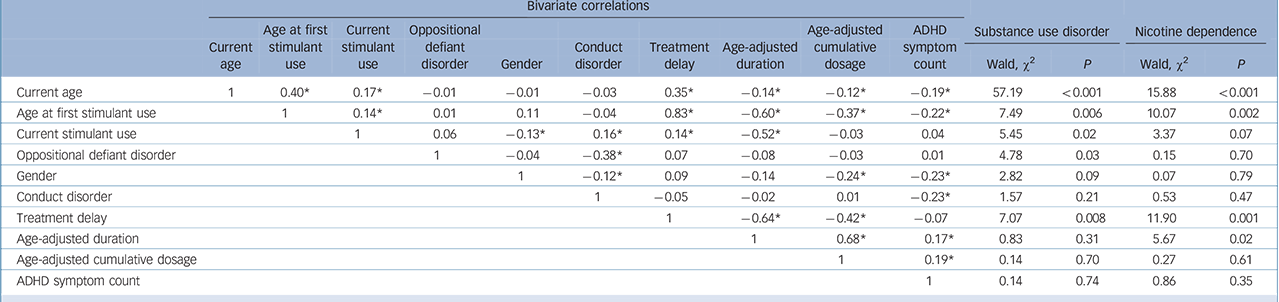
Correlations and results of the univariate analyses are displayed in Table 3. In the initial main effects' model for substance use disorders, seven main effects were retained, namely: current age, age at first stimulant use, treatment delay, current use of stimulants, oppositional defiant disorder, conduct disorder and gender. Because treatment delay and age at first stimulant use were highly correlated (r = 0.83), two models were built including all main effects and either age at first stimulant use or treatment delay. The main effects' model with the best fit indicated by QIC was the model including age at first stimulant use, oppositional defiant disorder and current age. Evaluation of this model showed that including oppositional defiant disorder, age at first stimulant use, current age, the interaction between age at first stimulant use and current age led to the most parsimonious model (QIC = 217.79). The protective effect of earlier age at first stimulant use on the development of a substance use disorder was found to decrease with increasing age (OR = 0.95, Wald χ2 = 13.78, P<0.001, Fig. 2).
Table 3 Bivariate correlations and univariate outcomes of possible predictors for substance use disorder and nicotine dependenceFootnote a
| Bivariate correlations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current age |
Age at first stimulant use |
Current stimulant use |
Oppositional defiant disorder |
Gender | Conduct disorder |
Treatment delay |
Age-adjusted duration |
Age-adjusted cumulative dosage |
ADHD symptom count |
Substance use disorder | Nicotine dependence | |||
| Wald, χ2 | P | Wald, χ2 | P | |||||||||||
| Current age | 1 | 0.40Footnote * | 0.17Footnote * | –0.01 | –0.01 | –0.03 | 0.35Footnote * | –0.14Footnote * | –0.12Footnote * | –0.19Footnote * | 57.19 | <0.001 | 15.88 | <0.001 |
| Age at first stimulant use | 1 | 0.14Footnote * | 0.01 | 0.11 | –0.04 | 0.83Footnote * | –0.60Footnote * | –0.37Footnote * | –0.22Footnote * | 7.49 | 0.006 | 10.07 | 0.002 | |
| Current stimulant use | 1 | 0.06 | –0.13Footnote * | 0.16Footnote * | 0.14Footnote * | –0.52Footnote * | –0.03 | 0.04 | 5.45 | 0.02 | 3.37 | 0.07 | ||
| Oppositional defiant disorder | 1 | –0.04 | –0.38Footnote * | 0.07 | –0.08 | –0.03 | 0.01 | 4.78 | 0.03 | 0.15 | 0.70 | |||
| Gender | 1 | –0.12Footnote * | 0.09 | –0.14 | –0.24Footnote * | –0.23Footnote * | 2.82 | 0.09 | 0.07 | 0.79 | ||||
| Conduct disorder | 1 | –0.05 | –0.02 | 0.01 | –0.23Footnote * | 1.57 | 0.21 | 0.53 | 0.47 | |||||
| Treatment delay | 1 | –0.64Footnote * | –0.42Footnote * | –0.07 | 7.07 | 0.008 | 11.90 | 0.001 | ||||||
| Age-adjusted duration | 1 | 0.68Footnote * | 0.17Footnote * | 0.83 | 0.31 | 5.67 | 0.02 | |||||||
| Age-adjusted cumulative dosage | 1 | 0.19Footnote * | 0.14 | 0.70 | 0.27 | 0.61 | ||||||||
| ADHD symptom count | 1 | 0.14 | 0.74 | 0.86 | 0.35 | |||||||||
ADHD, attention-deficit hyperactivity disorder.
