Prevalence and incidence rates of mild cognitive impairment vary as a result of different diagnostic criteria as well as different sampling and assessment procedures (Reference Petersen, Doody and KurzPetersen et al, 2001). Precise knowledge of the magnitude and pattern of mild cognitive impairment is of importance because of the prospect that early intervention might delay progression to dementia. People with mild cognitive impairment develop dementia at a rate of 10-15% per year, while the rate for healthy controls is 1-2% per year (Reference Petersen, Doody and KurzPetersen et al, 2001). This study reports and compares age-specific prevalence and incidence rates for four diagnostic concepts of mild cognitive impairment in the same sample. The validity of each concept as a predictor of future dementia is assessed.
METHOD
Sample
The data were derived from the Leipzig Longitudinal Study of the Aged (LEILA75+), a population-based study of the epidemiology of dementia and mild cognitive impairment (Reference Riedel-Heller, Busse and AurichRiedel-Heller et al, 2001). A total of 1500 community-dwelling individuals aged 75 years and over and resident in the Leipzig South district of Germany were identified by systematic random sampling from an age-ordered list provided by the local registry office. Individuals living in homes for the elderly were included in the study on a proportional basis (n=192).
Of the overall sample of 1692 persons, 242 (14.2%) declined to participate, 57 (3.4%) had died and 15 (0.9%) were not traceable. Information on 113 members of the study sample (6.7%) who were shielded by their relatives was obtained solely by proxy interviews. Clinical interviews incorporating neuropsychological assessment were conducted with 1265 (74.8%) participants; these people did not differ significantly from the remainder of the sample in terms of age (U=263 553, P=0.455), gender (χ2=0.391, d.f.=1, P=0.532) or marital status (χ2=5.027, d.f.=3, P=0.170). Two hundred and twenty (17.4%) of these 1265 participants were suffering from dementia according to DSM—IV criteria (American Psychiatric Association, 1994). The analysis is based on the remaining 1045 participants, who showed no DSM—IV dementia.
Instruments
Neuropsychological assessment
The main instrument employed was the Structured Interview for Diagnosis of Dementia of Alzheimer type, Multi-infarct Dementia and Dementia of other Aetiology according to ICD—10 and DSM—III—R (SIDAM; Reference Zaudig, Mittelhammer and HillerZaudig et al, 1991). The SIDAM consists of a neuropsychological test battery including the Mini-Mental State Examination (MMSE), a section for clinical judgement and third-party information on psychosocial impairment. The neuropsychological test battery covers six areas of neuropsychological functioning:
-
(a) orientation: assessment of orientation for time and place;
-
(b) memory: measured by delayed verbal recall of a word list and a fictitious name and address, and delayed visual reproduction;
-
(c) intellectual abilities: assessed by items of abstract thinking (differences, explaining the meaning of idiomatic expressions) and judgement (describing pictures representing actions, and plausibility judgement);
-
(d) verbal abilities and calculation: assessed by calculating serial sevens, spelling backwards, and digit span backwards;
-
(e) constructional abilities (visuospatial): assessed by copying figures;
-
(f) aphasia and apraxia: assessed by naming objects, reading and obeying a sentence, writing a sentence and performing a three-stage command.
For each cognitive domain, age-specific and education-specific norms were employed in the evaluation of impairment in cognitive function. The norms were developed on the baseline population (participants without dementia) from which the study sample was recruited.
Data on socio-demographic variables, mild cognitive impairment and possible risk factors for dementia were collected. A series of validated scales examining the capacity to perform a wide range of activities of daily living such as use of the telephone, feeding, dressing and personal hygiene were completed. Complaints of subjective memory impairment were assessed before cognitive testing by asking participants if they had any problems with their memory (answer ‘yes’ or ‘no’). Depressive symptoms were assessed by means of the Centre for Epidemiological Studies Depression (CES—D) scale (Reference RadloffRadloff, 1977) and the Structured Clinical Interview for DSM—III—R (SCID; Reference Spitzer, Williams and GibbonSpitzer et al, 1987).
