INTRODUCTION
Tuberculosis (TB) affects a variety of individuals, yet its prevalence in vulnerable populations is high. Overcrowding, poor economic conditions and barriers to access healthcare, facilitate the spread of TB. Therefore, equity in healthcare delivery is an essential component for adequate TB control in different population groups [Reference Frieden1, Reference Rasanathan2].
The overall burden of TB in industrialized countries has declined during the last decade. However, the incidence of TB in native ethnic minorities remains disproportionately high in most of these countries [3, Reference Tollefson4]. For instance, the rate of TB in US-born Afro-Americans is several-fold higher than the national TB incidence [5]. Similarly, the rate of TB in Canadian Indians is higher than that in Caucasian Canadians [Reference Long6].
Although Israel and other developed countries have reversed the trend of rising rates of TB, this accomplishment should not be underestimated. The healthcare system in the UK, for instance, has not succeeded in the last decade in reducing the incidence of TB [7]. Furthermore, a prominent feature of the Israeli National TB Program (NTP) is its reliance on the existing healthcare infrastructures of primary-care clinics, rather than setting up expensive programmes to tackle TB, thus improving cost containment of TB treatment.
The annual incidence of TB in Israel has steadily decreased for more than a decade, from 11 to 5·8 cases/100 000 population between 1998 and 2011, respectively. However, the rate TB in migrants who were born in countries with a high-incidence of TB is relatively high [Reference Mor8]. Israeli-born Arabs (IA) are the major ethnic minority in Israel, and comprise 21% of the country's almost 8 million total population [9]. IA express several characteristics which may be associated with an increased TB burden, such as a relatively lower income and education levels, greater unemployment and high-density living environments compared to the national Israeli average [10]. A previous study estimating the incidence of TB in Bedouins (a nomadic subpopulation of IA) in southern Israel demonstrated twofold higher TB incidence than that of the national Israeli population in 1987 [Reference Greene11]. However, there are no other reports regarding the trends of TB incidence in IA in Israel, particularly since the inauguration of the NTP in 1997 [Reference Chemtob12]. The NTP facilitates directly observed therapy for all TB patients free of charge for the entire period of their treatment [5].
This study aimed to compare the burden of TB between IA and Israeli-born Jews (IJ) during the last decade, as well as their demographic, clinical, laboratory and genotyping characteristics.
METHODS
This historical cohort study compared the incidence of TB in IA and IJ between 1999 and 2011. In Israel it is mandatory that all diagnosed TB patients are reported to the Ministry of Health (MoH). Each notification includes demographic, clinical, laboratory information and treatment outcome. All notifications are verified both at the regional and central levels of the MoH. The National TB and HIV registries are matched periodically, as both diseases are reported nominally to the same department [Reference Mor13].
A TB case was defined as culture-confirmed disease due to Mycobacterium tuberculosis complex identified from sputum, fluid or body tissue, or a clinician's decision to treat a patient with a full course of anti-TB treatment based on clinical or radiological findings compatible with TB [Reference Migliori14]. Pulmonary TB (PTB) was defined as M. tuberculosis involvement of the lung parenchyma, the tracheo-bronchial tree or the larynx, while extrapulmonary PTB (EPTB) was defined as M. tuberculosis disease at any other site. Treatment outcomes were classified according to World Health Organization criteria, while cure or completion of treatment was considered as ‘treatment success’ [Reference Veen15].
The denominators used to determine TB rates and other calculations were based on the official census published by the Israeli Bureau of Statistics [9]. All new M. tuberculosis isolates underwent antibiotic susceptibility testing, and since 2008 molecular genotyping has also been performed. Genotyping techniques were conducted using 43-spacer spoligotyping [Reference Kamerbeek16] and 16-loci mycobacterial interspersed repetitive units–variable number tandem repeats (MIRU-VNTR) typing [Reference Freidlin17] to characterize the genetic diversity of M. tuberculosis strains. Clades (families) were defined according to the spoligotyping patterns as described in the SpolDB4 international database [Reference Brudey18] and SITVIT [Reference Demay19]. Clusters were defined as strains that were identical in 43-spacer spoligotyping and in 16-loci MIRU-VNTR. Data were retrieved from the files of epidemiological investigations that were performed by the health departments at the time of reporting to ascertain possible contact between members of a cluster.
