Introduction and background research
Breast cancer (BC) is one of the most complex and diverse diseases, it represents the leading cause of death in women worldwide among other cancers and infectious diseases [Reference Jemal1]. Along with ovarian cancer, they constitute the most common forms of cancer in the developed and developing countries [Reference Abkevich2].
Since BC is considered as a public health problem in most countries as stated by the World Health Organization (WHO) [3]. Several studies have been conducted, either to establish the link between this malignant tumour and its host, also to understand its genesis and its response to treatments and drug effects [Reference Standish4]. According to the 2014 World Health Cancer report, they estimated 14 million new cases and 8.2 million deaths due to cancer worldwide [5]. Currently, 690 000 new cases are being diagnosed annually in the developed regions with around 92 000 new cases in Africa [6]. Even though the incidence for BC is high, the rate of mortality has been decreasing over the past 25 years [Reference Frisby7]. The last update of the global burden of cancer – International Agency for Research on Cancer (GLOBOCAN-IARC) [Reference Ervik8] also reported that the prevalence of BC in adult populations shows a high rate in Asia (36.7%), followed by Europe (29.1%), North America (17.2%), Africa (7.0%) and Latin America (8.8%). The incidence of mortality has been likely the same in Asia (39%), Europe (27.5%), North America (15.3%), Latin America (9.1%) and Africa (8%). By 2020, the forecast of BC will continue its increase slowly in the five continents from 1 651 872 in 2012 to 1 956 124 cases alongside with number of mortality going from 517 578 cases in 2012 to 617 479 [Reference Ervik8]. The growing prevalence of BC, especially in Africa, incriminates many factors, including hormonal, toxic or genetic factors. In this context, many genes such as BRCA1/2,TP53, HER2 and CHEK2 have been studied to characterize mutations specific to each population.
The BC screening by mammography can reduce the cancer-specific mortality. The value of other screening methods such as the genetic detection of familial and non-familial mutations is relevant and explain a large part of BC heredity. Although, the lack of observations of such gene mutations, particularly in Africa, is one of the reasons for the growing disparity between the sensitivity of diagnosis and rate of variation. Besides genetic variation associated with inheritance of some genes with defined penetrance of BC [Reference Standish4], the determination of other risk factors leading to the genetic variability of the disease is an area that needs to be considered for accurate detection of BC in Africa.
Epidemiological studies have provided great support to understand the genetic variability and risk factors among populations around the world to facilitate the accuracy of diagnosis, medical support and drug response. Therefore, the aim of this work is to provide a panel of genes associated with BC in the African populations. This panel of genes will be extracted through a literature review. Our investigation could be useful both for future epidemiological studies and enable decisions on new research and management strategies for BC genetic studies.
Methods
We searched in the databases PubMed, Scopus and Web of Science for studies reporting genetic variations implicated in BC in Africa. The search was conducted based on specific Medical Subject Headings (MeSH) and keywords, which are BC, genetics, gene, mutation, association and Africa. All languages were searched initially, but only English language studies were selected.
We extracted relevant information from each study using a standardized form and we included studies in this review only if the following criteria were met: a case-control study on the association between a variation (SNP, InDel, CNV…) and BC risk, and data were provided on mutations and polymorphisms associated with BC. We excluded case-only studies and clinical trials. The study selection process and flow diagram for identifying studies is detailed in Fig. 1.

Fig. 1. Flow diagram for identifying studies for assessment of breast cancer mutations in Africa.
In order to extract most of studies regarding BC and genetics, we identified published articles without fixing a specific date. The strategy adopted was applied for African populations to evaluate African BC specificities. We also searched the data concerning other continents, this served to compare and discuss African results.
Results
The African continent is commonly divided by the United Nations (UN) into five regions or sub-regions: Northern, Western, Central, Eastern and Southern Africa (http://www.un.org/esa/population/publications/worldageing19502050/pdf/96annexii.pdf). Therefore, we used this division to present the results retrieved from different databases.
Northern Africa region (NA)
For this region, we summarized studies on BC mutations in Algeria, Tunisia, Egypt and Morocco. Only Libya was excluded for lacking relevant genetic studies on BC. In total, 28 major studies have been conducted in NA region, including more than 3700 BC cases and showing that BRCA1/2 genes were the most investigated for their association with BC. Other genes such as HER2, P53, APOBEC3 and MTHFR were also studied.
In Algeria, three major studies have investigated the carried mutations on BRCA1 and BRCA2 genes in both sporadic and familial cases. Uhrhammer et al. [Reference Uhrhammer9], using complete gene sequencing found one BC founder mutation in BRCA1 gene (c.798_799delTT) in Algerian population with 9.8% familial cases and 36.4% sporadic cases. This mutation has been reported as the first non-Jewish founder mutation to be described in NA.
