Flaxseed, a widely used natural product, is marketed in different forms, including whole flaxseed, defatted flaxseed, flaxseed meal or oil, and flaxseed lignan-enriched complex. Flaxseed consumption is associated with a number of putative health benefits against chronic diseases such diabetes, CVD and cancer( Reference Adolphe, Whiting and Juurlink 1 – Reference Prasad 3 ), and flaxseed lignans are believed to contribute to these beneficial effects through diverse mechanisms. Secoisolariciresinol diglucoside (SDG) is the major lignan of flax, which is present in flaxseed as an oligomer of SDG molecules complexed with hydroxymethylglutaric acid. The SDG polymer acts as a source of the production of other lignans in the gut lumen( Reference Ford, Huang and Wang 4 ). Following oral consumption, the complex is believed to undergo hydrolysis with subsequent deglycosylation of SDG in order to form secoisolariciresinol (SECO)( Reference Clavel, Lippman and Gavini 5 ). Unabsorbed SECO undergoes further metabolism by the colonic microflora to produce the mammalian lignans (enterolignans) enterodiol (ED) and enterolactone (EL) (Fig. 1).

Fig. 1 Pathways for the conversion of the plant lignan secoisolariciresinol diglucoside (SDG)–hydroxymethylglutaric acid (HMG) polymer to the mammalian lignan enterolactone (EL). The SDG–HMG complex undergoes hydrolysis into its monomer units, 3-HMG and SDG, and the intestinal β-glycosidase enzyme cleaves glucose moieties from SDG to convert it to its aglycone form, secoisolariciresinol (SECO). The unabsorbed SECO undergoes further metabolism by the intestinal microflora to produce enterodiol (ED), metaresinol (MAT), lariciresinol (LAR) and EL.
Evidence from preclinical and clinical studies indicates that SDG and/or its metabolites have protective effects against chronic diseases such as cancer, CVD and inflammation( Reference Bloedon, Balikai and Chittams 6 – Reference Zhang, Wang and Liu 9 ). Epidemiological studies have also suggested an association of these health effects with serum EL concentrations. Many of these studies have reported no genotoxic, haematological or behavioural changes following consumption of flaxseed or flaxseed products( Reference Collins, Sprando and Black 10 – Reference Hemmings, Westcott and Muir 12 ). Billinsky et al. ( Reference Billinsky, Glew and Cornish 13 ) reported no evidence of hypoglycaemia or hypotension in healthy older adults when the flaxseed lignan-enriched complex, Beneflax, was administered for 6 months; however, other studies have reported some adverse reactions in special populations such as pregnant and lactating women( Reference Hutchins, Martini and Olson 14 – Reference Tou, Chen and Thompson 16 ). In general, safety and efficacy data on flaxseed lignans are inadequate. Although studies on pharmacokinetics (PK)( Reference Kuijsten, Arts and Vree 17 , Reference Setchell, Brown and Zimmer-Nechemias 18 ), toxicity and drug interaction potential( Reference Billinsky, Maloney, Krol and Vallisuta 19 ) exist, they are poorly described. Since safety and efficacy often correlate with bioavailability, characterisation of absorption, distribution, metabolism and excretion is necessary for any prospective evaluation of safety and efficacy of flaxseed lignan products.