Trend analysis was performed by χ 2 test to yield the linear-by-linear association test. Univariate analyses comparing the two ethnic groups (IA and IJ) and treatment success were performed by Pearson's χ 2 test for categorical variables (or Fisher's exact test when numerators were small). Student's t test was used for continuous variables that were distributed normally or the Kruskal–Wallis test when the distribution was not normal. P values <0·05 were considered statistically significant. Multivariate analysis was used to identify attributes predicting treatment success, and included all variables that were statistically significant in the univariate analysis by the logistic regression using the backward method to generate adjusted odds ratios and 95% confidence intervals. Data were analysed using SPSS software v. 19.0 (SSPS Inc., USA).
As this was a routine epidemiological analysis of non-nominal data from a standard reporting system, which was constant throughout the study period, ethical approval was not required.
RESULTS
During the study period, 4555 TB patients were reported in Israel, of whom 831 (18·2%) were Israeli born. The majority of the non-Israeli-born TB patients originated from the Horn of Africa (1725, 37·8%), Eastern Europe (1407, 30·9%), and South East Asia (452, 9·9%). Of the Israeli-born TB patients, 530 (64%) were IJ (308 males, 222 females), and 301 (36%) were IA (162 males, 139 females). The average annual TB rate in the general population of Israel during 1999–2011 was 7·0 cases/100 000 population, while in IJ and IA it was 1·1 and 1·6 cases/100 000 population, respectively (Fig. 1). TB rates in both ethnic groups were considerably lower than the national average and have consistently declined during the study period to ~1 case/100 000 residents (P < 0·01, χ 2 test for trend).

Fig. 1. Numbers and rates of newly diagnosed Israeli-born tuberculosis patients in Israel, by ethnic group, 1999–2011.
TB rates in both IJ and IA increased with age, from ~0·5 case/100 000 population in the 10–19 years age group to >2 cases/100 000 population in the >50 years age group (Fig. 2). Of 131 IJ aged <19 years, 61 (46·5%) TB patients were offspring of parents who were born in Ethiopia and immigrated to Israel.

Fig. 2. Rates of newly diagnosed Israeli-born tuberculosis patients, by ethnic group and age group, Israel, 1999–2011.
IA TB patients were more likely to be older, more commonly diagnosed with EPTB, incarcerated while diagnosed, and have inferior treatment success rates compared to IJ (Table 1). Death due to any cause during treatment was twice as high in IA as in IJ. TB-related deaths were higher in IA (n = 27, 84·4%) than in IJ (n = 17, 63·0%).
Table 1. Demographic, clinical, laboratory characteristics and treatment outcomes of Israeli-born tuberculosis patients in Israel, by ethnic group, 1999–2011
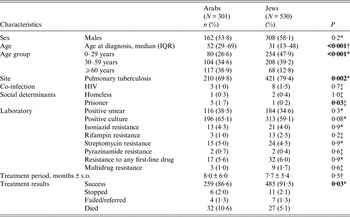
IQR, Interquartile range.
* χ 2 test.
† Kruskal–Wallis.
‡ Fisher's exact test.
Those who did not achieve treatment success were more likely to be IA, older patients, be co-infected with HIV and have a positive sputum culture (Table 2). By multivariate analysis, age and HIV infection status, but not ethnicity, were predictive of non-success in TB treatment (Table 3).
Table 2. Treatment results in all Israeli-born tuberculosis patients in Israel, 1999–2011
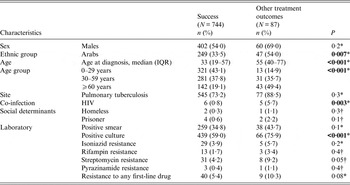
IQR, Interquartile range.
* χ 2 test.
† Fisher's exact test.
Table 3. Multivariate analysis for factors predicting treatment results other than success in all Israeli-born tuberculosis patients in Israel, 1999–2011
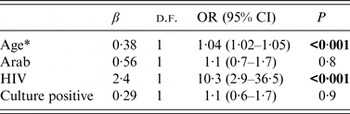
OR, Odds ratio; CI, confidence interval; d.f., degrees of freedom.
