Flax is one of the oldest domesticated crops (since 7000 BC) and flour from the seed was used in bread as early as 1000 BC(Reference Vaisey-Genser, Morris, Muir and Westcott1). Today, flaxseed is being increasingly used in the human diet because of its potential health benefits, particularly for cardiovascular protection(Reference Bloedon, Philippe and Szapary2–Reference Stavro, Marchie, Kendall, Vuksan, Jenkins, Thompson and Cunnane4). Flaxseed is the richest natural source of plant lignans, with secoisolariciresinol diglucoside (SDG) being the principal lignan compound. The concentrations of SDG in flaxseed vary with different cultivars. Eliasson et al. (Reference Eliasson, Kamal-Eldin, Andersson and Aman5) reported that SDG concentrations in twenty-seven flaxseed species ranged from 1·19 to 2·59 % for (+)-SDG and from 0·22 to 0·5 % (w/w) for its diastereoisomer, ( − )-SDG. Westcott et al. (Reference Westcott, Muir and Northrup6) presented a range of SDG concentrations from 0·97 to 3·09 % (w/w) in eight varieties of defatted flaxseed meals. Flaxseed lignan along with soyabean isoflavones are phyto-oestrogens commonly consumed in the human diet(Reference Hutchins, Slavin, Thompson and Cunnane7).
To date, a number of clinical trials have been conducted using dietary flaxseed which suggested that SDG lignan may lower plasma cholesterol concentrations. However, the results did not show consistent benefit. In most of these studies, the concentration of lignans was not determined. Differences of study designs, subject characteristics and treatment conditions could confound the outcomes and interpretation of the results(Reference Bloedon, Philippe and Szapary2). Nevertheless, multiple animal studies do indicate that dietary lignans, lignan-rich flaxseed extracts or flaxseeds themselves have effects on improving plasma lipid profiles or preventing atherosclerotic lesions(Reference Prasad8–Reference Sano, Oda, Yamashita, Shiramasa, Ijiri and Yamamoto14).
Attribution of lipid-lowering effects in whole flaxseed and flax flour studies solely to SDG is confounded by the presence of other potential lipid-lowering components. Flaxseed commonly contains 34–45·6 % fat(Reference Duan, Barthet, Chornick, Duguid, Thompson and Cunnane15), and α-linolenic acid represents 45–60 % of the total fat content in flax oil(Reference Cunnane, Thompson and Cunnane16). Although α-linolenic acid is an n-3 fatty acid, dietary α-linolenic acid did not show cholesterol-lowering effects in several clinical trials reviewed by Harris(Reference Harris17). Additionally, flaxseed contains insoluble fibres that may affect plasma lipids, and the impact cannot be easily adjusted when studying the effects of flaxseed lignan. Therefore, we conducted this clinical trial using an SDG-rich flaxseed extract (consisting of 33 % SDG) to investigate the possible effects of dietary SDG on plasma total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), TAG, as well as glucose concentrations during a period of 8 weeks.
Methods
Study design
The present study is a randomised, double-blind, placebo-controlled clinical study using repeated measurements for data collection, conducted by the Tumor Hospital and Institute (Beijing, China).
Subjects
For eligibility of enrolment, LDL-C level of each subject was equal to or greater than 3·62 mmol/l (1400 mg/l), and subjects did not use any drugs, herbs, flax-containing products or other non-prescription preparations for lowering plasma cholesterol levels 4 weeks before the present study. In order to monitor subjects' normal kidney and liver function, plasma creatinine concentrations were not over 133 μmol/l; and blood urea N concentrations were not over 7·1 mmol/l; subjects had normal γ-glutamyl transferase and/or alanine aminotransferase values. Subjects were not sensitive or allergic to components or ingredients of flax products. In total, sixty-six subjects were selected and each subject was randomly assigned to one of the treatment groups.
Treatment
Upon meeting eligibility criteria and signing a consent form, subjects were randomly assigned to either lignan extract treatment groups (treated with tablets providing 300 or 600 mg SDG) or the placebo group (tablets containing 0 mg SDG). Four flaxseed lignan extract or placebo tablets (each tablet weighing 1·35 g) were administered daily to each patient: two with breakfast and two with dinner (two tablets twice). Subjects were counselled not to change their regular dietary patterns and physical activities. The study period was 8 weeks. The study protocol was approved by the Clinical Trial Evaluation Board of the Tumor Hospital and Institute (Beijing, China). All subjects were requested to visit the clinic every 2 weeks. At the time of each return visit: (1) a 20 ml blood sample was drawn following overnight fasting and (2) any concerns and any adverse reaction of the subjects were recorded. Compliance was evaluated by patient inquiry, tablet counts and plasma lignan concentration changes.
