The discovery of an effective drug treatment for attention-deficit hyperactivity disorder (ADHD) is conventionally attributed to Charles Bradley, a North American child psychiatrist who ran the family nursing home for delinquents in Rhode Island (Reference BradleyBradley 1937). He described a case series of 30 children who received dexamphetamine, initially introduced as part of his idiosyncratic treatment for the headache that followed pneumoencephalography (Reference BrownBrown 1998). He believed that the newly discovered stimulant would promote the secretion of cerebrospinal fluid by the choroid plexus, which would prevent the headache. Dr Bradley was interested in organic causes for delinquent behaviour and routinely performed what was then state-of-the-art brain imaging and electroencephalograms on his patients. Although he did not identify any structural abnormalities, he did see an unexpected side-effect of dexamphetamine. He found ‘a spectacular change in behaviour’ and ‘remarkably improved school performance’ in 14 of the 30 children. He noted that the children started to refer to their ‘arithmetic pills’ and outlined the side-effects they encountered. History does not record the efficacy of the dexamphetamine for the headache.
As for many medications, the discovery was serendipitous, but over 70 years later, psychostimulants continue to be the most widely prescribed agents for ADHD. In many ways, our conception of ADHD is defined by the effect of low-dose stimulants on behaviour and cognition. Such is the link between the two that although ADHD does have all the features required for a diagnostic construct, including biological markers and predictive validity, cynics have described ADHD as a drug response masquerading as a diagnosis.
Psychopharmacology
A variety of medications, both licensed and unlicensed, are used in the treatment of ADHD in children and adolescents (young people). One way of grouping the drugs is by pharmacological action (Table 1), although there is a degree of overlap between the categories. For example, atomoxetine and bupropion, both noradrenergic reuptake inhibitors, are known to have additional dopaminergic effects. The majority of drugs appear to act through dopamine or noradrenaline systems. Modafinil's mode of action is unclear; although it has dopaminergic properties, some lines of evidence suggest effects via γ-aminobutyric acid pathways (Reference Ferraro, Antonelli and TanganelliFerraro 1999).
Psychostimulants
PsychostimulantsFootnote † or stimulants are drugs that temporarily increase alertness or awareness. They are variously defined pharmacologically but tend to act on the central nervous system via dopaminergic and noradrenergic mechanisms. Their routine medical use is complicated by the additional property of many of these agents: that of inducing euphoria. Methylphenidate, dexamphetamine and modafinil are stimulants, but taken orally they are not potent euphoric agents. In the UK, both methylphenidate and dexamphetamine come under controlled drugs legislation and are listed under Schedule 2 in the Misuse of Drugs Regulations 2001 (www.opsi.gov.uk/si/si2001/20013998.htm).
Stimulants are widely recognised as effective medications for the short-term amelioration of the core symptoms of ADHD. Most reviews have concluded that between 70 and 90% of young people with ADHD will respond favourably to methylphenidate, although side-effects may preclude their continuation (Reference Jadad, Boyle and CunninghamJadad 1998; Reference Greenhill, Halperin and AbikoffGreenhill 1999). The number whose hyperactive symptoms return to within the normal range is lower – between 50 and 60%.
Stimulants have a number of properties which have led to their widespread use: they are rapidly acting; they have a short half-life of several hours; they have a clear, linear dose–response relationship; they are generally well tolerated; and they have predictable side-effects (Box 1). Reference GualtieriGualtieri (2002) describes them pithily as ‘Yes or No’/digital drugs: they either work or they do not. At optimal doses, they usually have an effect obvious to all observers. Parents and teachers typically report improved attention, better task completion and organisation, and reductions in careless errors, distractibility and negative social interactions.
BOX 1 Side-effects of stimulants
Common
-
• Appetite suppression
-
• Sleep disturbance
-
• Abdominal pain
Uncommon
-
• Weight loss or restricted growth
-
• Worsening of tics
-
• Irritability
-
• Transient tearfulness
-
• Anxiety
-
• Behavioural rebound
-
• Headache
-
• Significantly raised blood pressure
-
• Tachycardia
-
• Skin picking and nail biting
-
• Skin rashes
Rare
-
• ‘Overfocus’
-
• Restricted attention
-
• Hallucinations
-
• Thought disorder
-
• Neutropaenia
Stimulant dosing
Dose-range studies suggest that the effective dosage for immediate-release methylphenidate is between 0.3 and 0.6 mg/kg per dose (Reference Rapport, Stoner and DuPaulRapport 1985), and this should be a useful guide for clinicians. However, body mass is not always predictive so there is considerable variation between individuals (Reference Rapport and DenneyRapport 1997). Young people with inattentive-type ADHD may require a lower dose (Reference Barkley, DuPaul and McMurrayBarkley 1991). Some individuals require doses of 1 mg/kg or more, but they are exceptional and usually supervised at specialist centres. In the USA, young people taking such high doses may undergo blood-level monitoring, but this is currently unavailable in the UK.