Some PK data on flaxseed lignans are available. Much of the reported PK information follows from nutritional intervention studies where different sources of flaxseed (e.g. flaxseed, whole flaxseed and ground flaxseed) were administered in vivo because of which a clear understanding of the exact lignan dose was not possible. However, such studies have indicated that flaxseed lignans are mainly distributed in the liver, prostate, kidneys and intestine( Reference Murray, Kang and Astheimer 20 ). These lignans undergo significant first-pass metabolism (mainly conjugative with glucuronic acid and sulphate, along with a small amount of cytochrome P450 enzyme-mediated metabolism to form principally monohydroxylated products) and enterohepatic recirculation( Reference Dean, Chang and Doss 21 , Reference Jansen, Arts and Nielen 22 ). Lin et al. ( Reference Lin, Krol and Alcorn 23 ) demonstrated that both intestinal and liver microsomes contributed significantly to the glucuronidation of EL. Flaxseed lignans are excreted mainly as enterolignan conjugates, glucuronides and sulphates, via urine, faeces and bile along with small amounts of lignan aglycones( Reference Axelson and Setchell 24 , Reference Bannwart, Adlercreutz and Wähälä 25 ). Following an oral administration of radiolabelled SDG ([3H]SDG, 3·7 kBq/g body weight) to female rats, 77 % of urine radioactivity was attributed to SECO, ED and EL and their conjugates; however, no measurable SDG was identified in the plasma or urine samples( Reference Rickard and Thompson 26 ). A human clinical study has reported the PK of ED and EL after the administration of purified SDG (1·31 μmol/body weight) to healthy volunteers( Reference Kuijsten, Arts and Vree 17 ). This clinical study has supported the in vivo conversion of SDG principally into ED and EL, but did not provide the bioavailability and comparative PK parameters of purified lignans. Setchell et al. ( Reference Setchell, Brown and Zimmer-Nechemias 18 ) reported the PK of SDG administration (oral, single and multiple doses) in healthy postmenopausal women, and concluded that there was a time-dependent metabolism of SDG to produce enterolignans; however, they could not report the bioavailability of these lignans because only one route of administration (oral) was used. In the present study, we investigated the oral bioavailability and the comparative PK of purified flaxseed and its associated mammalian lignans following oral bolus and intravenous administration in male Wistar rats. Such information will guide more focused investigations into relevant absorption and disposition characteristics of flaxseed lignans and into the mechanisms of lignan action and potential toxicity. Evaluation of preclinical safety and efficacy is an important step towards the application of accepted principles that allow the safe and effective use of this natural product.
Materials and methods
Chemicals and reagents
SDG and SECO (purity >95 %) were kind gifts from Agriculture and Agri-Food Canada, Saskatoon (A. D. M.). HPLC-grade acetonitrile was purchased from Fisher Scientific. Silastic tubing was purchased from VWR International. Diethyl ether was purchased from EMD Chemicals Limited. Methanol was purchased from Caledon Laboratories. ED, EL, umbelliferone (7-hydroxycoumarin), riboflavin, polyethylene glycol 300 (PEG 300), ethanol, Tween-80, benzyl alcohol and all other chemicals, unless otherwise indicated, were purchased from Sigma-Aldrich. Amicon Centrifree micropartition cartridges containing ultracel regenerated cellulose were purchased from Millipore. MilliQ water at 18·2 MΩ resistance was obtained from a MilliQ water purification system (Millipore). All other chemicals used were of analytical grade.
Single oral and intravenous bolus dose pharmacokinetics
Male Wistar rats (n 6 per dosing route) with a mean weight of 225 (sd 25) g were obtained from the Animal Resources Center, University of Saskatchewan. Rats were housed in a temperature- and humidity-controlled facility (22 ± 2°C) on a 12 h light–12 h dark cycle (07.00–19.00 hours), and had free access to a standard rodent diet and tap water. At 1 d before dose administration, rats were randomised based on the oral or intravenous administration route, and silastic cannulae (inner diameter 0·63 mm and outer diameter 1·19 mm; VWR International) were surgically implanted into the right jugular and left femoral veins of rats under isoflurane anaesthesia for the intravenous bolus dosing study, whereas only the right jugular vein was cannulated for the oral bolus dosing study. In a parallel study design, rats were randomised into two groups (n 6): one group received an oral bolus administration and the other group received an intravenous bolus injection (n 6). For the oral bolus administration route, flaxseed lignans SDG, SECO, ED and EL were administered via gastric administration (16 G, 3-inch stainless-steel feeding needle) to overnight fasted rats as a suspension in a vehicle containing formulation excipients, PEG 300, ethanol, Tween-80, benzyl alcohol and/or saline, in different proportions depending on the lignan (dose volume < 0·5 ml). SDG was dissolved in 100 % saline. SECO was dissolved in a mixture of PEG 300 (65 %), Tween-80 (8 %), benzyl alcohol (3 %) and ethanol (24 %). For ED and EL, PEG 300 (20 %), ethanol (10 %) and Tween-80 (15 %) were used in saline as a vehicle. The oral dose of SDG, SECO, ED and EL was 40, 40, 10 and 10 mg/kg, respectively. Blood samples (250 μl) were collected at 0 (pre-dose), 5, 10, 15, 20, 30 and 45 min, and at 1, 2, 4, 6, 8, 12 and 24 h post-dosing. SDG, SECO, ED and EL were dosed intravenously using the same formulation excipients (as described above) via the femoral cannula at doses of 20, 20, 5 and 1 mg/kg, respectively. Blood samples were collected from the jugular cannula at 0 (pre-dose), 5, 10, 15, 20, 30, 45 min, and at 1, 2, 4, 6, 8, 12 and 24 h post-dosing. Samples were left to coagulate for 40 min at room temperature and centrifuged at 37°C and 2000 g for 5 min to separate serum. Serum samples were transferred to microcentrifuge tubes and stored at − 80°C until analysis. Lignans were quantified using a HPLC fluorescence method( Reference Mukker, Kotlyarova and Singh 27 ). The bioanalytical assay was tested for within- and between-assay accuracy and precision, and quality-control (QC) samples were used as acceptance criteria for running an individual analysis. All procedures pertaining to animal handling and sample collection were approved by the Animal Research Ethics Board, University of Saskatchewan, and adhered to the Canadian Council on Animal Care guidelines for humane animal use (UCACS 20040070).