Data collection
Structured clinical interviews were conducted by trained psychologists and physicians during visits to the participants' homes. In addition, structured third-party interviews were conducted, in order to obtain information on cognitive and psychosocial functioning as well as subjective memory impairment. Baseline interviews were conducted between January 1997 and June 1998. Study participants were requested to take part in two follow-up assessments, which were conducted 18 months and 36 months after baseline assessment. If it was not possible to administer the SIDAM at follow-up (e.g. owing to death or severe weakness, or because relatives refused participation on behalf of the elderly person in their care), we offered the option of a fully structured proxy interview. This included the Clinical Dementia Rating (CDR) scale (Reference Hughes, Berg and DanzigerHughes et al, 1982) for assessment of cognitive functioning.
Definition of cases
Consensus conferences of physicians and psychologists were held for each subject. The clinical diagnosis of dementia was made according to DSM—IV criteria. The cognitive criteria for a dementia diagnosis were based either on cognitive testing or CDR data (in case of proxy interviews). The reported prevalence and incidence rates for mild cognitive impairment are based on individuals who performed cognitive testing at baseline (prevalence rates) and at least at one follow-up examination (incidence rates). Four diagnostic concepts for mild cognitive impairment were established: mild cognitive impairment (MCI), and a modification (MCI—modified); and age-associated cognitive decline (AACD), and a modification (AACD—modified). Reported predictive validities of these concepts include participants for whom only CDR data were available at follow-up.
Mild cognitive impairment
Mild cognitive impairment was diagnosed according to the criteria of Petersen et al (Reference Petersen, Smith and Waring1999); the condition is now described as ‘MCI—amnestic’, following a new sub-classification (Reference Petersen, Doody and KurzPetersen et al, 2001). These criteria were:
-
(a) the presence of a complaint about memory: participants or informants (or both) reported memory impairment;
-
(b) impaired memory function for age and education: like Ritchie et al (Reference Ritchie, Artero and Touchon2001), we operationalised MCI as an isolated memory loss and a test performance more than 1 s.d. below age- and education-specific norms, assessed by the ‘memory’ sub-test of the SIDAM (impairment on the ‘memory’ sub-test only and not on sub-tests relating to other cognitive functions);
-
(c) preserved general cognitive functioning: participants showed no impairment in the ‘intellectual abilities’ sub-test of the SIDAM (impairment was defined as a test performance more than 1 s.d. below age- and education-specific norms);
-
(d) intact ability to perform activities of daily living: forgetfulness did not compromise overall functional ability; impairment due to physical disease was not sufficient for exclusion;
-
(e) absence of dementia: assessed by DSM—IV criteria.
Age-associated cognitive decline
Age-associated cognitive decline was diagnosed according to Levy et al's (1994) criteria:
-
(a) report by the individual or a reliable informant that cognitive functioning has declined: onset of decline must be described as gradual and have been present for at least 6 months; either participants or informants (or both) reported memory impairment;
-
(b) impairment in any of five cognitive domains — memory and learning, attention and concentration, thinking, language, and visuospatial functioning: education-matched population; an impairment in any of the areas of neuropsychological functioning covered by the SIDAM was regarded as sufficient for diagnosis, and impairment was defined as a test performance 1 s.d. below the mean value for the age- and education-matched population;
-
(c) exclusion criteria — impairment should not be due to any present or past medical or psychiatric condition, or psychoactive substance use, that can cause cerebral dysfunction: exclusion criteria were investigated during the structured clinical interview with participants and informants.
Modifications
Modifications of the above states were also evaluated. These modifications were defined by the same criteria as the original concepts of MCI and AACD, with the exception of criterion (a), memory impairment. The importance of subjective memory impairment in the prediction of dementia is questionable and it may not be of additional predictive value (Reference Jorm, Christensen and KortenJorm et al, 1997).
Following these case definitions we find a diagnostic overlap: participants classified into the original concepts also meet criteria of the ‘modified’ concepts, and subjects with MCI are also identified as having AACD. Levy et al's exclusion criteria were applied for all four diagnostic concepts, to rule out the possibility of memory changes due to medical or psychiatric conditions.
