Generalised anxiety disorder (GAD) is a serious and chronic illness, defined by excessive and difficult-to-control worry. Somatic and mental symptoms include poor concentration, restlessness, sleep disturbance, fatigue and muscle tension. The prevalence of GAD is as high as 7.3% in community-dwelling older adults. Reference Beekman, Bremmer, Deeg, van Balkom, Smit and de Beurs1–Reference Gum, King-Kallimanis and Kohn3 Older adults with GAD worry about medical problems, family and finances; typical concerns in this age group but greatly amplified in duration, severity and distress. Reference Diefenbach, Stanley and Beck4 The disorder tends to be chronic in the absence of effective treatment, with an average duration of 20 years or more Reference Chou5–Reference Stanley, Beck, Novy, Averill, Swann and Diefenbach8 and strongly associated with functional disability. Reference Porensky, Dew, Karp, Skidmore, Rollman and Shear9
To our knowledge, no single study has provided a comprehensive examination of neuropsychological functioning in late-life GAD. Moreover, there is very little available data on whether neuropsychological function changes with treatment. Thus, we carried out a neuropsychological evaluation pre- and post-treatment, in a large group of older adults with GAD in a randomised placebo-controlled trial of the selective serotonin reuptake inhibitor (SSRI) escitalopram. We also compared participants with GAD (the GAD group) at baseline with a group of psychiatrically healthy older adults (comparison group), equated for gender, ethnicity, years of education and medical burden. The goal of this study was twofold: to characterise neuropsychological function among a large group of older adults with a principal diagnosis of GAD; and to identify any cognitive changes related to treatment of anxiety. Based on the published literature (including our own work) with the rationale that worry takes up cognitive capacity and leaves less attentional resources for the tasks at hand, we hypothesised that those in the GAD group would perform worse than the comparison group on measures of attention (digit span), information processing speed (coding), and executive functions including working memory (letter–number sequencing) and problem-solving, conceptual ability and mental flexibility (sorting test), as well as multiple measures of immediate and delayed recall. In addition, we examined measures of visuospatial function and language and we did not expect to find differences on these measures. We also hypothesised that impairments would improve with successful treatment of anxiety and that there would be a significant relationship between disability and cognitive performance, particularly in the executive domain.
Method
Study design
This was a National Institute of Mental Health sponsored 12-week double-blind randomised controlled trial of escitalopram v. placebo in older adults with a principal diagnosis of GAD, conducted in primary care practices and a specialty academic mental health centre in Pittsburgh, Pennsylvania, from 2005 to 2008 (trial registration NCT00105586).
Participants
This clinical trial recruited 177 individuals aged 60 or older meeting DSM-IV criteria for GAD. 10 We excluded a total of 17 participants, due to central nervous system disease (n = 13), physical impairment that precluded neuropsychological assessment (n = 3) or refusal to undergo neuropsychological assessment (n = 1). Thus in the present analysis there were 160 participants in the GAD group whose mean age at onset was 39.6 years (s.d. = 26.89) with a mean duration of 32.01 years (s.d. = 26.6). Participants were assessed at baseline (pre-treatment) and after the 12-week trial. For comparison, we also assessed 37 older adults without dementia and without a psychiatric history equated with the GAD participants on gender and ethnicity as well as years of education and medical (including vascular disease) burden with the same neuropsychological battery, at a single time point.
Details on participant recruitment, retention and evaluation have been described elsewhere. Reference Lenze and Wetherell11,Reference Mantella, Butters, Amico, Mazumdar, Rollman and Begley12 During screening, all potential participants were evaluated by a board-certified geriatric psychiatrist (E.J.L.). Psychiatric diagnosis was established with the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV) Reference First, Spitzer, Gibbon and Williams13 administered by formally trained master’s and doctoral degree level clinicians and a consensus diagnostic conference attended by the raters and at least two geriatric psychiatrists. Potential participants with an established diagnosis of dementia were excluded from the study; anyone with suspected dementia based on the screening evaluation was excluded. Moreover, the Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh14 was administered in this study and all data on anyone with a score <26 were closely reviewed by a geriatric psychiatrist (E.J.L.).
Measures
Participants underwent a broad-based pre-treatment assessment that included clinical, psychosocial and biological measures (described previously Reference Lenze and Wetherell11 ) as well as neuropsychological assessment (supervised by M.A.B.). We used a brief but comprehensive battery of neuropsychological measures that is widely used with older adults. The neuropsychological measures included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Reference Randolph15 Forms A and B were administered in a counter-balanced manner to minimise practice effects. The RBANS provides a total index score and subscale index scores measuring language (confrontation naming and category fluency), visuospatial construction skills (figure copy and line orientation tasks), attention (forward digit span and coding tasks), immediate memory (list and story recall) and delayed memory (list, story and figure recall). Because the RBANS does not assess executive functioning and other higher-level abilities such as working memory, we also administered the Delis–Kaplan Executive Function System (D–KEFS) Reference Delis, Kaplan and Kramer16 sorting test and used the confirmed correct sorts that measure problem-solving, conceptual ability and mental flexibility. We also used the colour–word interference score of the Stroop Neuropsychological Screening Test Reference Trennery, Crosson, DeBoe and Leber17 to measure the ability to inhibit automatic responses, and the Wechsler Adult Intelligence Scale (WAIS-III) Reference Wechsler18 letter–number sequencing subtest as a measure of working memory.