Pharmacokinetic data and statistical analyses
PK parameters were estimated from concentration v. time data for each individual rat using WinNonlin 4.1 (Pharsight). PK parameter estimates are presented as means and standard deviations. AUC0–∞ values were calculated by the linear trapezoidal rule-extrapolation method. Non-compartmental methods estimated systemic clearance (ClS), apparent volume of distribution (V d), half-life (T 1/2), maximum plasma concentration (C max) and time to C max (T max). Bioavailability (F) was calculated using equation 1. Graphs were drawn using the base package in R version 2.13.1 (R Foundation for Statistical Computing; http://www.R-project.org/)( 28 ):
where AUCoral is the estimated AUC extrapolated to time infinity following oral administration of a particular flaxseed lignan; AUCi.v. is the estimated AUC extrapolated to time infinity following intravenous bolus administration of a particular flaxseed lignan; dosei.v. and doseoral are the doses administered via the intravenous and oral routes, respectively. PK parameters of different lignans were compared using one-way ANOVA with Tukey's post hoc test. P value of 0·05 was considered as the threshold for significance testing.
Serum protein binding studies
SDG, SECO, ED and EL were tested for their serum protein binding capacity using Amicon Centrifree micropartition cartridges containing ultracel regenerated cellulose (14 mm, 30 kDa molecular-weight cut-off; Millipore). Blood samples were collected from anaesthetised (isoflurane) male Wistar rats (250 (sd 25) g) by cardiac puncture and allowed to clot for 30 min at room temperature. Subsequently, blank serum samples were collected by centrifugation at 3000 g for 5 min. An aliquot of 100 μl of lignan stock solution (500 μg/ml) was added into 900 μl of blank serum to obtain 50 μg/ml of lignans in serum, and equilibrated at 37°C for 20 min. After equilibration, the serum samples were transferred onto micropartition kit membranes and centrifuged using a fixed angle (25°) rotor (TA-14-50) in a Beckman Coulter Allegra 25R centrifuge (Beckman Coulter) for 8 min at 1000 g . About 70 μl of the filtrate were collected and analysed using the HPLC fluorescence method described below( Reference Mukker, Kotlyarova and Singh 27 ).
HPLC conditions
Lignan concentrations in serum samples were determined using a previously developed and validated HPLC fluorescence method( Reference Mukker, Kotlyarova and Singh 27 ). Briefly, the HPLC (Agilent Technologies) system consisted of a series 1200 quaternary pump with an online degasser, an autosampler and a fluorescence detector. Processed samples (50 μl) were injected onto a Waters Symmetry C18 column (4·6 × 150 mm, 5 μm) maintained at room temperature. The mobile phase consisted of water with 0·1 % formic acid (component A) and acetonitrile with 0·1 % formic acid (component B) in different ratios, and the analytes were eluted under a gradient mode delivered at a flow rate of 1 ml/min. Excitation and emission wavelengths were set at 277 and 617 nm, respectively.