Most British clinicians titrate upwards from 5 mg methylphenidate given two or three times daily and separated by about 4 h. Some prefer to start with the modified-release preparations where the dose range will be the equivalent of twice or three times daily dosing. For immediate-release methylphenidate, most clinicians use weekly increments of 2.5–5 mg; one week at each dosage level should be enough to obtain sufficient representative teacher and parent feedback in relation to response and side-effects. It is worth continuing the titration to the top of the weight-predicted dose range or until a good response or unacceptable side-effects are encountered.
Unfortunately, in adolescents, weight-predicted dose can be outside of the licensed dose range. This is because the licensing studies were performed on a younger age group and the doses were absolute rather than weight-based, a fact which should be discussed with the parents and the discussion entered into the clinical notes. Fortunately, the recent National Institute for Health and Clinical Excellence (NICE; 2008) guidelines acknowledge this deficiency for the first time.
One of the debates about methylphenidate use has been related to tolerance. Since tolerance can be a feature of addiction and dependency, this has been controversial. Most clinicians will be aware that closer attention to dosing is necessary in the first year of treatment than in later years, and that the dose usually increases during the first year. This clinical observation has been confirmed by a large open-label study undertaken over 2 years (Reference Wilens, McBurnett and SteinWilens 2005). The study, which included over 400 children across several sites, looked specifically at osmotic-release oral system (OROS) methylphenidate but the findings are likely to apply to all methylphenidate preparations. The authors found that the mean daily dose increased by 26%, predominantly in the first year, after which it levelled out. This was a significant increase even when normal growth was taken into account. The reasons for the increase remain obscure, although methylphenidate is not addictive in the conventional sense. I have found that patients are more likely to require encouragement and regular reminders to take their methylphenidate.
Side-effects
Most side-effects are dose related and subject to individual variation. Many diminish within a week or two of initiation and almost all will cease on discontinuation. Where side-effects persist, most become more tolerable with dose reduction. Both side-effects and treatment response will be related to the metabolism of methylphenidate, which is broken down predominantly by the enzyme carboxylesterase-1. Carboxylesterase-1 is known to have many polymorphisms and three haplotypes. The polymorphisms have been shown to be clinically relevant in the clinical response to the angiotensin-converting enzyme inhibitor imidapril (Reference Geshi, Kimura and YoshimuraGeshi 2005). These polymorphisms mean that some individuals metabolise the drug more slowly than individuals with the more common carboxylesterase-1 variant. The most common polymorphism occurs in only 10–15% of the general population of European ethnicity, but in 50% of those of African ethnicity. The relevance of these polymorphisms to clinical use of methylphenidate has not yet been clarified but does suggest that, in future, it may be possible to predict individuals likely to respond to low doses or those likely to develop early side-effects.
Two aspects of stimulants remain particularly controversial: their impact on young people's growth and their cardiac safety.
Effects on height and weight
Stimulants seem to suppress ‘normal growth’ in height and weight through two mechanisms: first, through appetite reduction, and second, through direct effects on growth hormone. Consequently, it is routine practice to monitor height and weight at review. The 3-year follow-up of the Multimodal Treatment Study of Children with ADHD (the MTA) clarified this area with some predictable and less predictable findings (Reference Swanson, Elliot and GreenhillSwanson 2007a). A total of 370 patients were allocated to one of four naturalistic subgroups (not medicated, newly medicated, consistently medicated and inconsistently medicated) on the basis of the duration of their exposure to stimulants. Analysis of variance was used to explore the relationship between stimulant treatment, growth and population norms. Swanson et al found that, over the 3-year study period, the 65 children who had never been medicated were an average of 2 cm taller and 2.7 kg heavier than the group of 88 children who had been consistently medicated. The effect was greatest in the first year and had approached zero by the third year. What was unexpected was that children with untreated ADHD were taller on average than the general population to begin with. This means that the magnitude of the lost growth for the treated group appears less marked when compared with the general population.