Measurement
Plasma TC, LDL-C, HDL-C, TAG, glucose, blood urea N, creatinine, alanine aminotransferase and γ-glutamyl transferase were determined using standard laboratory methods with the relevant experimental kits (Roche Diagnostics, Basel, Switzerland). These laboratory tests were conducted at baseline and at the end of weeks 2, 4, 6 and/or 8 of the study. Plasma secoisolariciresinol (SECO), enterodiol (ED) and enterolactone (EL) measurements were performed at baseline and at the end of weeks 2, 4 and 8, using a LC/MS/MS method (1100 Liquid Chromatography, Agilent and 14 000 MS; Applied Biosystems, Foster City, CA, USA)(Reference Du, Zhang, Zhang, Ding and Lin18). Briefly, a 100 μl plasma sample was mixed with 100 μl β-glucuronidase (containing 1200 units of the enzyme activity), the sample was incubated for 24 h at 37°C, and after adding 400 μl ethyl acetate, the test-tube was vortexed for 3 min. The sample mixture was further centrifuged for 2 min at 170 g, the upper ethyl acetate layer was collected and dried under N2. HPLC solvent (100 μl; methanol–ammonium acetate (5 mmol/l); 70:30, v/v) was added to dissolve the dried material. A 5 μl sample was injected into the LC/MS/MS system. The recovery rates of the method were from 79–105·6 % with CV < 19·4 %. The lower detection limits for plasma SECO, ED and EL were 5, 1 and 1 ng/ml, respectively.
Study material
The flaxseed lignan extract, containing approximately 33·0 % SDG, 15·1 % coumaric acid glucoside, 8·1 % ferulic acid glucoside, 15·6 % hydroxymethylglutaric acid, and the rest of water and other components (BeneflaxTM; Archer Daniels Midland Company, Decatur, IL, USA), was formulated to deliver the targeted daily SDG doses in four tablets (each tablet contains 0, 75 or 150 mg SDG). Placebo tablets containing food-grade maltodextrin (combined with food-grade colours to approximate the colour of the flaxseed extract) were prepared to match the size, shape and colour of SDG-containing tablets. In addition to flaxseed lignan extract or placebo material, tablets were formulated with commonly used excipient ingredients. Prepared tablets were analysed for SDG content then released to the study upon confirmation of appropriate SDG levels.
Statistical analysis
The null hypothesis was that there would be no differences on changes of plasma cholesterol and glucose concentrations between the treatment and placebo groups at the end of the study. The statistical power for rejecting this hypothesis was 0·94 based on an assumption that LDLC could be lowered by 15 % from baseline in the treatment group(s) and 5 % in placebo group with a standard deviation of 50 % of mean decrease (group n 20; α = 0·05; Dunnett adjustment). After performing descriptive statistics (mean values and standard deviations are used in the tables and mean values with their standard errors used in the figures), mixed model procedures were used to test the proposed treatment effects on changes or percentage changes of plasma lipid and glucose concentrations using the data from the placebo group as reference (Dunnett adjustment). The response variation of individual subjects to the treatment was considered as a random factor and the time points by weeks of the measurements were regarded as a repeated factor. General linear model repeated procedures were used to test the treatment effects within subjects, and baseline data were used as comparative reference. The plasma lignan concentrations were non-normally distributed (right skewed). The data were therefore logarithm transformed into approximately normal distribution before analysis. Spearman correlation was used to examine possible associations between the decreases of TC, LDL-C, HDL-C and TAG concentrations and plasma lignan concentrations. Further, subgroup analysis of the data in subjects with baseline glucose concentrations ≥ 5·83 mmol/l (1050 mg/l) was conducted using similar methods. SAS software version 9.1 (SAS Institute, Cary, NC, USA) was utilised for the data processing and analysis.