Analysis
The frequencies of all four diagnostic entities at baseline are described in terms of percentage prevalences. For analysis of incidence, the ‘person-years at risk’ method was used. Incidence rates were estimated as the number of new cases divided by person-years at risk. The at-risk population comprised those without a diagnosis of mild cognitive impairment at baseline. Age bands were based on age at the prevalence wave. Person-years for those without cognitive impairment were calculated as the time between baseline and the final follow-up examination at which the cognitive diagnosis was based on cognitive testing. For individuals with cognitive impairment or dementia, the time of occurrence of the diagnosis was assumed to be the midpoint between two examinations. Person-years were calculated accordingly. Study entrants who refused the incidence wave, could not be traced, died or did not perform cognitive testing were excluded from the analysis of incidence.
In order to analyse possible non-response bias, chi-squared analysis and the Mann-Whitney U test were applied. Possible differences in the prevalence rates between men and women were analysed by χ2 testing. For all analyses an α level of 0.01 was used.
To assess the validity of each concept, receiver operating characteristic (ROC) analysis was applied to evaluate the relative predictive powers of the different sets of diagnostic criteria in the predictive of future dementia. In addition, the positive predictive power for each concept was calculated as the proportion of participants who had received a diagnosis of mild cognitive impairment at baseline and developed dementia before follow-up (true positives) over all participants for whom information (including CDR data) were available at follow-up who had received a diagnosis of mild cognitive impairment at baseline (true and false positives).
RESULTS
Prevalence
Of the 1045 study participants without dementia, 116 met the exclusion criteria defined by Levy et al (1994); 929 participants remained for baseline examination.
Age-specific prevalence rates are summarised in Table 1. A diagnosis of MCI was assigned to 3.1% (95% CI 2.0-4.2) of the study participants, of MCI-modified to 5.1% (95% CI 3.7-6.5) AACD to 8.8% (95% CI 7.0-10.7) and of AACD-modified to 19.7% (95% CI 17.1-22.3). The prevalences of AACD and AACD-modified significantly increased with age. There was no significant change with age in the prevalences of MCI and MCI-modified. No difference in prevalence rates between men and women were found.
Table 1 Age-specific prevalence rates according to the different diagnostic criteria for mild cognitive impairment
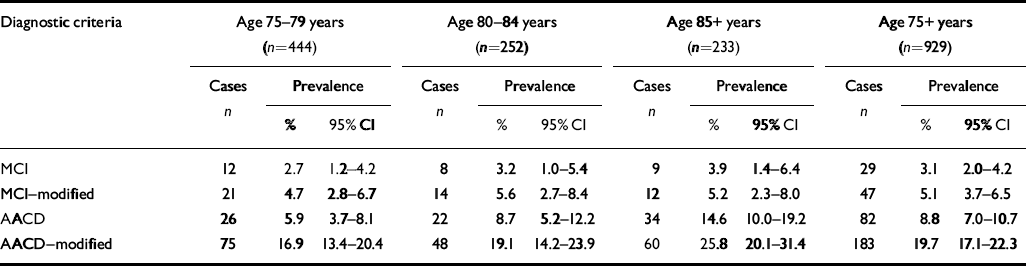
| Diagnostic criteria | Age 75-79 years (n=444) | Age 80-84 years (n=252) | Age 85+ years (n=233) | Age 75+ years (n=929) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases n | Prevalence | Cases n | Prevalence | Cases n | Prevalence | Cases n | Prevalence | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |||||
| MCI | 12 | 2.7 | 1.2-4.2 | 8 | 3.2 | 1.0-5.4 | 9 | 3.9 | 1.4-6.4 | 29 | 3.1 | 2.0-4.2 |
| MCI—modified | 21 | 4.7 | 2.8-6.7 | 14 | 5.6 | 2.7-8.4 | 12 | 5.2 | 2.3-8.0 | 47 | 5.1 | 3.7-6.5 |
| AACD | 26 | 5.9 | 3.7-8.1 | 22 | 8.7 | 5.2-12.2 | 34 | 14.6 | 10.0-19.2 | 82 | 8.8 | 7.0-10.7 |
| AACD—modified | 75 | 16.9 | 13.4-20.4 | 48 | 19.1 | 14.2-23.9 | 60 | 25.8 | 20.1-31.4 | 183 | 19.7 | 17.1-22.3 |
Incidence
Table 2 shows sample size and attrition at follow-up according to the four different diagnostic concepts. Participants who were investigated at follow-up were significantly younger and had a significantly higher MMSE score at baseline compared with those for whom no cognitive testing was performed at follow-up. There was no difference between those leaving the study and participants with regard to education and subjective cognitive complaints at the baseline assessment. The remaining participants had at least one follow-up assessment. The diagnosis at the last follow-up visit at which the participant had undergone cognitive testing was taken as the main outcome measure.