In another study performed by Cherbal et al. [Reference Cherbal10], over 101 individuals from 79 breast and ovarian cancer families were examined for unclassified variants (UVs) and polymorphisms in the BRCA1 and BRCA2 genes by Single-strand conformation polymorphism (SSCP) or High-Resolution Melting (HRM) curve analysis, followed by direct sequencing. The result revealed 168 UVs and polymorphisms in BRCA1/2 genes, 68 in BRCA1 and 100 in BRCA2. Cherbal and colleagues also performed BRCA1/2 mutation screening by HRM curve analysis and direct sequencing in 86 individuals from 70 Algerian BC families with history of BC, five mutations were detected in BRCA1 (c.83_84delTG, c.181T>G, c.798_799delTT, delEx2, delEx8) and 57 UVs/SNPs were revealed in both BRCA1 and BRCA2 [Reference Cherbal11]. In a recent study, Henouda et al. [Reference Henouda12] found five mutations in 40 different Algerian families with early BC. Among them, five mutations were identified in BRCA1 (c.1817del, Del exon 2, c.4065_4068del, c.5332 + 1G>A, c.5117G>C) and nine in BRCA2 (c.7654dupA, c.1528G>T, Del exons 19-20, c.6450del, c.7462A>G, c.1504A>C, c.5117G>C, c.5939C>T, c.1627C>A).
In Tunisia, a BRCA1 study on nine Tunisian patients with hereditary BC was carried out in order to evaluate the implication of the BRCA1 and DNA mitochondrial mutations and revealed that the mitochondrial mutation 315.insC was strongly implicated in two unrelated patients [Reference Troudi13]. Mahfoudh et al. [Reference Mahfoudh14] performed a screening for germline mutations of BRCA1 in 16 Tunisian high-risk BC families, where six families were found with BRCA1 mutations: three truncating mutations were described in BRCA1 (c.798_799delTT, c.3331_3334delCAAG, c.5266dupC), one specific to Tunisian population (c.212 + 2insG) and the c.798_799delTT was suggested to be a Tunisian founder mutation based on its frequency (18%). Mestiri et al. [Reference Mestiri15] screened 12 Tunisian women with familial or sporadic BC for BRCA1 gene mutations and the 1294del40 mutation of BRCA1 was found only in a patient with non-familial BC. In contrast, the 185delAG mutation was absent in all cases of BC. Troudi et al. [Reference Troudi16] studied the BRCA1/2 genes in 36 Tunisian patients with breast and/or ovarian cancer. The results revealed 90% of cases with deleterious mutations. In BRCA1 four mutations (c.211dupA, c.4041delAG, c.2551delG and c.5266dupC) were detected in two unrelated patients, the c.5266dupC mutation was described for the first time in a non-Jewish Ashkenazi population. In addition, two frame-shift mutations (c.1309del4 and c.5682insA) were observed in BRCA2. Furthermore, Riahi et al. [Reference Riahi17] performed a screening on BRCA1/BRCA2 genes in 48 patients by direct sequencing, the result revealed 12 mutations including three in BRCA1 (c.211dupA, c.5266dupC and c.1504_1508delTTAAA) and two novel mutations in BRCA2 (c.1313dupT and c.7654dupT). In a recent work, the same group proposed a cumulative mutation analysis with data from three Tunisian studies including 92 Tunisian women, the results revealed two recurrent mutations (c.211dupA and c.5266dupC) in 76% of BRCA1-related families and three recurrent mutations (c.1310_1313del, c.1542_1547delAAGA and c.7887_7888insA) in 90% of BRCA2-related families [Reference Riahi18]. Hadiji-Abbes et al. [Reference Hadiji-Abbes19] identified a novel in-frame deletion (5456del6 bp) in the BRCA2 gene in an early onset woman with BC without family history.