Results
Fifty-five of the sixty-six hypercholesterolaemic subjects (thirty-five men and twenty women) completed the study. Seven of them dropped out (three refused to give more blood samples, two needed other medical treatment, one relocated, and one did not give a reason). Participants in the three groups had a mean age between 53·5 and 58·3 years and an average BMI between 26 and 27 kg/m2 (Table 1). Mean baseline lipid values were between 6·6 and 7·0 mmol/l for TC, 4·3 and 4·5 mmol/l for LDL-C, 1·06 and 1·13 mmol/l for HDL-C, 2·67 and 3·35 mmol/l for TAG, and 5·9 and 7·1 mmol/l for fasting glucose (Table 1). Means of total plasma lignan concentrations at baseline were between 39·15 and 59·34 ng/ml (Table 2).
Table 1 Baseline parameters of all subjects (men and women)
(Mean values and standard deviations)
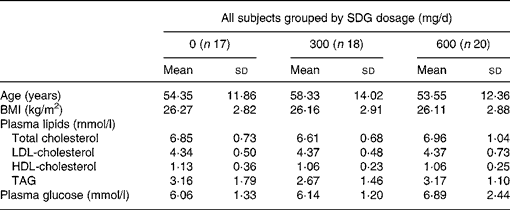
SDG, secoisolariciresinol diglucoside.
Table 2 Plasma lignan concentrations at baseline and after lignan supplementation*
(Mean values and standard deviations)
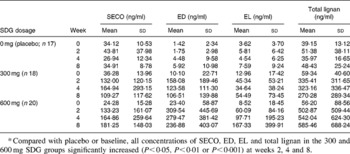
SDG, secoisolariciresinol diglucoside; SECO, secoisolariciresinol; ED, enterodiol; EL, enterolactone.
* Compared with placebo or baseline, all concentrations of SECO, ED, EL and total lignan in the 300 and 600 mg SDG groups significantly increased (P < 0·05, P < 0·01 or P < 0·001) at weeks 2, 4 and 8.
The data were analysed and compared between and within treatment groups using values of concentration change and percentage changes from baseline. Detailed treatment effects at individual time points on decreases and percentage decreases of plasma lipid and glucose concentrations are shown in Table 3 and Figs. 1 and 2, respectively. Significant overall treatment effects between groups on TC, LDL-C and glucose concentration decrease (P < 0·001, P = 0·003 and P = 0·019, respectively) were observed (Table 3). In the 600 mg SDG group at weeks 4, 6 and 8, statistical significance was achieved for TC concentration decrease and percentage decrease compared with both placebo and baseline (P < 0·05, P = 0·01 or P = 0·001). The mean TC decreases from baseline were − 1·04, − 1·79 and − 1·77 mmol/l (Table 3), and the mean TC percentage decreases from baseline were − 13·55, − 23·98 and − 24·2 % (Fig. 1 (A)). The mean LDL-C concentration decreases at week 6 and 8 were − 1·12 and − 1·0 mmol/l, and the mean percentage decreases were − 24·38 and − 22·0 % (P < 0·05 or P < 0·01 compared with the placebo or baseline; Table 3 and Fig. 1 (B)).
Table 3 Changes of plasma lipid and glucose concentrations from baseline values
(Mean values and standard deviations)

SDG, secoisolariciresinol diglucoside.
Changes compared with baseline were significant: *P < 0·05, **P < 0·01, ***P < 0·001.
Changes compared with placebo (0 mg SDG) were significant: †P < 0·05, ††P < 0·01, †††P < 0·001.
‡ P < 0·05 means that a statistically significant difference was reached among the three treatment groups over all data points.

Fig. 1 Effects of dietary flaxseed lignan on percentage changes in plasma lipids from baseline: (A) total cholesterol (TC); (B) LDL-cholesterol (LDL-C); (C) HDL-cholesterol (HDL-C); (D) TAG. (□), Placebo; (![]() ), 300 mg secoisolariciresinol diglucoside (SDG); (
), 300 mg secoisolariciresinol diglucoside (SDG); (![]() ), 600 mg SDG. Values are means, with their standard errors represented by vertical bars. Change was significant compared with baseline (week 0): *P < 0·05, **P < 0·01. Change was significant compared with placebo: †P < 0·05, †P < 0·01.
), 600 mg SDG. Values are means, with their standard errors represented by vertical bars. Change was significant compared with baseline (week 0): *P < 0·05, **P < 0·01. Change was significant compared with placebo: †P < 0·05, †P < 0·01.