Stock solutions (1 mg/ml) of the lignans, internal standards (umbelliferone and riboflavin) and QC samples were prepared by initial dissolution in methanol followed by dilution with the mobile phase (70 % component A:30 % component B for SECO, ED, EL and umbelliferone; 80 % component A:20 % component B for SDG and riboflavin) and storage at − 20 ± 5°C. Working solutions of the lignans (0·1–100 μg/ml) were prepared by a serial dilution of the stock solution with the mobile phase, while working solutions of the internal standard were prepared by a single dilution of the stock solution to a concentration of 100 μg/ml.
Calibration curve and QC samples were prepared on each day of analysis by adding 10 μl of individual working solutions to 90 μl of pooled rat blank serum with vortex mixing for 30 s. For SECO, ED and EL, 10 μl of umbelliferone (internal standard) solution (100 μg/ml) were added to 100 μl of calibration standards, QC samples or rat serum samples and vortex-mixed for 10 s. To all samples, 4 ml of diethyl ether were added, vortex-mixed for 10 min, centrifuged at 780 g at 4°C and the aqueous layer was snap-frozen with liquid N2, and the organic layer was transferred to glass tubes and evaporated to dryness under vacuum at 40°C in an evaporator (CentriVap Concentrator; Labconco Corporation). The residue was reconstituted in 100 μl of the mobile phase, vortex-mixed for 2 min and transferred to HPLC vials. For SDG, 10 μl of riboflavin (internal standard) solution (25 μg/ml) were added to 100 μl of calibration standards, QC samples or rat serum samples, and briefly vortex-mixed. The samples were transferred to centrifuge filters (Modified PES 10K, 500 μl; VWR International) and centrifuged at 13 300 g for 30 min. The filtrate was transferred to HPLC vials. The intra- and inter-day precision and accuracy were within 10 %, and all QC samples for a given run were within 10 % of their nominal value.
Erythrocyte partitioning
Fresh blood was collected from male Wistar rats under isoflurane anaesthesia by cardiac puncture. EL (1 mg/ml) solution was added to three different blood aliquots separately to make the final concentration of EL as 5 μg/ml. Aliquots of all blood samples were incubated at 37°C and 80 rpm. Blood samples were collected intermittently at different time points at 5, 10, 15, 30, 60, 90 and 120 min. Duplicate samples were centrifuged at 2000 g for 5 min and stored at − 80°C until analysis, while the third aliquot was stored as such for analysis. Erythrocytes were lysed by freezing the blood samples. All the samples were analysed using the HPLC fluorescence method as described above( Reference Mukker, Kotlyarova and Singh 27 ).
Results
Single oral and intravenous bolus dose pharmacokinetic studies
Since a comparative PK characterisation of pure flaxseed lignans in rats was not evaluated previously, we conducted single dose oral and intravenous dose PK studies in male Wistar rats (n 6). The mean intravenous and oral bolus serum concentration v. time profiles for SDG, SECO and ED are shown in Fig. 2. While the concentration of SDG in serum samples following oral administration was below the limit of quantification, SDG levels following intravenous dosing were detectable up to 4 h. SECO was detected up to 8 and 4 h, respectively, following both oral and intravenous administrations. The oral PK study of SECO exhibited a bimodal profile with very rapid absorption, such that C max was achieved at 5 min. Quantifiable levels of ED were obtained up to 4 h post-dose following both oral and intravenous administrations. The intravenous and oral administrations of EL at doses of 1 and 10 mg/kg, respectively, were fatal to rats within 2 h of dosing. In general, the rats were sluggish after administration of EL, and death was preceded by tremors, clawing, pawing, burying and jumping. Only two rats per dosing route were administered EL, and we conducted no further EL administration after the adverse outcome with the enterolignan. Natural log serum concentration v. time profiles of SDG, SECO and ED suggest two-compartment model characteristics (Fig. 2). No serum ED or EL was detected following the intravenous or oral bolus administration of SDG or SECO.

Fig. 2 Mean serum concentration v. time and mean serum log concentration v. time profiles of secoisolariciresinol diglucoside (SDG), secoisolariciresinol (SECO) and enterodiol (ED) upon oral (gastric administration) and intravenous (i.v.; femoral cannula) administration to male Wistar rats (n 6); the oral doses of SDG, SECO, ED and enterolactone (EL) were 40, 40, 10 and 10 mg/kg, respectively, and intravenous doses were 20, 20, 5 and 1 mg/kg, respectively. Values are means, with their standard deviations represented by vertical bars. Following oral administration, SDG was not detectable by HPLC; EL was fatal to rats following both oral and intravenous administrations.