Cardiovascular risks
In 2005 and 2006, decisions by two North American regulators received significant publicity in the British press, although the response to the later retractions was strangely muted (Box 2). Both decisions related to the evaluation of the risk of serious cardiac events for people taking stimulants. The differing opinions expressed relate as much to the professional background of the assessors as to the dearth of useful evidence. Child psychiatrists and paediatricians tend to overestimate the benefits, and cardiologists tend to overestimate the risks.
BOX 2 Stimulants and health scares
February 2005
Health Canada's New Drug Committee withdraws marketing authorisation for Adderall XR® owing to concerns about possible sudden death, heart-related deaths and strokes in adults and children taking the drug.
August 2005
Adderall XR® is reapproved with enhanced post-marketing surveillance and changes to the labelling warning against use in the presence of structural heart defects or amphetamine misuse.
February 2006
The Drug Safety and Risk Management Advisory Committee of the US FDA recommends a black box warning, describing the cardiovascular risks for all stimulant drugs.
March 2006
The Pediatric Advisory Committee of the FDA decides, on the basis of the risk–benefit arguments, that the black box warning is not warranted and the initial recommendation is not implemented.
April 2006
Dr Nissen, an adult cardiologist and a consultant for the Drug Safety and Risk Management Advisory Committee, takes the unusual step of defending the initial decision in a published article (Reference NissenNissen 2006). His principal concerns relate to the increasing use of stimulants for adult ADHD, known fatalities due to the unregulated use of related drugs, the known links between stimulants and raised blood pressure, and between raised blood pressure and a variety of cardiac events in adults.
August 2007
The 3-year outcome data from the MTA are published. The study confirms a small but measurable growth deceleration for those treated with stimulants and relatively high rates of delinquency and substance misuse despite treatment for ADHD. The clear-cut benefit of tailored medication treatment over other treatments is lost after the separate treatment protocols are ended (12 months into the study). All groups continue to show moderate benefits compared with those at the start of the study.
November 2007
British national newspapers, previewing BBC television's Panorama programme ‘What next for Craig’ (12 November 2007), announce ‘Drugs of “no benefit” to hyperactive children’ (Reference ClelandCleland 2007).
Most of the data on which the decisions were based come from post-marketing surveillance and the US Food and Drug Administration (FDA) Adverse Event Reporting System, a system similar to the British ‘Yellow Card’ arrangement (http://yellowcard.mhra.gov.uk/). These approaches have recognised inadequacies that lead to underreporting of adverse events. Nevertheless, the estimated annual sudden death rate for stimulants is 0.25 per 100 000 based on FDA data (Reference Rappley, Moore and DokkenRappley 2006). This compares with the background rate of between 0.6 and 6 sudden cardiac deaths per year per 100 000 of the general population (Reference Berger, Dhala and FriedbergBerger 2004).
A Florida study, using a more rigorous design, retrospectively analysed 10 years of health insurance data cross-linked to death registry information and found no cardiac deaths, sudden or otherwise, in 42 612 person-years of stimulant use (Reference Winterstein, Gerhard and ShusterWinterstein 2007). The authors found a background rate in Florida of 4 per 100 000 sudden cardiac deaths per year. From the current literature, it does not appear that stimulants significantly increase the risk of sudden cardiac death in young people.
The chance of abnormal cardiac events is increased by a family history of cardiac arrhythmia or syn cope. It is reasonable to request an electrocardiogram (ECG) prior to treatment in these circumstances or if the child complains of palpitations. This was the conclusion of the recent American Pediatric Association and American Heart Association guidance (Reference Vetter, Elia and EricksonVetter 2008) which was widely misreported initially. The recent NICE guidance (2008) has given us clear standards about the required clinical assessment necessary before initiating drug therapy (Box 3). It is very sensible, but if taken literally, most young people will require an ECG as there will be a history of serious cardiac disease (particularly atherosclerosis) in most families.
BOX 3 Pre-drug treatment assessment
Before starting drug treatment, children and adolescents with ADHD should have a full pre-treatment assessment, which should include:
-
• full mental health and social assessment;
-
• full history and physical examination, including:
assessment of history of exercise syncope, undue breathlessness and other cardiovascular symptoms heart rate and blood pressure (plotted on a centile chart)
height and weight (plotted on a centile chart)
family history of cardiac disease;
-
• an ECG if there is a past medical or family history of serious cardiac disease, a history of sudden death in young family members or abnormal findings on cardiovascular examination;
-
• risk assessment for substance misuse and drug diversion (where the drug is passed on to others).