Fig. 2 Effects of dietary flaxseed lignan on percentage changes in fasting plasma glucose from baseline: (A) results from all subjects (n 55); (B) results from subjects with glucose baseline ≥ 5·83 mmol/l (1050 mg/l; n 34). (□), Placebo; (![]() ), 300 mg secoisolariciresinol diglucoside (SDG); (
), 300 mg secoisolariciresinol diglucoside (SDG); (![]() ), 600 mg SDG. Values are means, with their standard errors represented by vertical bars. Change was significant compared with baseline (week 0): *P < 0·05, **P < 0·01. Change was significant compared with placebo: †P < 0·05.
), 600 mg SDG. Values are means, with their standard errors represented by vertical bars. Change was significant compared with baseline (week 0): *P < 0·05, **P < 0·01. Change was significant compared with placebo: †P < 0·05.
In the 300 mg SDG group, the TC concentration decreases were − 0·53, − 0·68, and − 1·03 mmol/l and percentage decreases were − 7·84, − 9·77 and − 15·47 % at the ends of week 4, 6, or 8, respectively. Only within-group (compared with baseline) statistical significance was reached for these values (Table 3 and Fig. 1 (A)). For LDL-C, the corresponding values were − 0·57 and − 0·74 mmol/l, and − 13·56 and − 17·04 % at the ends of week 6 or 8, respectively (Table 3 and Fig. 1 (B)). As with TC, only within-group statistical significance was reached for these values. Also, an obvious placebo effect appeared (Table 3 and Fig. 1).
For HDL-C concentrations, no overall significant changes appeared between treatment groups (P = 0·167). However, at week 8, a point statistical significance (P = 0·035) for percentage decrease was noted between the 600 mg SDG group and placebo. Within-group significances for concentration decrease or percentage decrease were observed in all groups starting from different weeks (Table 3 and Fig. 1 (C)). For TAG concentrations, the main significant effect on TAG lowering occurred only within the 600 mg SDG group (Table 3 and Fig. 1 (D)). For the TC:HDL-C ratios, no overall significant difference was reached between the groups (P = 0·116), but significant lowering effect on the ratios was noted at weeks 6 and 8 between the 600 mg SDG group and the placebo (P < 0·05; Table 3). Also, a significant within-group decrease occurred for all time points after week 2 (P < 0·05, P = 0·01, or P = 0·001; Table 3).
Significant concentration decrease or percentage decrease in fasting plasma glucose concentrations were found in the 600 mg SDG group at weeks 6 and 8 compared with either placebo (P = 0·019) or baseline (Table 3 and Fig. 2 (A)). This decrease was greater in subjects with baseline glucose levels ≥ 5·83 mmol/l or 1050 mg/l (Fig. 2 (B)). At weeks 6 and 8, the percentage decrease reached values of − 25·56 and − 24·96 % in the 600 mg SDG group (P = 0·022 and P = 0·021 compared with placebo and P = 0·003 and P = 0·005 compared with baseline, respectively). Although a noticeable fasting glucose concentration-lowering effect was also observed in the 300 mg SDG group, this change did not reach statistical significance.
As noted above, all treatment groups achieved significant TC and LDL-C lowering either between or within groups by the end of the 8 weeks. This was accomplished despite the fact that the placebo group also exhibited decreases in lipid concentrations. It should be noted that most of the observed treatment effects followed a dose-dependent pattern. At the end of 8 weeks' treatment, the BMI means for the placebo, 300 mg SDG group and 600 mg SDG group were 25·94 (sd 3·20), 26·23 (sd 2·94) and 25·89 (sd 3·34) kg/m2, respectively; and the corresponding baseline BMI values were 26·27 (sd 2·82), 26·16 (sd 2·91) and 26·11 (sd 2·88) kg/m2. These BMI data were not significantly different between or within groups. In addition, all subjects maintained normal plasma creatinine, blood urea N, alanine aminotransferase and γ-glutamyl transferase levels throughout the trial, and there were no adverse events reported by subjects.