The PK parameters are summarised in Tables 1 and 2. SDG exhibited the lowest apparent volume of distribution (0·76 (sd 0·29) litres/kg), systemic clearance (1·11 (sd 0·46) litres/h per kg) and half-life (0·52 (sd 0·17) h). SECO and ED exhibited large apparent volumes of distribution, but systemic clearance of SECO was lower (7·82 (sd 1·11) litres/h per kg), which attributed to its longer half-life (4·0 (sd 1·50) h) relative to ED (1·80 (sd 1·20) h) (Table 1). SECO exhibited the highest oral bioavailability at approximately 25 % in rats, while SDG and ED showed poor oral bioavailability ( < 1 %) (Table 2).
Table 1 Pharmacokinetic parameter estimates calculated by a non-compartmental pharmacokinetic analysis using WinNonlin 4.1 following an intravenous bolus administration of secoisolariciresinol diglucoside (SDG), secoisolariciresinol (SECO) and enterodiol (ED)‡ (at doses of 20, 20 and 5 mg/kg, respectively) in male Wistar rats (Mean values and standard deviations, n 6)
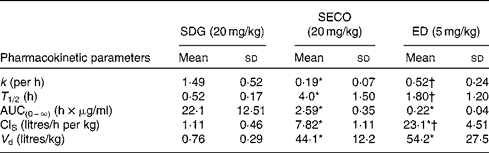
k, Elimination rate constant; T 1/2, half-life; AUC(0–∞), area under the serum concentration v. time curve extrapolated to infinity; ClS, systemic clearance; V d, apparent volume of distribution.
* Mean value was significantly different from that of SDG administration (P< 0·05).
† Mean value was significantly different from that of SECO administration (P< 0·05).
‡ Enterolactone was administered intravenously at a dose of 1 mg/kg, but rats died within 2 h of administration.
Table 2 Pharmacokinetic parameter estimates calculated by a non-compartmental pharmacokinetic analysis using WinNonlin 4.1 following a single oral dose administration of secoisolariciresinol diglucoside (SDG), secoisolariciresinol (SECO) and enterodiol (ED)‡ (at doses of 40, 40 and 10 mg/kg, respectively) and an intravenous bolus administration of SDG, SECO and ED‡ (at doses of 20, 20 and 5 mg/kg, respectively) (for systemic clearance (ClS) and volume of distribution (V d) calculations) in male Wistar rats (Mean values and standard deviations, n 6)
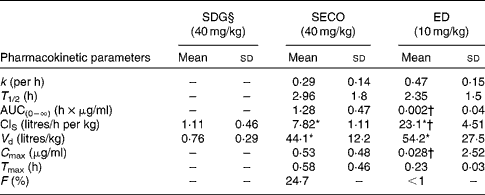
k, Elimination rate constant; T 1/2, half-life; AUC(0–∞), area under the serum concentration v. time curve extrapolated to infinity; C max, maximum serum concentration; T max, time at which serum concentration of lignans reaches C max; F, oral bioavailability.
* Mean value was significantly different from that of SDG administration (P< 0·05).
† Mean value was significantly different from that of SECO administration (P< 0·05).
‡ Enterolactone was orally administered at a dose of 10 mg/kg, but rats died within 2 h of administration.
§ SDG concentration was below the limit of quantification (50 ng/ml).
Serum protein binding studies and erythrocyte partitioning (enterolactone)
Since only the unbound form of a molecule is free to exert a pharmacological effect, the extent to which lignans bind to serum protein was determined in rat serum using ultrafiltration. The percentage of serum protein binding was reported for flaxseed lignans (SDG and SECO) and associated mammalian lignans. SDG demonstrated very minimal serum protein binding as determined by the assay used in the present study, while SECO, ED and EL showed increasing serum protein binding characteristics in ascending order (SECO 67 %, ED 93 % and EL 98 %). An erythrocyte partitioning study has indicated that EL did not accumulate in erythrocytes (blood concentration:plasma concentration ratio approximately 1).