(National Institute for Health and Clinical Excellence 2008)
The MTA 3-year follow-up
The MTA is the largest ADHD treatment study that has ever been undertaken. When the initial findings were published (MTA Cooperative Group 1999a,b) they were highly influential on the development of national guidelines on both sides of the Atlantic (National Institute for Clinical Excellence 2000; American Academy of Child and Adolescent Psychiatry 2002) and on individual clinical practice. The key findings related to the enhanced efficacy of carefully tailored stimulant treatment for the core symptoms of ADHD over a complex package of psychosocial interventions or standard US ‘community’ treatment (largely stimulant treatment that was less closely monitored). Controversially, the research group found no statistically greater benefits for the combination of a psychosocial intervention and stimulant treatment over treatment with medication alone (Reference Conners, Levin and SparrowConners 2001).
The 3-year follow-up data were published in the form of four separate papers in the summer of 2007 (Reference Jensen, Arnold and SwansonJensen 2007; Reference Molina, Flory and HinshawMolina 2007; Reference Swanson, Elliot and GreenhillSwanson 2007a,Reference Swanson, Hinshaw and Arnoldb). The main findings from these studies are set out in Box 4. The publication passed without much notice outside of medical circles, until a BBC television Panorama programme in November 2007. The conclusions of the programme were previewed in several UK national newspapers with typically alarmist and inaccurate headlines (e.g. ‘Drugs of “no benefit” to hyperactive children’; Reference ClelandCleland 2007).
BOX 4 Key findings of the MTA 3-year follow-up studies
Overall, 485 (83.8%) of the original 579 children took part in the follow-up study. Their mean age was 11.9 years.
The primary outcome measures were ADHD and oppositional defiant disorder symptoms, reading scores, social skills, level of impairment and diagnosis.
At the end of the first year, the controlled treatment protocols (medical management, behavioural treatments, combination treatment and community care) were dropped, allowing for a more naturalistic study.
Reference Jensen, Arnold and SwansonJensen et al (2007)
-
• All of the groups showed symptom improvement over baseline assessments
-
• There was no significant difference between the severity of ADHD symptoms between the four initial groups at 3 years
-
• There was an increase in the number of children taking medication overall, particularly in the group treated behaviourally (increased from 14 to 45%)
-
• The clear advantage of tailored medication over the other treatments, seen in the first MTA study, was lost.
Reference Swanson, Elliot and GreenhillSwanson et al (2007a)
-
• There is a small but detectible reduction in overall growth in height for children who remain on stimulants
-
• Loss of growth is maximal in the first year of treatment.
Reference Swanson, Hinshaw and ArnoldSwanson et al (2007b)
-
• There was substantial variation between individual response to stimulants over time
-
• Three patterns of effect were seen:
34% showed a moderate and gradual improvement over the 3-year study period
52% showed a larger improvement which was sustained to year 3
14% initially responded well but deteriorated over years 2 and 3.
Reference Molina, Flory and HinshawMolina et al (2007)
-
• Despite drug treatment, children with ADHD continue to show higher than normal rates of delinquency (27.1% v. 7.4%) and substance use (17.4% v. 7.8%).
Several more-balanced conclusions can be reached from the MTA 3-year follow-up data. The fact that the medication group's clear advantage was lost 2 years after the end of the controlled phase can be understood by a combination of at least two likely explanations. First, the loss of the controlled conditions after the first year meant that in the second and third years the groups became more heterogeneous, particularly in terms of medication use. It is unsurprising that treatment effects were closer. Second, it is likely that the core ADHD symptoms improved over time for most children as a result of increased maturity. This makes it harder to demonstrate differences between groups.
The study also indicates the high rates of delinquency and substance misuse that occur in ADHD despite treatment of any kind. However, we have no untreated or placebo group with which to compare. Given rates of untreated ADHD in adults with substance misuse (Reference WilensWilens 2004) and in adolescent delinquent populations, it is likely that even these high levels represent an improvement on the untreated course.