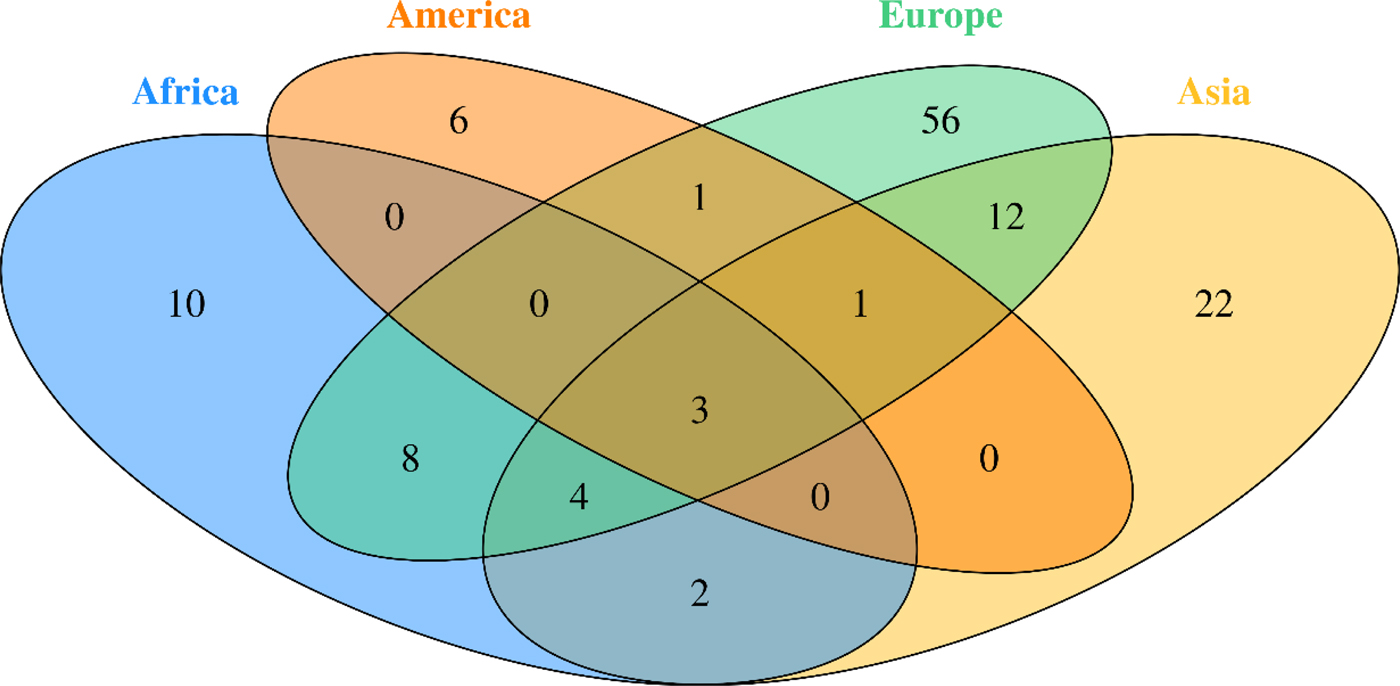
Fig. 2. Venn diagram of studied Breast Cancer genes in different continent
HER2 gene has been reported as major factor in BC development and progression [Reference Kallel20]. Therefore, Kallel et al. [Reference Kallel20] focused on this gene by analysing three polymorphisms in 148 cases and 290 controls from the Tunisian population. The Ile(655)Val mutation was found to be significant in 90% of cases. The noncoding SNP (rs903506) and the H(AC)I4 mutation were also implicated. The association of Ile(655)Val with BC has been confirmed by a further meta-analysis from 27 case-control studies [Reference Lu21].
A study concerning the P53 genewas conducted in order to investigate the association of p53 Arg72Pro, Ins16 bp and G13964C polymorphisms and their haplotypes with BC risk in 159 Tunisian female patients. The results revealed that these mutations were associated with familial BC risk in this population [Reference Trifa22].
The ESR1 and ESR2 genes were also studied by Kallel et al. [Reference Kallel23] in 148 Tunisian BC patients and 303 controls using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The ESR1 2014 G allele showed significant association with BC risk (p = 0.025).
In Egypt, Bensam et al. [Reference Bensam24] screened BRCA1 and BRCA2 in 20 Egyptian patients with BC. Mutations were detected in 44% of the studied population, with 18% in the BRCA1 gene (185AGdel, 624C>T) and 26% attributed to BRCA2 (999TCAAAdel, 2256T>C, 8934G>A).
The P53 gene has also been studied in the Egyptian population by El-Ghannam et al. [Reference El-Ghannam, Arafa and Badrawy25], their study focused on mutation detection in 30 patients with BC using flow-cytometry, PCR-SSCP and sequencing. P53 mutations including A218 T, R279G, S297X and Y159X were detected in 17% of patients.
In another study, Hussein et al. [Reference Hussein26] examined the relation between the PON L55 M and Q192R polymorphisms with BC risk in Egyptian females and analyzed their relation to clinic-pathological parameters of BC. Both SNPs were shown to be significantly associated with an increased risk of BC. However, when they conducted a study in order to evaluate the association of two polymorphisms in XPD (Asp312Asn) and XRCC1 (A399G) on 100 BC Egyptian females, they did not detect an association between the XRCC1 gene mutation and BC [Reference Hussien27].