a. Correlations were calculated using Pearson correlation coefficient with the exception of oppositional defiant disorder, gender and conduct disorder; these correlations were calculated using Spearman rank correlation.
* Significant at P<0.05.
The initial main effects' model for nicotine dependence retained five possible predictors: current age, age at first stimulant use, age-adjusted duration of stimulant use, current use and symptom count at baseline. It appeared that including current age and age-adjusted duration of stimulant use led to the most parsimonious model (QIC = 163.66). Higher current age was significantly related to an increased risk of developing nicotine dependence (OR = 1.17, Wald χ2 = 10.89, P = 0.001), whereas percentage of time treated was not significantly associated with the risk of developing nicotine dependence (OR = 0.99, Wald χ2 = 3.77, P = 0.052).
Discussion
Main findings
The current study investigated the effects of stimulant medication on the development of alcohol- and drug-related substance use disorders and nicotine dependence in ADHD. A protective effect of stimulant therapy on the development of the substance use disorders was found. No difference was found in the risk of developing substance use disorder between the stimulant therapy group and the healthy controls, suggesting normalisation. In contrast, no difference in the risk of developing nicotine dependence was found between participants not treated with stimulants and participants who were treated. Specific moderators were investigated in order to further unravel the mechanisms through which stimulant use influences the later development of substance use disorders. It was found that children who start stimulant medication at a younger age are better protected against the later development of substance use disorders. However, the effect of age at first stimulant use on substance use disorder development diminished with age, and seemed to reverse around the age of 18.
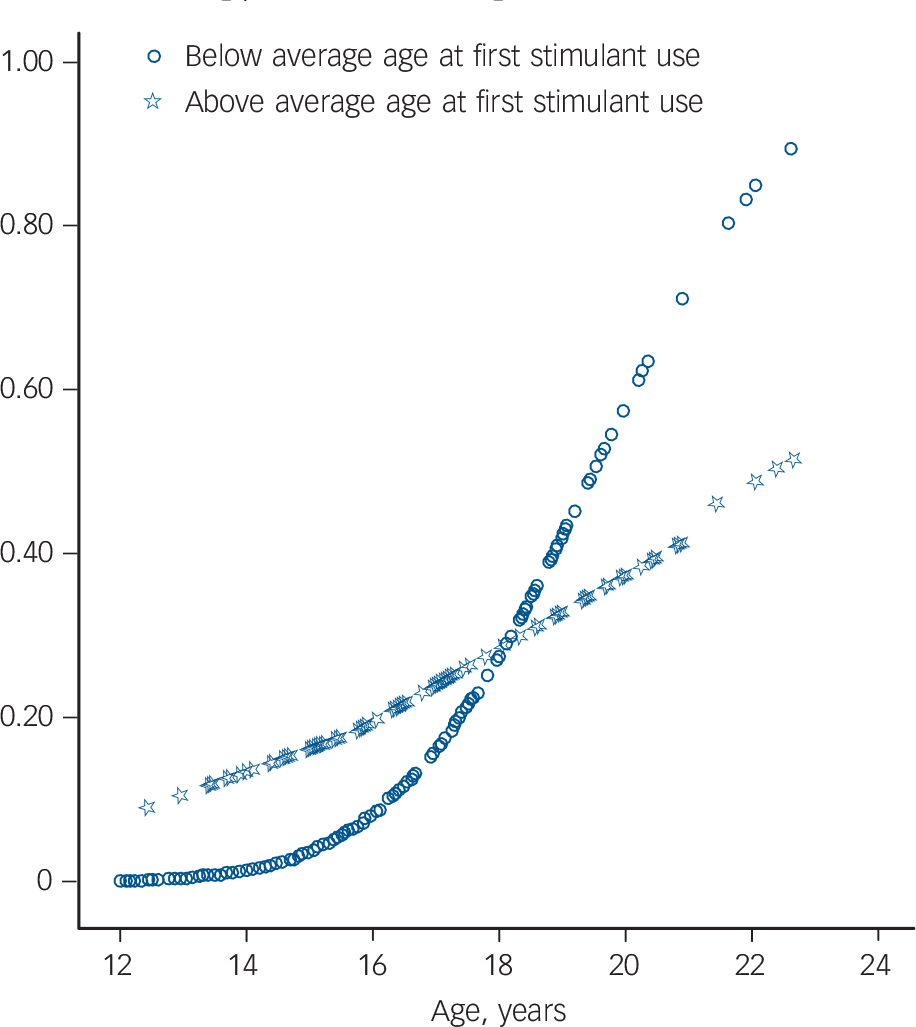
Fig. 2 Predicted probability of substance use disorders within stimulant-treated group with attention-deficit hyperactivity disorder.