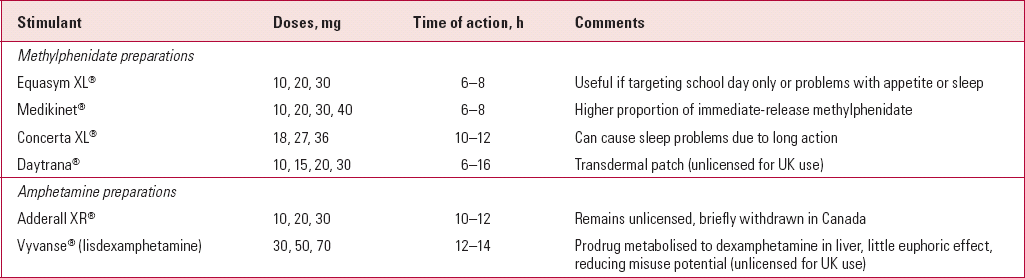
Range of stimulant preparations
The range of preparations and formulations of stimulants has increased considerably over the past 10 years (Table 2). One of the most useful developments has been the advent of effective modified-release stimulants. Most of the preparations have been developed for the North American market and most have then gone on to receive a licence for UK use. Odd exceptions unlicensed in the UK include Adderall®, a long-acting combination of dexamphetamine and amphetamine salts, and Dexedrine spansules®, a long-acting dexamphetamine preparation. Both can be imported from the USA and can be useful in treating individuals with known sensitivity to methylphenidate.
The modified-release psychostimulants vary in length of action. This makes some more appropriate for coverage of the school day and others more useful for adolescents with homework or for more home-based behavioural problems. Parents often describe their effects as smoother or less ‘jerky’ than immediate-release preparations, probably a reflection of more gradual changes in stimulant blood concentration. Other advantages include improved adherence with fewer tablets to remember, reduced stigma (as not taken at school) and the reduced potential for misuse and diversion (where the drug is passed on to others for non-prescription use).
Other novel preparations awaiting a UK licence include a methylphenidate transdermal patch and the prodrug lisdexamphetamine dimesylate. The former may have a niche for younger children who cannot swallow tablets and the latter will be useful when substance misuse is an important consideration.
Modafinil
Modafinil was initially developed as a therapy for narcolepsy, which remains its only approved indication. As a psychostimulant without euphoric effects it was an obvious candidate for use in treatment-resistant ADHD, but proving its efficacy in formal trials has taken time. It is more effective in treating children than adults, with four positive child studies (Reference Biederman, Swanson and WigalBiederman 2005). It appears to be more effective at higher doses (>300 mg/day) and is well tolerated at these doses. The most common side-effects are insomnia, headache and reduced appetite. The FDA has accepted the evidence of efficacy but has demanded further studies to establish the risk of the serious skin reaction Stevens–Johnson syndrome after one child in a study group of 900 demonstrated the condition (Reference KatzKatz 2006). It is not licensed in the UK for ADHD treatment, but may become more prescribed in future.
Nonstimulant drugs
Atomoxetine
Atomoxetine is a potent inhibitor of presynaptic noradrenergic transporters. It was originally developed as an antidepressant with a lower rate of the anticholinergic side-effects of tricyclic anti-depressants (TCAs), but the success of fluoxetine meant that the drug was shelved. However, TCAs were known to be useful agents for ADHD and, although unlicensed for this indication, were in routine use in specialist centres on both sides of the Atlantic. One of the main problems of the use of the tricyclics imipramine and amitriptyline is the high rate of adverse reactions, particularly in children. Atomoxetine offered the potential of efficacy in ADHD and low side-effect profile. It was therefore evaluated for use in ADHD and gained a US licence in 2003 and a UK licence in 2004.
Atomoxetine is a useful addition to the clinician's toolbox. Although it is slower to take effect than stimulants, it can provide all-day cover and has other wider effects (such as mood stabilisation and treatment of initial insomnia) than those on the core ADHD symptoms. It has been shown to improve measures of attention and behaviour in a number of large US studies (Reference Michelson, Faries and WernickeMichelson 2001; Reference Spencer, Biederman and HeiligensteinSpencer 2001) and one large UK study (Reference Prasad, Harpin and PoolePrasad 2007). Reference Michelson, Buitelaar and DanckaertsMichelson and colleagues (2004) have also demonstrated that continuation of atomoxetine treatment prevents the relapse of ADHD symptoms.