In Morocco, three studies targeted BRCA1/2 mutations, the first one was conducted by Laarabi et al. [Reference Laarabi28], and was interested in analyzing BRCA1/2mutations in five healthy women belonging to three families with an elevated risk of BC, this investigation revealed that three asymptomatic women were found to be carriers of BRCA1/2 mutations. The second study was performed by Tazzite et al. [Reference Tazzite29] on a Moroccan cohort of 40 women, diagnosed with BC and a familial history of breast/ovarian cancer, or aged less than 40 years old showed that 25.64% of patients carried BRCA1/2 mutations. The third study, conducted by Laraqui et al. [Reference Laraqui30] on 121 Moroccan women diagnosed with BC, only BRCA1 status was investigated and revealed that mutations were found in 36.1% of familial cases and 1% (1/102) of early-onset sporadic. The result of these three studies showed 14 BRCA1/2 point mutations; nine in BRCA1 (c.68_69delAG, c.181T>G, c.798_799delTT, c.3279delC, c.2805delA, c.1016dupA, c.4942A>T, c.5062-5064delGTT, c.5095C>T) and five in BRCA2 (c.517-1G>A, c.3381delT, c.5073dupA, c.7110delA, c.7235insG).
Considering the potential function of driver and APOBEC3 genes in the process of tumorigenesis in BC, it is possible that germline variations and copy number variations (CNV) in those genes could influence the risk of BC. In this term, a case-control study was conducted in a group of Moroccan women targeting 36 SNPs in 13 genes (APOBEC3A, APOBEC3B, ARID1B, ATR, MAP3K1, MLL2, MLL3, NCOR1, RUNX1, SF3B1, SMAD4, TBX3, TTN). The analysis showed that 12 SNPs in eight driver genes, four SNPs in APOBEC3B and one SNP in APOBEC3A were associated with BC risk and/or clinical outcome at the significance level of 0.05. RUNX1_rs8130963 (p = 0.0005), TBX3_rs8853 (p = 0.0003), TBX3_rs1061651 (p = 0.0002), TTN_rs12465459 (p = 0.0009), were the most significantly associated SNPs with BC risk. A strong association with clinical outcome was detected for SMAD4_rs3819122 with tumour size (p = 0.009) [Reference Marouf31].
Another study was conducted to investigate if MTHFR C677T polymorphism modulates the risk of developing BC in Moroccan women. Results showed a positive correlation between the MTHFR C677T polymorphism and progesterone receptor expression (p = 0.04). There was a significant association between C677T polymorphism and BC risk in both additive (p = 0.007) and dominant (p = 0.008) models [Reference Diakite32].
Many studies were also conducted to investigate the implication of three genes described as a high penetrance BC susceptibility. The PIN3 Ins16 bp polymorphism of the TP53 gene [Reference Tazzite33], the ABCB1 C3435T polymorphism [Reference Diakite32] and the +936C/T VEGF-A polymorphism [Reference Rahoui34]. However, there was no evidence of a significant association between these polymorphisms and risk of BC.
Southern Africa (SA) region
No founder genes have been described in BC patients of the four countries (Botswana, Lesotho, Namibia and Swaziland) included in SA's region. Whereas, the studies performed among South Africa's BC patients revealed variants in the two major genes BRCA1&BRCA2 and in five other genes deemed intermediate (CHEK2, PALB2) and minor (RAD50, MTHFR, hMLH).
Reeves et al. [Reference Reeves35] were the first to report the role of BRCA1 in 90 SA BC families in 2004. Thereafter, Francies et al. [Reference Francies36] studied 108 sporadic patients with 78 women premenopausal and 30 triple negative breast cancer (TNBC) from the four South African ethnic groups, to determine the mutations frequency in BRCA1, BRCA2 and PALB2 and evaluate the presence of the CHEK2. This study used the next-generation sequencing (NGS) approach in combination with Multiplex Ligation-dependent Probe Amplification (MLPA) to detect large rearrangements in BRCA1 and BRCA2. The result revealed the BRCA2 (c.7934delG) Afrikaner founder mutation, the BRCA2 variant (c.9875C>T) and the CHEK2 mutation (c.1100delC). PALB2 variants (c.118A>G, c.2845T>C) were also described as probably damaging [Reference Francies36]. The two studies mentioned previously and carried out on the BRCA1 gene reported nine following mutations in a South African population: c.181T>G, c.212G>A, c.3593T>A, c.1155G>A, c.1953_1954insA, c.1843_1845delTCT, 1493delC, 185delAG, 4957insC, 5382insC, E881X and S451X [Reference Reeves35, Reference Francies36].
Sluiter et al. [Reference Sluiter, Mew and van Rensburg37] also identified PALB2 as a BC susceptibility gene, and the related mutations double the BC risk with moderate to low penetrance. In a cohort of 48 young South African BC patients unselected for family history of BC, the authors determined the involvement of PALB2 mutations and they identified a novel truncating mutation, c.697delG (V233fs).
MTHFR, RAD50 and hMLH1 genes were also showed to be involved in the development of BC in this region of Africa [Reference Blokhuis38, Reference van der Merwe39].
Western Africa region
In this region of Africa, few studies focused on the genetic risk factors for BC. These studies were performed only in Senegal and Nigeria, and were restricted on the description of the mutations in BRCA1, BRCA2 and LEPR genes.