Discussion
Flaxseed lignans are gaining research interest due to their putative health benefits against a number of chronic diseases such as cancer, CVD and diabetes( Reference Adolphe, Whiting and Juurlink 1 , Reference Prasad 29 , Reference Prasad 30 ). Our research group has previously reported the dose-dependent reduction in serum and hepatic cholesterol levels when SDG and SECO were administered chronically to high-cholesterol diet-fed rats( Reference Felmlee, Woo and Simko 7 ). Whether SDG, SECO or the enterolignans mediated the reduction in cholesterol levels is not known. To promote the safe use of this natural product in human populations and to understand the in vivo pharmacology of lignans, investigations into the relevant absorption and disposition characteristics of flaxseed lignans and their bioavailability are necessary. As an important step towards an understanding of their preclinical safety, we evaluated the oral bioavailability and PK characteristics of purified flaxseed lignans in the rat, which is an often-used animal model for such investigations. Most of the PK data reported in the literature follow from administration of flaxseed products, which contain multiple constituents that might substantially modulate the PK of lignans. PK of ED and EL after per oral administration of purified SDG (1·31 μmol/kg body weight) in healthy human subjects and healthy postmenopausal women( Reference Kuijsten, Arts and Vree 17 , Reference Setchell, Brown and Zimmer-Nechemias 18 ), as well as of radiolabelled SDG in rats have been reported( Reference Rickard and Thompson 26 ). To the best of our knowledge, no other studies have reported the administration of purified SECO, ED or EL. An understanding of their oral bioavailability is important to know whether these metabolites of SDG may be the putative bioactive lignans mediating the health benefits of flaxseed lignan supplementation.
In the present study, flaxseed lignans exhibited low to no oral bioavailability in male Wistar rats. Poor oral bioavailability may be attributed to poor intestinal permeability or high first-pass metabolism. For SDG, serum concentrations after oral administration were below the limit of quantification. In a previous study in our laboratory, an apparent permeability value for SDG in the Caco-2 cell monolayer system (an in vitro model of oral absorption) could not be calculated as we failed to detect the transepithelial flux of SDG across the Caco-2 cell monolayer( Reference Mukker, Michel and Muir 31 ). This was not unexpected because of its polar nature (clog P= − 1·338), as a result of the presence of two glucose moieties, and lack of reported specificity for uptake transporters that might be present in the Caco-2 cell monolayer. Furthermore, the Caco-2 cell monolayer phase II enzyme metabolism of SDG was less than 3 %, indicating that SDG does not undergo significant intestinal wall metabolism( Reference Mukker, Michel and Muir 31 ). The lack of transport across the Caco-2 cell monolayer and minimal phase II enzyme metabolism suggests poor permeation characteristics that limit the oral absorption of SDG. This is consistent with the lack of oral bioavailability in male Wistar rats in the present study, and these findings question SDG as the bioactive lignan, and, thus, the health benefits are probably associated with the metabolites of SDG.
The aglycone form of SDG (SECO) demonstrated moderate oral bioavailability (approximately 25 %), while the bioavailability of the metabolite of SECO (ED) was less than 1 %. Despite greater apparent permeability values in the Caco-2 cell monolayer( Reference Mukker, Michel and Muir 31 ), lower oral bioavailability of ED may be due to more extensive first-pass metabolism as the Caco-2 cell monolayer showed more efficient metabolism of ED relative to SECO( Reference Mukker, Michel and Muir 31 ). Jansen et al. ( Reference Jansen, Arts and Nielen 22 ) also found extensive glucuronidation and sulphation of ED and EL after in vitro incubation of ED and EL in HT-29 and Caco-2 cell lines. The ability of the gastrointestinal mucosa to contribute significantly to pre-systemic metabolism is also supported by the finding that ED and EL are largely found as glucuronic acid and sulphate conjugates in the portal vein of rats following an oral administration of flaxseed lignans( Reference Axelson and Setchell 32 ). A relatively higher contribution of intestinal glucuronidation compared with liver glucuronidation has been suggested for EL in an in vitro liver microsomal enzyme kinetic study( Reference Lin, Krol and Alcorn 23 ). Other studies have also suggested the contribution of both phase II enzyme conjugates and, to a minor extent, aromatic hydroxylated metabolites of ED and EL in rat, pig, rhesus monkey and human liver microsomes( Reference Dean, Chang and Doss 21 , Reference Heinonen, Nurmi and Liukkonen 33 – Reference Niemeyer, Honig and Lange-Böhmer 36 ). However, the exact disposition of these purified lignans in rats is not known.