Atomoxetine has found a niche in the treatment of young people who do not respond or develop significant side-effects to stimulants, and as an alternative for those with comorbid conditions such as anxiety or tic disorder. It is also useful for those with sleep problems or substance misuse. The NICE guidelines (2008) suggest that atomoxetine should be considered an alternative first-line treatment to methylphenidate in ADHD with these comorbidities and should be used second line for individuals intolerant of or non-responsive to methylphenidate. In a 6-week, head-to-head comparison, 43% of those who did not respond to good therapeutic doses of methylphenidate went on to benefit from atomoxetine therapy (Reference Newcorn, Kratochvil and AllenNewcorn 2008).
There is emerging evidence that atomoxetine has at least two mechanisms of action. This would account for the immediate and delayed cognitive and behavioural effects that are seen with the drug (Reference Chamberlain, del Campo and DowsonChamberlain 2007). In clinical practice, this means that patients should be warned that the benefits of atomoxetine may take 4–6 weeks to become apparent, although there are some children who show a much earlier response.
The most common side-effects of atomoxetine are sedation and loss of appetite or gastrointestinal irritation. Dry mouth and palpitations have also been reported. To reduce the impact of these, it is recommended that a half dose is used for the first week of treatment. As with most dosing in paediatrics, the atomoxetine dose is calculated by body mass. The initial dose should be around 0.5 mg/kg, rising to 1.2 mg/kg after a week. For some children the side-effects are problematic even with this approach and a slow increase over several weeks is more tolerable. It is worth waiting for up to 8 weeks at full dose before assessing the response. The original dosing studies showed further benefits but increased side-effects at doses up to 1.6 mg/kg (Reference Michelson, Faries and WernickeMichelson 2001); however, the licensed UK maximum dose remains at 1.2 mg/kg. The NICE guidance proposes increasing up to 1.8 mg/kg per day in poor responders but with consultation from a tertiary or regional centre (National Institute for Health and Clinical Excellence 2008).
Since the launch of atomoxetine, two further rare side-effects have been described: liver dysfunction and suicidal ideation. Although the risks appear to be very low, the Committee on Safety of Medicines (CSM) has made specific recommendations (Reference DuffDuff 2005). In 2005, one confirmed and one suspected case of hepatitis and 39 cases of raised liver enzymes or bilirubin were picked up by post-marketing surveillance in 3.4 million patients (Eli Lilly 2005). The CSM has advised that patients and carers be warned of the possibility of hepatitis and that they be made aware of the symptoms and the need for urgent medical review.
A possible increase in suicidal ideation was picked up in pooling data from 12 placebo-controlled studies involving 1357 children (Eli Lilly 2005). There was one attempted, but not completed, suicide in the treated group and none in the placebo group. The significance of this is unclear but as atomoxetine was developed as an antidepressant, a link to mood variability remains a possibility. The CSM recommended that patients and carers should be informed of the risk and the need for review should irritability or suicidal thinking develop.
Many children and adolescents with ADHD will already be on a methylphenidate preparation before switching to atomoxetine. Owing to the delayed onset of action of atomoxetine, some form of cross-tapering of medication is preferred. Different strategies for this are reviewed elsewhere (Reference Prasad and SteerPrasad 2008). The possibility of augmenting stimulant therapy with atomoxetine is also being explored, but very limited data are available (Reference Carlson, Dunn and KelseyCarlson 2007).
Treatment choice
When initiating drug treatment for ADHD, there are a number of factors to take into account. These are succinctly summarised in the most recent NICE guidelines (Box 5) (National Institute for Health and Clinical Excellence 2008). The guidance is not unduly restrictive and does allow scope for clinician and patient choice. Essentially, there are only three UK-licensed medications to choose from, although there are several formulations of these. It is interesting to note that the guidance highlights the comorbidities of epilepsy and tic disorder specifically. Presumably, this reflects the caution in the prescribing information provided by the manufacturers regarding use in these circumstances.
BOX 5 Factors to be considered when initiating ADHD treatment
Where drug treatment is considered appropriate, methylphenidate, atomoxetine and dexamphetamine are recommended, within their licensed indications, as options for the management of ADHD in children and adolescents.