In Nigeria, genetic studies revealed that BRCA1 and BRCA2 are the most prevalent cause of BC. The overall BRCA1/2 mutation rate is 11%, which is the highest reported rate for any BC cohort from a non-founder population unselected for family history, ethnicity or age of onset in Nigeria. Unexpectedly, only 7.1% of patients carried BRCA1 mutations and 3.9% individuals were BRCA2 mutation carriers. A total of 48 mutations were found in BRCA1/2 (31 in BRCA1 and 17 in BRCA2), including non-sense, missense, frame-shift and splice-site mutations. Deleterious mutations like Y101X, 1742insG, C64Y, 4241delTG and del Ex 21 were the most commonly carried BC-related mutations in exons 11, 12 and 21 of the BRCA1 gene. The BRCA2 gene in the Nigerian population carried the 1538delAAGA, 2630del11 and 9045delGAAA mutations in exons 10, 11 and 22, respectively [Reference Fackenthal40].
The LEPR Gln223Arg polymorphism was also investigated in premenopausal Nigerian women carrying at least one LEPR 223Arg allele. This investigation revealed that the heterozygote Gln223Arg and mutant homozygote Arg223Arg have no association with postmenopausal BC risk (p = 0.68) [Reference Okobia41]. Premenopausal Nigerian women carrying at least one LEPR 223Arg allele were at a modestly increased risk of BC (p = 0.07) [Reference Okobia41].
In Senegal, BC studies revealed that the BRCA1 gene is the most common genetic risk factor for the disease development in Senegalese women. Indeed, a novel deleterious mutation (c.1949_1950delTA) has been described [Reference Diez42].
Eastern Africa region
In this region, our bibliographic research revealed that only Ethiopia and Sudan contributed to the determination of the genetic risk factors of BC.
In Sudan, two studies were performed, the first one reported 33 BRCA1 point mutations, found in 59 Central Sudanese premenopausal BC patients. [Reference Biunno43]. The second study characterized germline BRCA1/2 mutations in patients (34 females, one male) selected by diagnosis within age 40 years or male gender. A total of 33/35 patient were found to carry 60 BRCA1/2 variants, among them, 17 were novel, 22 reported in populations from various geographic areas and 21 reported worldwide. The most frequent mutations found are in BRCA1 (c.3999delT, c.4065_4068delTCAA, c.557C>A, c.2458A>G, c.5090G>A) and in BRCA2 (c.3195_3198delTAAT, c.6406_6407delTT, c.8642_8643insTTTT, c.6101G>A, c.68-7delT) [Reference Awadelkarim44].
The most relevant studies in Ethiopia targeted the implication of the HER gene. This Proto-oncogene plays an important role in the carcinogenesis and the prognosis of BC [Reference Lu21]. Many studies have been conducted to explore the association between the HER2 Ile655Val polymorphism and BC risk, a significant association among Africans was found for Val/Val v. Ile/Ile genotypes: odds ratio = 78, 95% confidence interval = 1.94–39.72, p = 0.35 for heterogeneity; for the recessive model Val/Val v. Ile/Val + Ile/Ile: odds ratio = 8.60, 95% confidence interval = 1.92–38.48, p = 0.31 for heterogeneity [Reference Lu21].
Central Africa region
In this region, only Democratic Republic of Congo have studied the BRCA1/2 genes in a family with a severe history of BC at young ages. This genetic analysis revealed the presence of the c.2389_2390delGA mutation at the heterozygous state in all BC family members, the mutation leads to a frameshift at codon 797 of the BRCA1 gene (p.Glu797fs) [Reference Luyeye Mvila45].
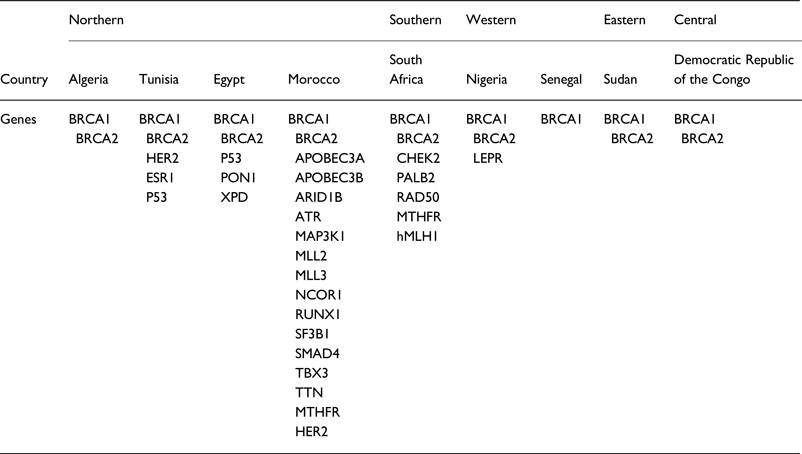
A summary of the extracted data from our literature review, the panel of genes illustrated in Table 1 represents the genes associated with BC in the different African populations.