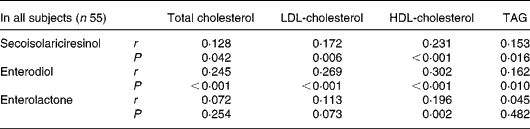
Plasma concentrations of SECO, ED, EL and total lignans (the sum of SECO, ED and EL) are reported in Table 2. Compared with the placebo or baseline, all measured lignan concentrations in the SDG treatment groups increased significantly. Significant correlations (both Spearman and Pearson) were observed between total lignan concentrations and the concentrations of SECO, ED or EL. Also, correlations were observed between the decrease of plasma lipids and the lignan concentrations. Because of the non-normal distributions of the lignan data, a non-parametric correlation method (Spearman) was used to explore possible relationships. Significant correlations were mainly observed with SECO and ED. The Spearman correlation coefficients with their corresponding P values are presented in Table 4.
Table 4 Spearman correlations (r) between plasma lignan concentrations and decreases of plasma lipid concentration from baseline
It was noted that the values of lipid lowering in men seem to be greater than those in women (data not shown). However, the statistical power and evidence are not sufficient to suggest a difference of lipid lowering between sexes (very small group sample sizes in women; five in placebo, eight in the 300 SDG group and seven in the 600 mg SDG group).
Discussion
The present study found significant plasma lipid-lowering effects in hypercholesterolaemic subjects, especially on TC and LDL-C, following daily supplementation of tablets containing a flaxseed SDG lignan extract. Decreases of HDL-C and TAG concentration were also noted; statistical significances mainly occurred in the comparisons within subjects. Although the HDL-C decrease is not an expected effect, the significant reductions of TC:HDL-C ratio indicate that non-HDL-C was more favourably decreased by the treatment. A substantial plasma glucose-lowering effect was found in the 600 mg SDG dose group after 6 weeks of treatment, particularly in subjects who had higher baseline glucose concentrations. There were also noticeable placebo effects on cholesterol lowering. Since no noticeable changes in BMI occurred before and after the treatment, the placebo effects may not be related to changes of daily energy intake.
The mechanism for the observed cholesterol-lowering effect of flaxseed lignan has not been clearly elucidated. It has been suggested by Bloedon et al. (Reference Bloedon, Philippe and Szapary2) that lignans may lower plasma cholesterol by modulating 7α-hydroxylase and acyl CoA cholesterol transferase, both of which are involved in cholesterol metabolism. However, Bloedon's suggestions were based on studies in rabbits and rats. Some phyto-oestrogens are considered to be selective oestrogen receptor modulators(Reference Brzezinski and Debi19), and selective oestrogen receptor modulators have been shown to reduce plasma LDL-C concentrations in human subjects and rats. Insull et al. (Reference Insull, Davidson, Kulkarni, Siddhanti, Ciaccia and Keech20) reported that 12-month intakes of raloxifene (at 60 mg/d) reduced LDL-C by 10·5 % in postmenopausal women. It was also reported by Lemieux et al. (Reference Lemieux, Gelinas, Lalonde, Labrie, Cianflone and Deshaies21) that acolbifene, another selective oestrogen receptor modulator, increased liver LDL receptor protein 2-fold and decreased TC, HDL-C and non-HDL-C about 50 % after 4 weeks of treatment (at 0·5 mg/d by oral administration) in rats. The phyto-oestrogen lignan may share a similar mechanism with the selective oestrogen receptor modulators for a cholesterol-lowering effect.
A number of human studies have investigated the effects of flaxseed or flaxseed meal (defatted flaxseed) on plasma lipids. In 2004, Bloedon et al. (Reference Bloedon, Philippe and Szapary2) conducted a systemic review of nine clinical trials. Six of the nine trials observed significant concentration decreases of TC, LDL-C, or both, after dietary flaxseed supplementation. Two other clinical observations(Reference Demark-Wahnefried, Robertson, Walther, Polascik, Paulson and Vollmer22, Reference Demark-Wahnefried, Price, Polascik, Robertson, Anderson, Paulson, Walther, Gannon and Vollmer23) in subjects with prostate disorders noted that intake of 30 g flaxseed/d with a low-fat diet reduced plasma cholesterol levels by 12 % to 13·4 % after 6 months or 34 d of treatment, respectively. Stuglin and Prasad(Reference Stuglin and Prasad24) recently reported that a 4-week intake of 32·7 g flaxseed did not substantially alter plasma TC concentration in fifteen healthy men by a self-controlled trial. Another study by Dodin et al. (Reference Dodin, Lemay, Jacques, Legare, Forest and Masse25) noted that consumption of 40 g flaxseed/d delivering an unexpectedly low amount of lignan (21 mg) for 12 months did not significantly lower cholesterol levels compared with baseline in eighty-five postmenopausal women with normal plasma lipid profiles. Recently, Hallund et al. (Reference Hallund, Ravn-Haren, Bugel, Tholstrup and Tetens26) reported that a 6-week treatment of 500 mg SDG/d (in muffins) did not significantly affect plasma lipid concentrations in twenty-two normal postmenopausal women in a cross-over trial using the same flaxseed lignan extract as in the present study (BeneflaxTM-Flax Lignan Concentrate; Archer Daniels Midland Company). Besides dissimilarity of study design, two key differences between Hallund's study and ours exist. The Danish subjects in Hallund's study were normocholesterolaemic. Treatment efficacy on cholesterol lowering has been observed to be significantly related to baseline lipid concentrations, particularly in studies examining the effect of a dietary treatment(Reference Chen, Ferng, Yang, Peng, Lee and Chen27, Reference Henkin and Shai28). Second, all subjects were postmenopausal females in Hallund's study. A different response to flaxseed lignan treatment on lipid lowering may exist between sexes. These factors probably explain the overall different outcome from the present study.