Table 2 Sample size and attrition according to the different diagnostic criteria for mild cognitive impairment
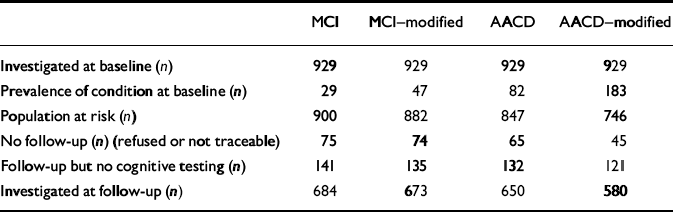
| MCI | MCI—modified | AACD | AACD—modified | |
|---|---|---|---|---|
| Investigated at baseline (n) | 929 | 929 | 929 | 929 |
| Prevalence of condition at baseline (n) | 29 | 47 | 82 | 183 |
| Population at risk (n) | 900 | 882 | 847 | 746 |
| No follow-up (n) (refused or not traceable) | 75 | 74 | 65 | 45 |
| Follow-up but no cognitive testing (n) | 141 | 135 | 132 | 121 |
| Investigated at follow-up (n) | 684 | 673 | 650 | 580 |
Age-specific incidence rates are summarised in Table 3. Gender-specific rates are not given owing to the small number of incidence cases. The annual incidence rate for the MCI condition for individuals aged 75 years or more was 8.5 (95% CI 4.8-14.1) per 1000 person-years and for MCI-modified it was 12.2 (95% CI 63.3-92.9). Although the incidences for AACD and AACD-modified significantly increased with age, incidence rates for the other two diagnostic concepts did not.
Table 3 Age-specific incidence rates according to the different diagnostic criteria for mild cognitive impairment
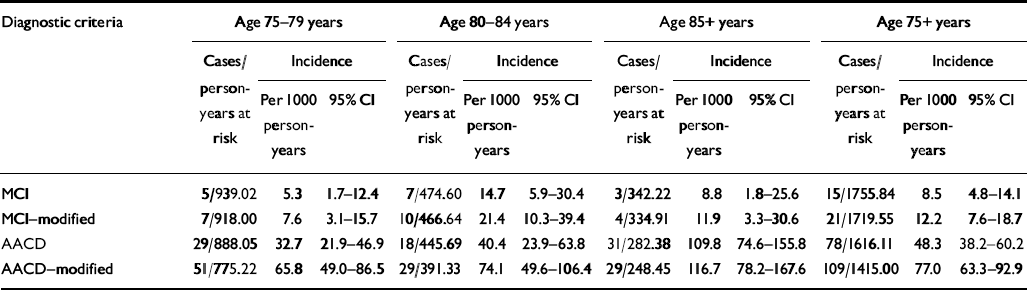
| Diagnostic criteria | Age 75-79 years | Age 80-84 years | Age 85+ years | Age 75+ years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases/person-years at risk | Incidence | Cases/person-years at risk | Incidence | Cases/person-years at risk | Incidence | Cases/person-years at risk | Incidence | |||||
| Per 1000 person-years | 95% CI | Per 1000 person-years | 95% CI | Per 1000 person-years | 95% CI | Per 1000 person-years | 95% CI | |||||
| MCI | 5/939.02 | 5.3 | 1.7-12.4 | 7/474.60 | 14.7 | 5.9-30.4 | 3/342.22 | 8.8 | 1.8-25.6 | 15/1755.84 | 8.5 | 4.8-14.1 |
| MCI—modified | 7/918.00 | 7.6 | 3.1-15.7 | 10/466.64 | 21.4 | 10.3-39.4 | 4/334.91 | 11.9 | 3.3-30.6 | 21/1719.55 | 12.2 | 7.6-18.7 |
| AACD | 29/888.05 | 32.7 | 21.9-46.9 | 18/445.69 | 40.4 | 23.9-63.8 | 31/282.38 | 109.8 | 74.6-155.8 | 78/1616.11 | 48.3 | 38.2-60.2 |
| AACD—modified | 51/775.22 | 65.8 | 49.0-86.5 | 29/391.33 | 74.1 | 49.6-106.4 | 29/248.45 | 116.7 | 78.2-167.6 | 109/1415.00 | 77.0 | 63.3-92.9 |
Prediction of dementia
Of the 929 participants available for baseline examination, 77 were lost to followup (refused assessment or not traceable). These 77 individuals did not differ significantly from the remainder of the sample (n=852) as regards age (U=29 823, P=0.186), gender (χ2=0.337, d.f.=1, P=0.068) or subjective cognitive complaints at baseline assessment (χ2=2.773, d.f.=3, P=0.428). However, they were slightly less educated (χ2=7.941, d.f.=3, P=0.019) and had a significantly lower MMSE score at baseline (U=26 919, P=0.009). The remaining 852 participants attended at least one follow-up assessment. Participants were followed for an average of 2.6 years (s.d.=0.73). The diagnosis at the last follow-up visit attended by the participant (or where an informant interview could be conducted) was taken as the main outcome measure.