Table 1. Panel of genes associated with BC in African populations
Discussion
BC is the most commonly diagnosed cancer in African women and the main cause of death from cancer diseases. The evaluation of the BC genetic risk factor provided support to understand the relationship between genomics and cancer. Only nine of 54 countries in Africa have studied few genes involved in BC. In this review, data showed 27 distinct genes in Africa (Table 1). Mutations in BRCA1 and BRCA2 genes were identified in all five regions of the continent.
According to our literature review, the BRCA1 c.798_799delTT mutation was found recurrent in the three North African countries, Algeria [Reference Cherbal11] Tunisia [Reference Mahfoudh14] and Morocco [Reference Marouf31] but absent in other African regions. Moreover, the mutation c.181T>G on BRCA1 was commonly reported only in the Algerian [Reference Cherbal11] and Moroccan [Reference Marouf31] populations. Another BRCA1 mutation c.2612C>T was identified in Algeria [Reference Cherbal11] and Tunisia [Reference Riahi17]. The BRCA2 mutations c.-26G>A, c.7242A>G and c.8503T>C were identified in two NA countries, Algeria [Reference Cherbal11] and Tunisia [Reference Mahfoudh14].
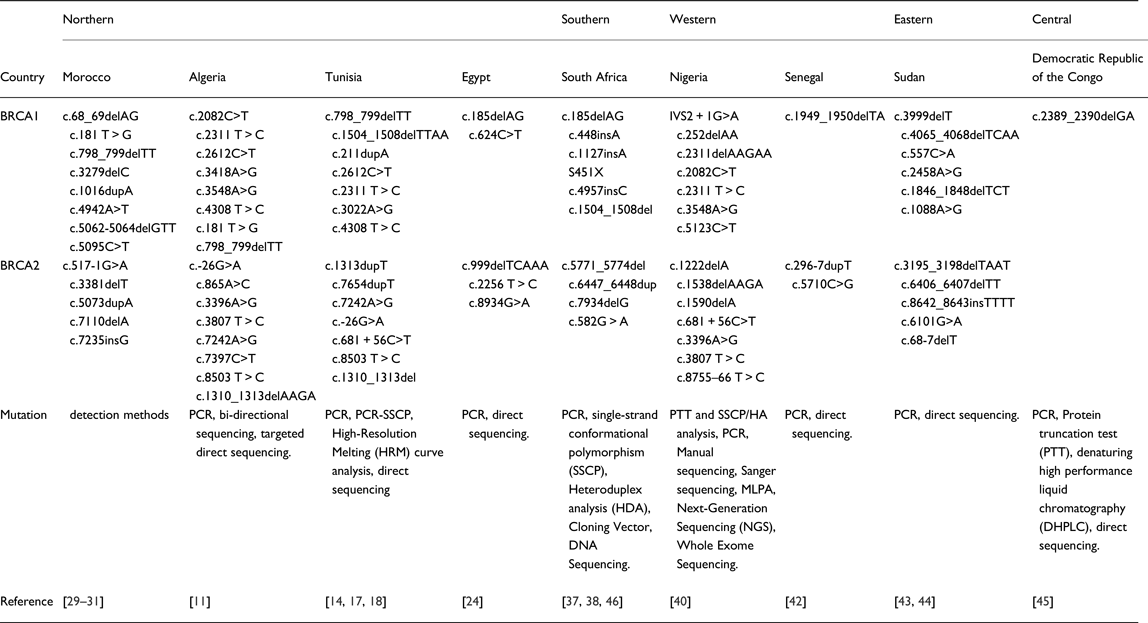
Furthermore, many common mutations in BRCA1/2 were found in other regions, especially in Western Africa, where three common mutations with BRCA1 (c.2082C>T, c.2311T>C, c.3548A>G) and two common BRCA2 mutations (c.3396A>G, c.3807T>C) were identified [Reference Fackenthal40]. These inter-regions similarities were also observed between South Africa and two NA countries: the c.1504_1508del BRCA1 mutation is common between South Africa [Reference De Leon Matsuda46] and Tunisia [Reference Riahi17], and the c.185delAG BRCA1 between South African [Reference Sluiter, Mew and van Rensburg37] and Egyptian [Reference Bensam24] populations. More details concerning mutations retrieved in African countries are listed in Table 2.
Table 2. Mutation detection methods used in African Studies
Our literature review revealed that the majority of genes were studied in the NA (18 genes) . Whereas, only six genes were studied in SA. We performed a functional analysis using the online tool ToppGene (https://toppgene.cchmc.org) to explore the biological function and pathways of the studied genes in both of NA (Table 3) and SA (Table 4). The functional analysis revealed a strong similarity between the candidate genes from NA and SA regions. Furthermore, the biological pathways ‘Role of BRCA1, BRCA2 and ATR in Cancer Susceptibility’ and ‘Breast Cancer’ were found in the top enriched terms for NA and SA regions with a p of 1.074×10−8 (2.09×10−10) and 2.804×10−5 (2.69×10−3), respectively.