Based on the findings by Prasad(Reference Prasad8, Reference Prasad9, Reference Prasad, Mantha, Muir and Westcott29), Lucas et al. (Reference Lucas, Wild, Hammond, Khalil, Juma, Daggy, Stoecker and Arjmandi30), Clandinin et al. (Reference Clandinin, Foxwell, Goh, Layne and Jumpsen31), Nestel et al. (Reference Nestel, Pomeroy, Sasahara, Yamashita, Liang, Dart, Jennings, Abbey and Cameron32) and Cunnane et al. (Reference Cunnane, Hamadeh, Liede, Thompson, Wolever and Jenkins33), effects of dietary flaxseed on plasma cholesterol lowering were less likely due to its high content of α-linolenic acid or fibre, although either of their effects cannot be absolutely excluded. Thus, the present flaxseed SDG lignan extract was chosen as a test material and the SDG in the extract was considered as the functional compound in the present trial. The lignan dose levels of the present study are precisely known in contrast to studies using whole flaxseed or defatted flax flour in previous works. As mentioned above, SDG content can range 1·19–2·59 % for (+)-SDG(Reference Eliasson, Kamal-Eldin, Andersson and Aman5) in whole flaxseeds, and can range 0·97–3·09 % (w/w) in defatted flaxseed meals(Reference Westcott, Muir and Northrup6). Thus, if an individual consumed 40 or 50 g whole flaxseed/d as reviewed by Bloedon et al. (Reference Bloedon, Philippe and Szapary2), the SGD intake levels could reach 1·036 or 1·295 g/d at the high end. The treatment doses used in the present study, 0·3 and 0·6 g SDG/d, were within the range or lower than those used in previous studies. It should also be noted that consumption of whole flaxseed in amounts higher than 30 g/d may lead to gastrointestinal distress, potentially limiting the use of flaxseed for plasma cholesterol management.
To date, no peer-reviewed data have been found on the effect of lignan-rich flaxseed extract on fasting plasma glucose concentrations in human subjects. Based on limited publications relevant to this subject(Reference Cunnane, Hamadeh, Liede, Thompson, Wolever and Jenkins33–Reference Cunnane, Ganguli, Menard, Liede, Hamadeh, Chen, Wolever and Jenkins35), we did expect that the flaxseed extract or lignan may have some favourable effect on fasting blood glucose. A dietary flaxseed study by Lemay et al. (Reference Lemay, Dodin, Kadri, Jacques and Forest34) reported that 40 g crushed flaxseed/d consumed over 2 months significantly lowered fasting glucose concentration by 5·3 % from pretreatment levels in twenty-five menopausal women. Two previous publications(Reference Cunnane, Hamadeh, Liede, Thompson, Wolever and Jenkins33, Reference Cunnane, Ganguli, Menard, Liede, Hamadeh, Chen, Wolever and Jenkins35) reported that dietary flaxseed could decrease postprandial blood glucose response by 27 % and increase glucose tolerance after a glucose load in young healthy subjects (area under the curve of glucose level increment was 73 mmol/l × 90 min with a flaxseed diet and 92 mmol/l × 90 min with a control diet.). In the present study, the mechanism for the observed dose-dependent lowering effect on fasting blood glucose concentrations deserves to be further explored. Nevertheless, its clinical significance is important, particularly in those subjects with higher baseline glucose concentrations ( ≥ 5·83 mmol/l or 1050 mg/l).