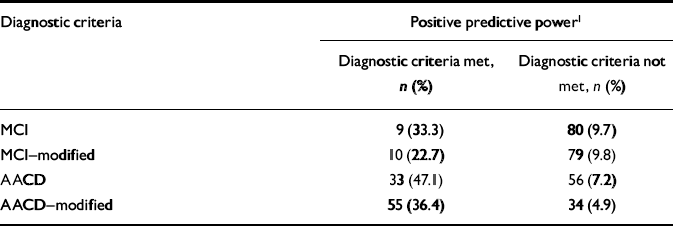
Eighty-nine people in the study developed dementia. The conversion rates to dementia over 2.6 years are similar for those in the MCI (n=9, 33%) and AACD-modified (n=55, 36%) groups (Table 4). The conversion rate was highest for AACD (n=33, 47%) and lowest for MCI-modified (n=10, 23%). The conversion rate for participants who did not fulfil the diagnostic criteria was between 5% and 10%, depending on which set of diagnostic criteria was applied.
Table 4 Predictive power of the different diagnostic criteria for mild cognitive impairment: development of dementia within 3 years
| Diagnostic criteria | Positive predictive power1 | |
|---|---|---|
| Diagnostic criteria met, n (%) | Diagnostic criteria not met, n (%) | |
| MCI | 9 (33.3) | 80 (9.7) |
| MCI—modified | 10 (22.7) | 79 (9.8) |
| AACD | 33 (47.1) | 56 (7.2) |
| AACD—modified | 55 (36.4) | 34 (4.9) |
There was no difference in the duration of follow-up between participants who had become demented by the follow-up examination and those who had not, between participants with mild cognitive impairment and those without, and between the different diagnostic groups of mild cognitive impairment.
The ROC curves indicate an inability of the MCI and MCI-modified criteria to predict dementia (Table 5): area under the curve (AUC) is 0.539 (P=0.231) and 0.534 (P=0.295) respectively. However, the ROC curves for the other concepts indicate a significant predictive power: for AACD, AUC=0.661 (P=0.000); for AACD-modified, AUC=0.746 (P=0.000). The AACD-modified criteria show the highest sensitivity (62%) and the MCI criteria the lowest (10%). The AACD-modified criteria have the highest relative predictive power for the development of dementia (sensitivity 62%, specificity 87%).
Table 5 Results of the receiver operating characteristics analysis conducted to evaluate the relative predictive powers of the different sets of diagnostic criteria for mild cognitive impairment used to predict the onset of dementia

| Diagnostic criteria | Sensitivity (%) | Specificity (%) | AUC | s.e. | Asymptotic significance | 95% CI |
|---|---|---|---|---|---|---|
| MCI | 10.1 | 97.6 | 0.539 | 0.034 | 0.231 | 0.472-0.605 |
| MCI—modified | 11.2 | 95.5 | 0.534 | 0.034 | 0.295 | 0.468-0.600 |
| AACD | 37.1 | 95.2 | 0.661 | 0.035 | 0.000 | 0.592-0.730 |
| AACD—modified | 61.8 | 87.4 | 0.746 | 0.032 | 0.000 | 0.684-0.808 |
DISCUSSION
Prevalence of AACD
Two population-based studies have examined the prevalence of AACD, finding a prevalence rate of 27% in people aged between 68 and 78 years (Reference Hänninen, Koivisto and ReinikainenHänninen et al, 1996) and a rate of 21% in people aged 60 years or more (Reference Ritchie, Artero and TouchonRitchie et al, 2001). Our prevalence rate for AACD was only 9% because many participants did not meet the criteria of a subjective impairment in cognitive functioning as reported by the participant or significant other. Our population was older than in the other two studies. Stereotyped views of old age (e.g. that cognitive impairment is an inevitable process of ageing) might result in people not reporting cognitive impairment because they did not recognise it as such. Our prevalence of AACD-modified (20%) was comparable with the results of the studies by Hänninen et al (Reference Hänninen, Koivisto and Reinikainen1996) and Ritchie et al (Reference Ritchie, Artero and Touchon2001).