Oral bolus administration of SECO demonstrated a bimodal serum concentration v. time profiles in all rats investigated in the present study. This was not observed following the intravenous administration. A bimodal serum concentration v. time curve usually arises as a result of enterohepatic recirculation or absorption from two different absorption sites or windows. Since the time of appearance of the second peak in the serum concentration–time profile was shorter than the gastric emptying time of rats (approximately 60 min)( Reference Trudel, Tomasetto and Rio 37 ), it is unlikely that the bimodal profiles are due to enterohepatic recirculation. We fitted a two-compartment model with absorption from different sites in WinNonlin; however, the model was unidentifiable due to over-parameterisation and lack of sufficient time points during the ascendency of the second serum concentration peak. Further studies with a greater number of serial blood samples in the early portion of the serum concentration–time profile are necessary to unambiguously determine whether two absorption sites are possible.
The flaxseed lignans demonstrated rapid elimination characteristics in rats. Expectedly, systemic clearance increased with decreasing polarity of lignans (with SDG being the most polar and ED being the least polar). A meta-analysis of 47 018 Pfizer compounds has shown that an increase in log P values (to a certain limiting value) corresponds to an increase in membrane permeability and liver microsomal clearance( Reference Johnson, Dress and Edwards 38 ). The increase in permeability probably increases access to metabolic enzymes, and lower solvation energy results in higher metabolic clearance. The systemic clearance values of the flaxseed lignans showed a similar trend with increasing log P values (SDG < SECO < ED). Interestingly, the systemic clearance of ED exceeded the blood flow rate to the rat liver (13·8 ml/min)( Reference Davies and Morris 39 ), yet mass balance principles suggest that hepatic clearance cannot exceed hepatic blood flow (i.e. hepatic blood flow is the limiting value of hepatic clearance)( Reference Pang and Rowland 40 ). Since systemic clearance is the sum of all clearance mechanisms contributing to the elimination of a compound, additional non-hepatic mechanisms of elimination are probably involved in the systemic clearance of ED in rats. These high systemic clearance values also further support a significant role of the intestine in not only presystemic metabolism but also overall disposition of the lignans in rats. The involvement of the intestine in lignan disposition may explain the predominance of the faecal excretory route in lignan elimination, as reported in the literature( Reference Bach Knudsen, Serena and Kjaer 41 ). Interestingly, Setchell et al. ( Reference Setchell, Brown and Zimmer-Nechemias 18 ) reported high clearance values for SECO (23·2 (sd 2·1) litres/h) in healthy postmenopausal women, but could not report for ED and EL.
As with systemic clearance, the apparent volume of distribution and extent of serum protein binding of the flaxseed lignans increased with decreasing polarity (SDG < SECO < ED). Although SDG did not bind serum protein, its poor permeability characteristics would probably limit its tissue distribution relative to SECO and ED. Both SECO and ED had a very large apparent volume of distribution values, indicating an extensive distribution into peripheral organs, a finding consistent with the literature( Reference Setchell, Brown and Zimmer-Nechemias 18 , Reference Murray, Kang and Astheimer 20 , Reference Saarinen and Thompson 42 ). Extensive serum protein binding characteristics of ED coupled with its very large volume of distribution are somewhat surprising, as only the unbound concentration is free to permeate across biological membranes. Tissue partitioning is a significant factor determining the volume of distribution, and these data suggest that ED has a high affinity for tissue binding sites relative to its affinity for serum protein binding. Additionally, these lignans have been reported to have high binding affinities towards steroid hormone binding globulins( Reference Schottner, Gansser and Spiteller 43 , Reference Schottner, Spiteller and Gansser 44 ). If a significant proportion of steroid hormone binding globulins is located extravascularly (mainly in the testis and prostate), binding to these extravascular steroid hormone binding globulins may partially contribute to a high volume of distribution. Several studies have suggested the accumulation of ED and EL in certain tissues such as the liver, prostate and breast( Reference Murray, Kang and Astheimer 20 ), which is consistent with the distribution characteristics of ED identified in the present study.