Predicted probability of substance use disorder according to generalised estimated equations model, that included age, gender, and the interaction age×age at first stimulant use. Below average age at first stimulant use: participants started before age 8.1 years; above average age at first stimulant use: participants started after age 8.1 years.
Our results argue against the sensitisation hypothesis. This hypothesis states that stimulant therapy would increase the risk of developing substance use disorders and nicotine dependence in ADHD, by increasing the sensitivity to substances, through alterations in the dopamine system. Rather, our results support previous findings that stimulant therapy has a protective effect on the development of substance use disorders. Reference Wilens11-Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg13 The protective effect of stimulant treatment on the development of substance use disorder could not be explained in terms of the impact of comorbid oppositional defiant disorder or conduct disorder symptoms and ADHD severity, as findings remained essentially unchanged when adjusting for these possible confounds. Furthermore, we found that the stimulant treatment group did not significantly differ in the risk of developing substance use disorders compared with the healthy control group, whereas the no-stimulant treatment group did. This suggests normalisation of the risk of developing substance use disorders in the stimulant treatment group, but not in the no-stimulant group. As outlined above, possibly, stimulant treatment may protect against substance use disorders by decreasing the core symptoms of ADHD (for example impulsivity) and associated problems (for example poor self-esteem, school failure, academic or occupational failure) that may lead to drug and alcohol use. Reference Wilens11 Although our results show a less robust protective effect of stimulant therapy on the development of substance use disorders (HR = 2.00) than indicated by an earlier meta-analysis by Wilens et al Reference Wilens, Faraone, Biederman and Gunawardene33 (HR = 5.8), our findings are of great clinical significance. The present study shows that the participants treated with stimulants were two times less likely to develop substance use disorders than participants that did not receive stimulant treatment.
Interestingly, previous studies have shown that the protective effect of stimulant treatment on the development of substance use disorders is much stronger in adolescence Reference Biederman, Wilens, Mick, Faraone, Weber and Curtis4,Reference Katusic, Barbaresi, Colligan, Weaver, Leibson and Jacobsen12,Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg13 than in adulthood. Reference Faraone, Biederman, Wilens and Adamson14,Reference Biederman, Monuteaux, Spencer, Wilens, MacPherson and Faraone15 This might mean that substance use disorder development is delayed rather than being altered by stimulant treatment. The present study reports on an adolescent sample (mean age 16.4), and we can therefore not draw firm conclusions about the effect of stimulant therapy on substance use disorders in adulthood. We did find, however, that the protective effect of age at first stimulant use was only true for children up to 18, and that the risk of developing substance use disorders may even reverse around the age of 18 (Fig. 2). Apart from the direct effect of stimulant medication on the development of substance use disorders, an alternative explanation of our findings is possible. Participants who start stimulant medication early might have greater parental support, however, this parental support may diminish once the individual reaches adulthood. Indeed, parental support has been found to be inversely related to substance use in a large sample of high school students. Reference Wills, Resko, Ainette and Mendoza34 Clearly, future studies are warranted to assess whether parental support mediates the protective effect of stimulant therapy on the development of substance use disorders.