* Per single year of age
Of all 136 (47 IA, 89 IJ) Israeli-born citizens who were reported as having TB between 2008 and 2 011 91 (66·1%) had positive sputum culture results (31 IA, 60 IJ) and 88 (30 IA, 58 IJ) were spoligotyped. Ten mixed IA and IJ spoligotyping clades were identified (Beijing, CAS1_DELHI, H1, H3, H4, LAM9, LAM10_CAM, T1, T2, U), with a total of 64 (72·7%) isolates (Table 4). T1 and Beijing were the most dominant ethnically mixed clades. Using the 16-loci MIRU-VNTR technique, 11 clusters were identified (data not shown). Three of these were ‘mixed clusters', each included isolates of one IA and one IJ. Only two members of an IA–IJ identical isolate could recall mutual contact on epidemiological investigation, as the IA was a taxi driver living in east Jerusalem and travelling between Israel and the Palestinian Authority, while the IJ was a soldier who was stationed at the checkpoints at the border. The strain of this cluster is part of the H4 spoligotyping family, SIT 262.
Table 4. Clades by ethnic group of 88 (30 Arabs, 58 Jews) Israeli-born tuberculosis patients with positive sputum culture results between 2008 and 2011
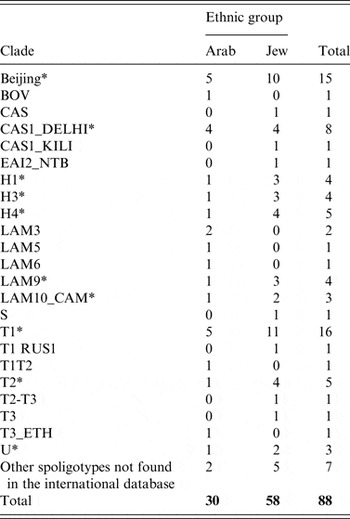
* Mixed Israeli Arabs and Jews clade.
DISCUSSION
This study showed that the incidence of TB in IA was higher than that in IJ and declined during the study period, converging to nearly 1 case/100 000 residents in 2011 in both groups. Moreover, both groups had a low rates of TB compared to the Israeli national rates reported during the study period, as the majority of all TB patients in Israel are foreign born, mostly originating from countries with a high incidence of TB [Reference Mor8].
IA TB patients were older and more commonly diagnosed with EPTB than IJ. Nearly half of all the IJ TB patients aged <19 years were of Ethiopian descent. However, the population of Ethiopian descendants in this age group comprised of 35 000 citizens, which is only 1·3% of the respective Israeli-born population in 2011 [9]. Jewish migrants from Ethiopia are known to have a relatively higher rate of TB than the general population [Reference Mor8]. These migrants probably infected their Israeli-born offspring [Reference Mor20], which suggests a dynamic propagation of the infection in this population. The older age of IA TB patients compared to IJ suggests that TB in IA is more likely to be reactivation of latent infection rather than a recent infection [Reference Shea21]. This may also be related to the lower rate of rifampin resistance in IA TB patients.
Fewer IA achieved treatment success compared to IJ, while a greater proportion of IA died during treatment. It is possible that the older age [Reference Shuldiner22] of IA and the higher rates of EPTB, which is usually associated with late diagnosis, indicate a more disseminated or severe disease [Reference Mor23].
The major spoligotype families (clades) that were found in the genotyped isolates were T1, Beijing and CAS1_DELHI. These are the most predominant clades that were found in the Israeli population during 2008–2010 [Reference Goldblatt24]. Most Beijing strains in Israel originated from Former Soviet Union patients, both tourists and IJ migrants, while the T clade is more heterogeneous, and includes strains from patients originating from the Former Soviet Union and the Horn of Africa. The CAS1_DELHI clade is most predominant in Israel in patients originating from the Horn of Africa.
Eleven clusters were identified in all of the cases diagnosed with TB between 2008 and 2010, including 25 patients. Of those, three IA–IJ members of identical strains belonged to three different clusters, yet a possible transmission was identified in only a single case of IA and IJ TB patients who shared an identical cluster. Residential segregation between IA and IJ which subsists in Israel, and rare inter-ethnic social interactions or marriages may be the cause of the limited transmission between the two ethnic groups.