Prevalence of mild cognitive impairment
Several research centres use the term ‘mild cognitive impairment’ (Reference Petersen, Doody and KurzPetersen et al, 2001), although there seems to be little agreement on its diagnostic algorithms (Reference Ritchie and TouchonRitchie & Touchon, 2000). Prevalence studies of mild cognitive impairment (Reference Frisoni, Fratiglioni and FastbomFrisoni et al, 2000; Reference Kivipelto, Helkala and HänninenKivipelto et al, 2001; Reference Ritchie, Artero and TouchonRitchie et al, 2001) used different operational criteria with different outcomes. For example, the study by Ritchie et al (Reference Ritchie, Artero and Touchon2001), which related mild cognitive impairment to an isolated memory loss, reported a prevalence rate of only 3% in people aged 60 years and over. This is comparable with our results for MCI and MCI—modified.
Kivipelto et al (Reference Kivipelto, Helkala and Hänninen2001) defined mild cognitive impairment as ‘an objective impairment of memory or in one other area of cognitive function’ and recorded a prevalence rate of 6% in people aged 65-79 years. Only people scoring 24 or less on the MMSE were subjected to a full diagnostic evaluation, which might have underestimated the true prevalence in this population. Frisoni et al (Reference Frisoni, Fratiglioni and Fastbom2000) defined mild cognitive impairment as a score 1 s.d. below the mean of age- and education-specific norms on the MMSE, and reported a prevalence rate of 15% for their study sample, aged 75-95 years.
Age- and gender-specific prevalence rates
According to our study the prevalences of AACD and AACD—modified increase with age. An increase in prevalence with age was also found in other studies (Reference Coria, Gomez de Caso and MinguezCoria et al, 1993; Reference Di Carlo, Baldereschi and AmaducciDi Carlo et al, 2000; Reference Unverzagt, Gao and BaiyewuUnverzagt et al, 2001). A general decline with age was found in one study (Reference Koivisto, Reinikainen and HänninenKoivisto et al, 1995) but others found no significant influence of age on the frequency of mild cognitive impairment (Reference Hänninen, Koivisto and ReinikainenHänninen et al, 1996; Reference Frisoni, Fratiglioni and FastbomFrisoni et al, 2000). Like the study by Hänninen et al (Reference Hänninen, Koivisto and Reinikainen1996) we found no gender difference in the prevalence of mild cognitive impairment. However, higher prevalence rates for men (Reference Koivisto, Reinikainen and HänninenKoivisto et al, 1995) and women (Reference Di Carlo, Baldereschi and AmaducciDi Carlo et al, 2000) have been reported.
Potential bias
Our results might underestimate prevalence rates, since 25% of those originally selected were lost to the study. Although there was no significant difference in age between the participants and the remainder of the study sample, there could still be a bias — particularly as 7% did not participate because they were shielded by their relatives, and these people might have been more physically and cognitively impaired than the participants.
Incidence
To our knowledge, there is no incidence study that has applied MCI or AACD criteria. Like the prevalence rates, in our study the incidence rates for MCI and MCI—modified were very low. Incidence rates for AACD—modified were significantly higher than those for AACD. The incidence of AACD was comparable with dementia incidence rates reported in a meta-analysis on incidence data (Reference Jorm and JolleyJorm & Jolley, 1998). Our incidence rates should be considered as conservative estimates because it has been shown that the effect of people leaving the study was selective in favour of younger and cognitively less-impaired study participants.