Although we did find a protective effect of stimulant use on the development of substance use disorders, such a protective effect was not found for the development of nicotine dependence. This is in accordance with another study that also failed to find a protective effect of stimulant use on nicotine dependence, but did find a protective effect on substance use disorders. Reference Biederman, Wilens, Mick, Spencer and Faraone35 Other studies have found protective effects on smoking initiation and regular smoking (for example Wilens et al, Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg13,Reference Wilens, Adamson, Monuteaux, Faraone, Schillinger and Westerberg17 Huss et al Reference Huss, Poustka, Lehmkuhl and Lehmkuhl36 ), but these studies did not look at nicotine dependence. These findings suggest that stimulant therapy does have a protective effect on the early stages (initiating and regular smoking) of nicotine dependence, but there is no effect on later stages of full-onset nicotine dependence. However, due to the relatively small number of participants with nicotine dependence in our sample (7%), caution should be used when interpreting the null findings concerning nicotine dependence.
Overall, the relationships between ADHD and related externalising disorder, and nicotine dependence and substance use appear to be complex. Attention-deficit hyperactivity disorder is associated with earlier initiation of smoking and higher rate of regular smoking, and longitudinal twin modelling indicates that the covariance between ADHD and smoking is foremost as a result of common environmental risk factors. Reference Korhonen, Latvala, Dick, Pulkkinen, Rose and Kaprio37 Covariance between smoking and substance use was because of both additive genetic and common environmental influences. Further, about half of the covariance between externalising disorders and substance use was as a result of shared genetic factors and half as a result of shared environmental factors. Reference Korhonen, Latvala, Dick, Pulkkinen, Rose and Kaprio37 According to the gateway theory, smoking precedes use of substances in many cases. Reference Mayet, Legleye, Chau and Falissard38 However, in a minority of instances, evidence for a reverse gateway is found in that marihuana users had a higher risk for subsequent tobacco use. Reference Mayet, Legleye, Chau and Falissard38 Future prospective studies on the specific trajectories from first nicotine use to nicotine dependency, in ADHD medication-treated and medication-naive patients, are warranted to further elucidate the effects of stimulant treatment on the development of nicotine dependence.
Limitations
Our findings should be viewed in the light of some limitations. First of all, our study design is naturalistic and non-randomised and this makes it impossible to control for all possible confounding factors. The best method of determining the effect of stimulant medication on the development of substance use disorders and nicotine dependence would be a randomised controlled trial. However, due to practical and ethical issues such studies are not feasible. The current study design makes it difficult to draw conclusions about causality and one should be cautious in interpreting the results. Second, participants were of White descent, which limits the possibility of generalising our results to other ethnicities. Furthermore, we used multiple measures and multiple informants to assess substance and nicotine use and misuse rather than clinicians' diagnostic judgements. This approach might have influenced our estimates of the prevalence. Finally and importantly, our no-stimulant treated group was relatively small compared with the stimulant-treated group, which may have reduced the power of our analyses or the generalisability of our results.
Implications
Despite some limitations, our large European sample of well-defined participants with ADHD provided us with a unique opportunity to examine the relationship between treatment with stimulant medication and substance use disorders and nicotine dependence. This study adds two important insights to the available literature. First, we found that the elevated risk of drug- and alcohol-related substance use disorders and nicotine dependence in individuals with ADHD could not be attributed to the use of stimulant medication. Stimulant treatment has a protective effect on the development of drug- and alcohol-related substance use disorders. Furthermore, we showed that early age at stimulant treatment initiation had a protective effect on the development of substance use disorders, but that this effect appears to reverse after the age of 18.
Funding
This work was supported by an unrestricted grant from Shire Pharmaceuticals (to S.V.F.) and by a grant from The Netherlands Organisation for Health Research and Development (ZonMw) (60-60600-97-193 to J.K.B.).








eLetters
No eLetters have been published for this article.