Table 3. Top enriched terms and biological pathways identified by functional analysis of breast cancer candidate genes studied in Northern Africa
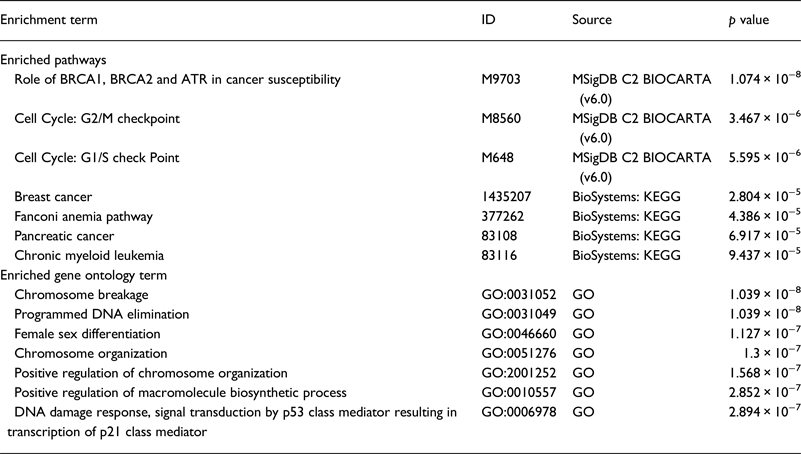
Table 4. Top enriched terms and biological pathways identified by functional analysis of breast cancer candidate genes studied in southern Africa
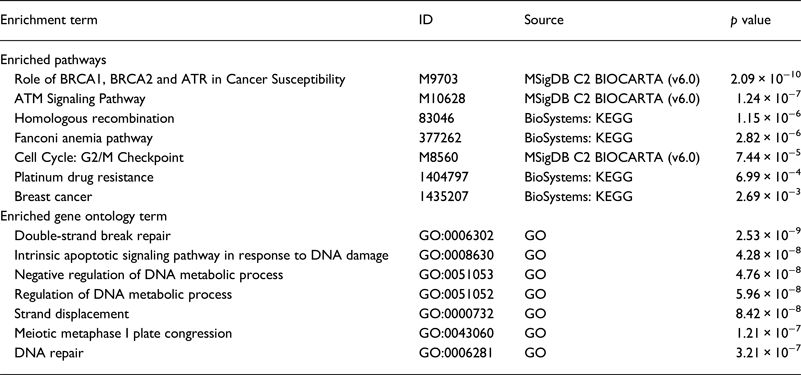
GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; MSigDB, The Molecular Signatures Database.
In the remaining regions, a modest number of genes have been studied only in few countries (Nigeria, Senegal, Sudan, etc.), this may reflect the lack of clinical monitoring for BC patients and control of the disease.
The screening for different types of gene mutations to determine their origin, either somatic or germinal, or either founder or recurrent mutations helped to characterize the population specificities toward genes and mutations found in populations across the continent. Researchers in American continent have studied the genetic factors of BC through decades using different types of technologies, from RFLP-PCR to Whole Exome Sequencing, these studies have largely contributed to define major and minor genes and mutation incidence. Several mutations, mainly in BRCA1/2, have been described in both American and African populations, such 185delAG [Reference De Leon Matsuda46], 1742insG, 2630del11, 4241delTG, 9045delGAAA and delEx12 [Reference Villarreal-Garza47]. A wide spectrum of mutations in other genes associated with BC have been identified but have not been significantly reported in both continents [Reference Amemiya48, Reference Ossa and Torres49]. Many mutations described in the American studies have not been described or are rarely found in the African continent, such as VS511G.A, S955X, R1443X, E1644X, 943ins10, C64Y, M1775R, Q1090X and Y101X [Reference Ossa and Torres49]. A common phylogenetic relation between American and African populations would explain the recurring mutations found among them, and the genetic history between women with BC found in North or South America. The diversity of ethnicity among the American populations has an important role in explaining these findings. Indeed, a significant number of women diagnosed with BC in North America have African or Latino-Hispanic origins and a relevant number of mutations found in these women have been described in African women with BC [Reference Ossa and Torres49]. However, many detected mutations in the American women with African-related origins have not been described in African studies. Considering these undescribed mutations in African BC women, it would be relevant to complete the African genetic risk factor panel in forthcoming BC genetic studies.