Choice of drug should take into consideration:
-
• comorbid conditions (such as tic disorders, Tourette syndrome and epilepsy)
-
• different adverse effects of the drugs
-
• specific issues of adherence (e.g. problems created by midday doses at school)
-
• potential for drug diversion and misuse
-
• preferences of the child and carers
(National Institute for Health and Clinical Excellence 2008)
ADHD and tic disorders
The relationship between stimulants and tics has been controversial for many years. What is not controversial is the high rate of ADHD among young people and adults with tic disorders; nor is the effectiveness of stimulants in the treatment of ADHD in doubt. Although most clinicians will have experience of patients whose tics are exacerbated when they start stimulants or after an increase in dose, the evidence does not show methylphenidate to be worse for tics than placebo or even clonidine (Box 6) across populations (Tourette's Syndrome Study Group 2002). It seems reasonable to consider using methylphenidate for ADHD with tic disorder unless there has been a previous exacerbation with a close temporal relationship. There is no reason to believe that dexamphetamine should be any different from methylphenidate although there is less evidence.
BOX 6 Clonidine
Clonidine is an α2a-noradrenergic agonist originally developed as a hypertensive agent. It has been routinely used in the treatment of ADHD for at least 30 years, despite limited evidence for efficacy except perhaps for Tourette syndrome (Reference Singer, Brown and QuaskeySinger 1995). It was enthusiastically taken up in the USA after one highly influential study suggested that it had a similar effect to methylphenidate (Reference Hunt, Minderaa and CohenHunt 1985). The authors found that it was more effective against core hyperactivity than against attentional deficits, but suggested a niche for highly overactive children for whom methylphenidate was unhelpful. They also suggested replication of the study, although it was many years before a well-designed placebo-controlled trial was performed; this was much less positive, but by then clonidine had become fashionable.
A modern reappraisal suggests that clonidine can be useful for symptoms of overactivity and impulsivity, presumably mediated through its sedative effects and impact on arousal. However, its limited impact on academic functioning and its high level of side-effects, which include sedation, headaches, depression and rebound hypertension, mean that it has a restricted place in ADHD treatment. Its use is now on the wane except in specific situations such as treatment resistance and comorbid tic disorder.
Atomoxetine, on the other hand, may actually reduce tic severity in some individuals. This should not be surprising given that the tricyclic nortriptyline was used for this purpose in Tourette syndrome clinics in the 1990s (Reference Spencer, Biederman and WilensSpencer 1993). Two medium-scale studies, involving more than 70 children in both cases, were insufficiently powered to demonstrate that atomoxetine can cause a statistically significant improvement in tics (Reference Allen, Kurlan and GilbertAllen 2005; Reference Spencer, Sallee and GilbertSpencer 2008). Nevertheless, both studies showed atomoxetine to improve ADHD symptoms and hinted at efficacy in tic reduction. This suggests that atomoxetine can have a useful role in the common comorbidity of ADHD in the context of tic disorder. In my Tourette syndrome clinic, I am more likely to treat the ADHD than the tic disorder for which young patients were referred.
ADHD and epilepsy
Treating ADHD in the presence of epilepsy is also a contentious issue. Child psychiatrists appear more reluctant than child neurologists to consider the use of stimulants in the context of epilepsy, despite mounting evidence about safety and efficacy (Scottish Intercollegiate Guidelines Network 2005). This is a shame, since up to 40% of children with epilepsy will have symptoms of ADHD (depending on the population studied and selection criteria). The inattentive subtype of ADHD appears more common among children with epilepsy (Reference Dunn, Austin and HarezlakDunn 2003).
In one review, two paediatric neurologists surveyed the available evidence on the impact of stimulants on seizure control (Reference Tan and AppletonTan 2005). They came to a number of useful conclusions. First, they found no evidence that short- or long-term methylphenidate treatment increases the risk of developing seizures. Second, they state that the existing studies suggest that methylphenidate appears safe to use in children who have ‘active or well-controlled epilepsy’. Third, they advise against the routine use of electroencephalogram screening before methylphenidate treatment. They conclude with the caveat that it is important to monitor seizure frequency in the first few weeks and months after initiating therapy.
Atomoxetine has been in use for a shorter time and so it is unsurprising that data on which to base treatment decisions are sparse. In January 2006, the Medicines and Healthcare products Regulatory Agency led a review on the risks and benefits of atomoxetine. They recommended that epilepsy be added as a caution to the product data sheet (Reference DuffDuff 2006). This decision appears to be based on the absence of evidence and the need for further assessment rather than evidence of risk.