Metabolically, ingested SDG is broken down into SECO, ED, and further to EL in the intestine and colon. Although some publications exist related mainly to EL or ED concentrations in human plasma, no reports have revealed plasma SECO concentrations or how these three compounds are distributed in the circulation under SDG supplementation. The data of the present study indicate that all three compounds can be absorbed and enter the circulation, and a steady-state concentration is reached within 2 weeks. At baseline, the means of total lignan concentrations among the three treatment groups were relatively similar, from 39·15 to 59·34 ng/ml. Multiple researchers reported plasma EL concentrations with a range of 1·84–17·97 (ng/ml, means or median) without supplementation of SDG or flaxseed products(Reference Vanharanta, Voutilainen, Lakka, van der Lee, Adlercreutz and Salonen36–Reference Hausner, Johnsen, Hallund and Tetens44). Our EL data at baseline and in the placebo group shown in Table 2 are comparable with these values. With flaxseed extract supplementation, concentrations of SECO, ED and EL all substantially increased, and the profile of lignan distribution changed from baseline. The obtained plasma lignan data also indicate that EL concentration seems to be the minor component of total lignans, and SECO and ED may be more important for the potential benefits of dietary flaxseed or its extract. It is not clear whether the distribution of plasma lignans solely depends upon absorption, further metabolism in the circulation, or both. The large standard deviations of the means in the present study indicate that variation in response to lignan supplementation is appreciable between individuals. Large variance of plasma ED and EL concentrations in subjects with flaxseed or SDG supplementation was also reported by Nesbitt et al. (Reference Nesbitt, Lam and Thompson45) and Morton et al. (Reference Morton, Wilcox, Wahlqvist and Griffiths46). The metabolism of dietary SDG is an area that deserves further study.
The observed significant correlation between cholesterol lowering and plasma SECO and ED concentration suggests that they can be functional agents. However, we cannot rule out that some other compounds presented in the flaxseed lignan extract may play a role, such as coumaric acid glucoside or ferulic acid glucoside. Both compounds provided about 15 and 8 % of the composition of the extract, respectively. Each has antioxidant activity due to their phenolic chemical structures. Also, it is unknown if synergic effects exist among the lignans, coumaric acid and ferulic acid.
The present study has limitations. The study population consisted of entirely Chinese nationals and, as such, would be more genetically homogeneous compared with a Western population. Effect of SDG lignan should be confirmed in a study using subjects with wider genetic diversity. Second, the authors intentionally conducted the study using a free-living population without dietary restriction with the consideration of measuring effects under typical living conditions, as one would normally experience. However, dietary intake data should have been collected. If the dietary data had been available, it might have provided an explanation for the observed placebo effects. This would be an insufficiency of the present study.
In conclusion, outcomes of the present study indicate that dietary SDG lignan-rich flaxseed extract significantly lowered plasma cholesterol concentrations in these hypercholesterolaemic subjects. The cholesterol-lowering effect was more significantly observed for TC and LDL-C in a dose-dependent manner. A greater fasting plasma glucose-lowering effect was also noted in subjects with baseline glucose concentration ≥ 5·83 mmol/l. In addition, the presented data of plasma lignan concentrations provide a primary understanding about absorption and plasma distribution of dietary flaxseed lignans. The correlations between the plasma SECO and ED concentrations and the decreases of cholesterol concentration suggest that they may be the key functional components for the effects observed. Considered together with the safety data, the flaxseed lignan extract should be further evaluated as another natural alternative for plasma cholesterol and glucose concentration management or intervention.
Acknowledgements
We thank the Department of Environmental Chemistry, Center of Disease Control and Prevention of China, for analysing plasma lignan concentrations.
In the study, funding and the test material (flaxseed lignan extract) were provided by Archer Daniels Midland Company. Three of the authors, B. F., M. W. E. and S. Z. S., are employed by Archer Daniels Midland Company (Decatur, IL, USA). The contributions from them were mainly on testing material preparation, statistical support, and English language correction for the manuscript. The study was conducted by researchers at the Chinese Academy of Medical Sciences and Peking Union Medical College, Tumor Hospital and Institute, in Beijing, China.