In contrast to the low TB rates that were found in IA and IJ in this study, the rate of TB in the USA is 5·8 times higher in US-born Afro-Americans than in Caucasians (5·7 vs. 0·8/100 000, respectively) [5]. Similar findings were reported for American Indians in 2008, with a TB rate of 5·9/100 000 residents, which is more than five times greater than for non-Hispanic white individuals. In Canada, the age- and sex-adjusted incidence of TB in registered Indians is 52·6/100 000 person-years, which is 38 times higher than in Canadian-born non-Indians [Reference Long6]. In 2014, the WHO issued a new global TB strategy, which plans to reduce the global incidence of TB by 90% between 2015 and 2035 and aims to achieve TB elimination (<1 case/million population) by 2050 [25]. Developed countries and Israel are expected to achieve this goal in their native-born population.
M. tuberculosis usually affects males more than females. Our study showed that 53·8% of the IA and 58·1% of the IJ patients were males. This slight majority is in line with other reports from different regions of the world [26, Reference Khan27].
The converging TB rates in the two different ethnic groups suggest a reduction in disparities of TB in Israeli-born individuals, which is probably a multi-factorial process. The main reasons for the reduction in disparities include the inauguration of national healthcare coverage to all Israeli citizens in 1995, establishment of the NTP in 1997 and improved socioeconomic and education levels of IA [Reference Long6]. Moreover, several other factors probably contributed to closing the TB ethnic gap in Israel compared to other Western countries, such as the USA or Canada. First, there was an initially relatively lower TB rate in IA. It should be noted that TB clinics are paid for treating TB patients, access to healthcare is different for IA and IJ. Second, the low prevalence of TB risk factors (e.g. homelessness, alcoholism, poverty and HIV infection) in IA compared to North American Indians and US-born Afro-Americans, reduced their risk of contracting TB and improved treatment success rates [Reference Khan27, Reference Sandgren28]. Third, IA have a lower exposure to ‘imported’ TB than IJ, because of Israeli migration laws, where the majority of the migrants are of Jewish descent [Reference Feske29]. Therefore, IJ are exposed to Jewish migrants, because they have social and family ties, while IA are living in other cities or neighbourhoods, and are disconnected from Jewish migrants.
This study is subject to several limitations. First, there might have been information bias regarding classification of the two ethnic groups. However, the possibility for ethnic misclassification was low because an ethnic group is a regularly registered component in the national civil census for each citizen, while inter-ethnical marriages in Israel are very rare [9]. Second, molecular epidemiology of M. tuberculosis complex has been performed at the National Mycobacterium Reference Laboratory for all new culture sputum-positive cases in Israel since 2008. Therefore, data were available only for the last 3 years of the study period. Third, clustering might have been overestimated because genotyping was based on 16-loci MIRU-VNTR rather than the more discriminatory 24-loci MIRU-VNTR. The 24-loci MIRU-VNTR might have identified an even lower number of IA–IJ mixed clusters. Last, due to the retrospective nature of the study, we did not have data regarding smoking, diagnosis of other lung diseases, drug and alcohol use.
CONCLUSIONS
The TB rates were higher in IA than in IJ, However, the incidence of TB consistently declined and converged during the study period to nearly 1 case/100 000 in both groups. Although treatment success rates were higher in IJ compared to IA, it was unrelated to ethnicity according to the results of the multivariate analysis. Inauguration of national healthcare coverage to all Israeli citizens in 1995, establishment of the NTP in 1997 and the improved living conditions of IA were probably the main reasons for diminishing health disparities in TB in the two Israeli-born populations, who are rather segregated, and minimal transmission could be identified.
ACKNOWLEDGEMENTS
The authors thank Ms. Miri Yehuda from the Department of Tuberculosis and AIDS at the Ministry of Health in Jerusalem, Israel for her meticulous record keeping and Ms. Noa Cedar for her assistance in data collection and the Mycobacteria Reference Laboratory team for their excellent analytical expertise.
No financial support was provided for this study.
DECLARATION OF INTEREST
None.