As with dementia, incidence rates of mild cognitive impairment seem to increase with age (Reference Paykel, Brayne and HuppertPaykel et al, 1994; Reference Andersen, Nielsen and LolkAndersen et al, 1999). However, in our study, although the incidence rates for the AACD and AACD—modified groups significantly increased with age, the incidence rates for the other two diagnostic groups did not. In old age, memory impairment commonly occurs together with other cognitive deficits (Reference Ritchie and TouchonRitchie & Touchon, 2000), which excludes participants from the MCI and MCI—modified categories.
Prediction of dementia using MCI criteria
The annual rate of conversion of MCI to dementia in our study falls within the range of results from clinical samples (10-15%) (Reference Petersen, Doody and KurzPetersen et al, 2001). However, as indicated by the ROC analysis, the MCI diagnostic concept does not have a significant relative predictive power. We found a small percentage of MCI cases in our non-dementia population (3%). Thus, MCI criteria have a low sensitivity in the detection of dementia. This outcome supports the results of Ritchie et al (Reference Ritchie, Artero and Touchon2001), in whose study the sensitivity of MCI—amnestic criteria for the prediction of dementia was 5%.
Prediction of dementia using AACD criteria
To our knowledge, the only population-based study that has applied the AACD criteria to predict dementia reported a 29% conversion rate within 3 years (Reference Ritchie, Artero and TouchonRitchie et al, 2001). A clinical study applying criteria comparable with the AACD criteria revealed a 2-year conversion rate to Alzheimer's disease of 28% (Reference Celsis, Agniel and CardebatCelsis et al, 1997). In our study the conversion rate to dementia within 2.6 years was higher (47%), probably because of the greater age of our population.
The AACD—modified criteria yielded the best relative predictive power and the best relation of sensitivity and specificity. The 20% prevalence of AACD—modified found in our study is similar to the AACD prevalence established by Ritchie et al (Reference Ritchie, Artero and Touchon2001). Our results suggest that in older people with evidence of objective cognitive impairment, the diagnostic criterion of subjective cognitive complaints has no additional predictive power. The predictive power of subjective memory complaints has been questioned because of their multiple determinants and situational variables affecting the interaction between clinicians and patients (Reference Jorm and ChristensenJorm & Christensen, 2001). Jorm et al (Reference Jorm, Christensen and Korten1997) concluded that it is inappropriate to include cognitive complaints in diagnostic criteria for mild cognitive impairment. Neuropsychological screening at the primary care level could detect people at risk even if they did not report subjective complaints. Cases of mild cognitive impairment could be missed if the elderly person did not admit cognitive impairment and no third-party information could be obtained. However, in people without demonstrable cognitive impairment, subjective memory complaints might be of prognostic value for future dementia. This may apply especially to highly educated elderly people, owing to the ceiling effect of some cognitive tests (Reference Jonker, Geerlings and SchmandJonker et al, 2000).
In sum, the AACD—modified criteria represent the best compromise as regards sensitivity and specificity and yield a high conversion rate of 36% within 2.6 years. Moreover, the criterion of deficits in cognitive domains other than memory has been supported by recent research on the prediction of dementia (Reference Bozoki, Giordani and HeidebrinkBozoki et al, 2001). Since subjective cognitive impairment does not seem to be very useful for the prediction of dementia, it might be preferable to omit it as a criterion for mild cognitive impairment if objective data on cognitive performance are available.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Since individuals with mild cognitive impairment are at risk of developing dementia, it is important to recognise the condition and offer adequate options for further assessment.
-
▪ Precise knowledge of the magnitude and pattern of mild cognitive impairment in the older population is of importance because of the prospect that early intervention might delay progression to dementia.
-
▪ Since incidence and prevalence rates for mild cognitive impairment are highly dependent on the diagnostic criteria applied, consensus on these criteria should be obtained and considered for integration into psychiatric classificatory systems.
LIMITATIONS
-
▪ Diagnosis of mild cognitive impairment was determined under field study conditions.
-
▪ Non-response bias cannot be excluded completely.
-
▪ Prevalence and incidence rates are partly based on relatively few cases of mild cognitive impairment.








eLetters
No eLetters have been published for this article.