Our literature search revealed that only nine African countries had studied the genes involved in BC. Unlike, in Europe, where the studies covered 15 countries, which gives a valuable information regarding BC genetics in Europe. Indeed, different studies have been carried out through the European continent to determine the role and the implication of BRCA1 and BRCA2 genes in BC, as well as detect novel mutations [Reference Adank50–Reference Willems72]. Other genes have been studied in both African and European continents and revealed to be associated with BC, such as CHEK2 [Reference Armaou52, Reference Peixoto65, Reference Bisof73–Reference Cybulski75], TP53 [Reference Bisof73, Reference Bisof76, Reference Zhang77], MTHFR [Reference Papandreou78], PALB2 [Reference Dansonka-Mieszkowska79–Reference Silvestri81]. Whereas, other genes have been proved involved in BC development solely in Africa such as APOBEC3A, APOBEC3B, ARID1B, NCOR1, SMAD4, MAP3K1, HER2, HER and RAD50 or only in Europe such as RAD51 [Reference Gao82–Reference Vuorela84], COMT [Reference Ding85, Reference Ghisari86], CYP17 [Reference Ghisari86], MRE11A [Reference Loizidou61, Reference Loizidou87], CYP19 [Reference Loizidou87] and MDM2 [Reference Zhao88].
The ethnic similarity between the different regions of Europe and Africa can guide African scientists to well investigate the genes involved in BC. For instance, research in North Africa should explore other genes, such as GSTM1, GSTT1 found in Portugal [Reference Ramalhinho, Fonseca-Moutinho and Breitenfeld89] and PALB2, ATM, TNFRSF11A found in Spain [Reference Garcia80, Reference Grana90, Reference Bonifaci91].
The investigation in Asia were more important. Indeed, many studies exploring the major and minor genes involved in BC were performed in different Asian countries. Several Meta-analyses and reviews were also conducted [Reference Lu21, Reference Liede92, Reference Kim93].
For BRCA1 and BRCA2 genes, several germline mutations were identified and listed in the National Comprehensive Cancer Network (NCCN) guidelines, however, no data were reported concerning African studies [94]. The genetic aetiology of hereditary BC has not yet been fully elucidated. Although germline mutations of high-penetrance genes such as BRCA1/2 were shown implicated in development of BC hereditary, at least half of all BC families are not linked to these genes [Reference Yang95]. For example, in China 42 deleterious germline mutations were identified in 21 genes, including 18.2% BRCA1 or BRCA2 mutations, 3% TP53 mutations, 5.1% DNA mismatch repair gene mutations, 1% CDH1 mutations, 6.1% Fanconi anemia pathway gene mutations, and 9.1% mutations in other genes [Reference Yang95].
CYP, CHEK2 and MTHFR were also studied and their involvement in BC showed that they may modify susceptibility to BC [Reference Ma70, Reference Baloch96, Reference Qi97]. TNF-alpha, Enos, RAD50, CCND1, NBS1 and SULT1A1 genes were studied in Asia and the results exclude the possible role of RAD50 and NBS1 in familial BC [Reference He59]. SULT1A1 may be a low-penetrant risk factor for developing BC in the Asian population [Reference Wang66]. Although the clinical significance of newly identified low-penetration genetic mutations has not yet been fully appreciated in Asia, these new findings provide valuable epidemiological information for future studies of BC in the Asian population.
Unlike Africa, many studies in Asia have used recent techniques such as NGS and Whole Exome Sequencing that have contributed to the development of genetic screening panels [Reference Lee55, Reference Cai98, Reference Guo99]. For example, a test panel has been developed in accordance with NCCN, which has proposed guidelines for the genetic testing of the BRCA1 and BRCA2 genes, based on studies in western populations. However, like in Africa, there is still a gap in the availability of genetic counselling and genetic testing in Asian countries due to financial facilities, access and inaccurate reporting of a family history of cancer.
The variety of options now accessible for the patient and physician in making appropriate and timely decisions in hereditary breast and ovarian cancer has triggered a daily increase in the demand for mutation analysis of the BRCA1/2 genes on the other continents. Therefore, it is necessary for African scientists to implement these testing and analysis, to better characterize BC genetic risk factors.
The analysis of the African genetic studies reported in our literature search revealed that the screening of genes and mutations related to BC in African countries is less illustrative compared with other continents. Therefore, it is necessary to encourage researchers in Africa to characterize more genes involved in BC, to better target the diagnosis and guide specific and efficient therapeutic strategies for African community.
The insufficiency of reliable data on BC in Africa and the increase of mortality prevalence and incidence rates could be linked with precarious socioeconomic criteria. For instance, the weakness of the health system, the lack of health insurance and coverage, the limited access to medication, the scarcity of care facilities and counselling, the deficiency of genetic testing and low income. In addition, the lack of support for scientific research in several African countries also contributed to the spread of the disease.
Acknowledgements
We acknowledge Pr. Nicola Mulder's (University of Cape Town) support, advises and manuscript revision.
We acknowledge greatly H3Africa, H3ABioNet networks And the African Genomic Medicine Training (AGMT) committee
Declaration of interest
None.