The half-life of flaxseed lignans is rather short in rats. SDG had the shortest half-life, followed by ED and SECO. Since half-life is a hybrid function of the volume of distribution and systemic clearance, a lower volume of distribution of SDG relative to the other lignans contributed to its shorter half-life. Despite a similar apparent volume of distribution values for ED and SECO, higher systemic clearance of ED contributed to its shorter half-life relative to SECO. Although we could not evaluate EL, Kuijsten et al. ( Reference Kuijsten, Arts and Vree 17 ) reported a longer half-life for EL in comparison with ED when pure SDG (1·31 μmol/kg body weight) was administered as a single oral dose to healthy human subjects. Similarly, Setchell et al. ( Reference Setchell, Brown and Zimmer-Nechemias 18 ) also reported a similar trend for the half-lives of EL, ED and SECO. However, these changes may be explained due to species differences. Interestingly, we detected no serum levels of ED and EL following bolus administration of SDG or SECO in rats. This is not surprising given the very low oral bioavailability of the enterolignans, and the need for their conversion from SDG and SECO by the microflora of the gastrointestinal tract.
The PK parameters of EL could not be established because EL was fatal to rats (death within 1–2 h post-administration) when administered at 1 mg/kg (intravenously) and 10 mg/kg (orally administered). Interestingly, EL was not detected in serum samples collected before death following either the intravenous or oral administration. An erythrocyte partitioning study has suggested that EL did not accumulate in erythrocytes and, therefore, does not explain the undetectable levels of EL in the serum. Possibly, EL underwent very rapid and extensive distribution into the tissues and the HPLC fluorescence method used in the present study lacked sufficient analytical sensitivity to detect low EL serum levels that may result from a very high apparent volume of distribution. To confirm these assertions, a more sensitive analytical method (e.g. LC–MS/MS) is required. Nonetheless, EL may accumulate in a vital organ(s), leading to organ dysfunction and rapid death. Just before death, rats exhibited symptoms such as tremor, pawing and burying. These symptoms of toxicity were observed in rats after administration of bifenthrin, a pesticide that acts on the central and peripheral nervous systems( Reference Andrew 45 ). One study has reported very low lignan concentrations in the brain, which might suggest that EL or a toxic metabolite of EL acted on the peripheral nervous system (or some other critical organ system) to cause toxicity( Reference Saarinen and Thompson 42 ). The exact cause of death remains unknown and requires further investigation.
In conclusion, the PK of flaxseed lignans is characterised by high systemic clearance and apparent volume of distribution values and short half-lives. SDG did not undergo significant absorption and is, thus, not likely to be directly responsible for the putative health benefits of flaxseed lignan consumption. SECO demonstrated the highest oral bioavailability. The systemic clearance, apparent volume of distribution and extent of serum protein binding of ED were higher than those of SDG and SECO. The high systemic clearance values suggest a significant involvement of non-hepatic clearance mechanisms, and extensive distribution characteristics indicate a high affinity of lignans for extravascular tissues. Oral (10 mg/kg) and intravenous (1 mg/kg) administrations of purified EL caused acute death (within 2 h of administration), but the cause of this acute toxicity remains unknown. Our general PK characterisation of the lignans administered in their respective purified forms is consistent with the reported literature involving administration of flaxseed lignans in complex mixtures; however, many questions remain regarding the absorption and disposition and the putative bioactive lignan form of this interesting class of compounds.
Acknowledgements
The present study was supported by the Rexall Research Trust Fund and College of Pharmacy and Nutrition, University of Saskatchewan. J. K. M. was funded by a graduate student scholarship from the University of Saskatchewan. The Rexall Research Trust Fund had no role in the design and analysis of the data or in the writing of this article.
The authors' contributions are as follows: J. K. M. made substantial contribution to the study conception and design, completed the PK study and summarised the data, analysed and interpreted the data, drafted the manuscript, and critically revised the manuscript; R. S. P. S. made substantial contribution to the study conception and design, completed the study and summarised the data, analysed and interpreted the data, and revised the manuscript; A. D. M. made substantial contribution to the study conception and design, analysed and interpreted the data, and critically revised the manuscript; E. S. K. made substantial contribution to the study conception and design, analysed and interpreted the data, critically revised the manuscript, and approved the final version of the manuscript; J. A., as the principal investigator, made substantial contribution to the study conception and design, surgical implantation of jugular and femoral cannulas, oversight on the PK data analysis, and the drafting of the manuscript, and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.