The available data come from the adverse-event recording in post-marketing surveillance, with its known shortcomings, and the relatively small clinical trials (Reference Wernicke, Holdridge and LinWernicke 2007). The post-marketing surveillance picked up a possible 180 seizure-like events over a 2-year period, representing 2.2 million child and adult exposures. This yields a rate of 8 per 100 000 exposures. Using the clinical trials database, Wernicke et al found 12 children, among 5083 studied, who had at least one seizure over the study period. This gives a crude incidence of 0.2% or 2.3 per 1000 patient-years. Although not directly measured, neither of these figures is dissimilar to background population rates. Thus, the risk of inducing or exacerbating seizures appears low and at least similar to that for methylphenidate. We will need to wait for larger, more definitive prospective studies.
Conclusions
Medication will remain a central part of the treatment of ADHD in children and adolescents. The positive effect of stimulants and atomoxetine on measures of attention and impulsive behaviour is beyond doubt. Serious side-effects are rare, despite recent health scares. The increasing range of medications available gives clinicians more choice, but brings with it increased complexity. The recent NICE guidelines are comprehensive, well researched and pragmatic, and can help with these treatment decisions (National Institute for Health and Clinical Excellence 2008). However, young people with ADHD almost always have additional diagnoses or wider difficulties with peer and family relations, learning and self-esteem. Medication alone cannot solve these problems and clinicians should explain clearly to parents, carers and the young people themselves the likely symptomatic benefits and what will be left untreated. Unfortunately, the outcome remains poor for many even with treatment, but this is not an excuse not to diagnose and treat appropriately.
MCQs
-
1 Psychostimulants improve alertness and awareness through effects on:
-
a γ-aminobutyric acid
-
b dopamine only
-
c dopamine and noradrenaline
-
d noradrenaline
-
e serotonin.
-
-
2 Atomoxetine:
-
a is unsafe to use in the treatment of ADHD and tic disorder
-
b is a controlled drug under current UK legislation
-
c should never be used in combination with stimulants
-
d was originally developed as an antidepressant
-
e commonly causes irritability as a side-effect.
-
-
3 The MTA 3-year follow-up study demonstrated that:
-
a stimulant drugs are of no benefit to children with ADHD
-
b the outcome for all children treated with medication is good
-
c long-term medication use will restrict eventual height
-
d delinquency and substance misuse are unusual comorbidities
-
e the media will accurately depict scientific research.
-
-
4 The following drug has not been used in the treatment of ADHD:
-
a clonidine
-
b dexamphetamine
-
c nortriptyline
-
d modafinil
-
e imidapril.
-
-
5 Regarding drug treatments in ADHD:
-
a stimulants should not be used if ADHD is complicated by epilepsy
-
b modafinil seems more useful in adults than in children
-
c the dose of methylphenidate is likely to rise in the first year of treatment
-
d methylphenidate is metabolised by a cytochrome P450 isoenzyme
-
e the efficacy of stimulants was discovered by one of the princes of Serendip.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | f | a | f | a | f | a | f |
| b | f | b | f | b | f | b | f | b | f |
| c | t | c | f | c | t | c | f | c | t |
| d | f | d | t | d | f | d | f | d | f |
| e | f | e | f | e | f | e | t | e | f |
TABLE 1 Mode of action of ADHD drugs

| Pharmacological action | Drug |
|---|---|
| Dopamine reuptake inhibition and direct release | Methylphenidate,a dexamphetamine,a L-amphetamine, modafinil |
| Noradrenaline agonism | L-amphetamine |
| Noradrenaline reuptake inhibition | Atomoxetine,a imipramine, bupropion |
| α2-adrenergic agonism | Clonidine, guanfacine |
TABLE 2 Long-acting stimulant preparations
| Stimulant | Doses, mg | Time of action, h | Comments |
|---|---|---|---|
| Methylphenidate preparations | |||
| Equasym XL® | 10, 20, 30 | 6–8 | Useful if targeting school day only or problems with appetite or sleep |
| Medikinet® | 10, 20, 30, 40 | 6–8 | Higher proportion of immediate-release methylphenidate |
| Concerta XL® | 18, 27, 36 | 10–12 | Can cause sleep problems due to long action |
| Daytrana® | 10, 15, 20, 30 | 6–16 | Transdermal patch (unlicensed for UK use) |
| Amphetamine preparations | |||
| Adderall XR® | 10, 20, 30 | 10–12 | Remains unlicensed, briefly withdrawn in Canada |
| Vyvanse® (lisdexamphetamine) | 30, 50, 70 | 12–14 | Prodrug metabolised to dexamphetamine in liver, little euphoric effect, reducing misuse potential (unlicensed for UK use) |





eLetters
No eLetters have been published for this article.