Among individuals with anxiety disorders both depression and anxiety symptoms are confounded making it extremely difficult to tease the two apart. Moreover, many of the most widely used mood scales (e.g. the Hamilton Rating Scale for Depression (HRSD) Reference Hamilton19 ) contain items that measure symptoms common to both depression and anxiety. In order to help tease apart the effects of depression v. anxiety, we examined depressive symptoms with the least confounded measure, the HRSD core depression items (q1 + q2 + q3 + q7). Reference Dombrovski, Blakesley-Ball, Mulsant, Mazumdar, Houck and Szanto20
We assessed disability with the Function and Disability Instrument (FDI) limitations and frequency subscales. Reference Jette, Haley, Coster, Kooyoomjian, Levenson and Heeren21 These subscales measure a person’s self-reported performance of socially defined life tasks that are expected of an individual within a typical environment, along two domains: how much difficulty he or she has performing activities (activity limitations subscale) and how often he or she performs activities (frequency subscale). The limitation and frequency subscales of the FDI correspond to the disability domains of activity limitation and participation restriction, consistent with the International Classification of Function, Disability and Health. 22 Both of these domains of function are impaired in late-life GAD. Reference Porensky, Dew, Karp, Skidmore, Rollman and Shear9
Statistical analysis
Prior to statistical analysis, we examined the data for normality and used transformations where necessary. If the distribution could not be normalised a non-parametric test was used. We generated descriptive statistics to characterise the GAD and comparison groups on key demographic and clinical characteristics.
Neuropsychological functioning of GAD and comparison groups at baseline
To characterise neuropsychological function among older adults with a principal diagnosis of GAD, we compared neuropsychological functioning of the GAD and comparison groups at baseline using analysis of variance. Seven main neuropsychological measures were chosen a priori to represent the domains of attention (digit span forward), information processing speed (coding), and the specific executive functions of inhibition (Stroop), working memory (letter–number sequencing), and problem-solving, conceptual ability and mental flexibility (sorting test), as well as immediate memory (RBANS immediate memory index) and delayed memory (RBANS delayed memory index). Age and education were used as covariates in these models except for those involving the RBANS memory scores, because these scores are age-adjusted based on test norms. We also examined baseline differences in index scores of the remaining two RBANS domains, visuospatial construction and language. In these models only education was used as a covariate since the derived scaled scores are adjusted for age. We report the results both with and without correction for multiple comparisons. Generalised anxiety disorder is frequently accompanied by depressive symptoms so to examine the effect of depression on neuropsychological functioning without obscuring the effect of GAD, we re-ran the analyses, once excluding the participants who also met criteria for major depressive disorder (MDD, n = 15) and once covarying for MDD diagnosis.
Functional correlates of neuropsychological performance at baseline
Because group and depression scores are highly multicollinear and could not be entered into the same model, to further examine the potential influence of depression, we examined the correlations of HRSD total score and the core HRSD depression items score with the neuropsychological data. The correlations were not significant and therefore we did not control for either in the analyses comparing cognitive performance between the groups.
To examine the relationship between cognitive function and everyday functioning in late-life GAD, we calculated Spearman correlation coefficients between the nine neuropsychological variables and the FDI frequency and limitations subscales.
Comparing neuropsychological functioning pre- and post-treatment in the GAD group
As cognitive function in cognitively normal individuals may not improve with efficacious treatment for anxiety, and to identify any cognitive changes related to treatment of anxiety, we compared neuropsychological functioning pre- and post-treatment in the low-scoring GAD treatment completers group using a repeated measures mixed-effect model with random intercept and slope. We examined treatment (drug v. placebo), time, and treatment×time interactions. A participant was considered to be a low cognitive scorer at baseline if he or she performed in the lower half of a median split of the GAD group’s RBANS total index scores.
We also examined neuropsychological change scores among the GAD group in participants whose anxiety symptoms significantly improved, to see which, if any, neuropsychological measures also improved, using the Wilcoxon Signed Rank Exact test. A participant was considered ‘improved’ if he or she reported having ‘much improved’ or ‘very much improved’ on the Clinical Global Impressions (CGI) Reference Guy23 scale (CGI score ≤2) post-treatment.
Results
Descriptive analyses
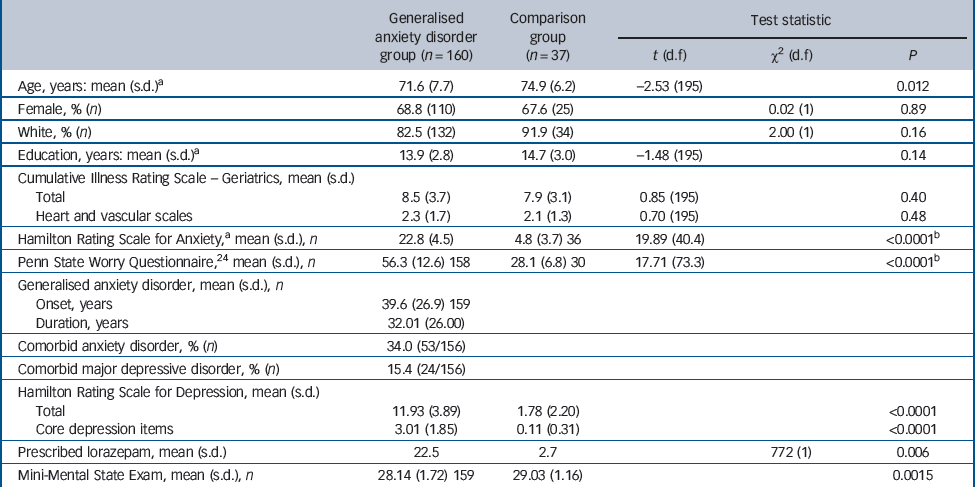
Individuals in the GAD group were younger than those in the comparison group (71.6 (s.d. = 7.7) v. 74.9 (s.d. = 6.2) years, P = 0.012; Table 1). Otherwise, the GAD and comparison groups did not differ in terms of relevant demographic and clinical characteristics, including medical and vascular disease burden, as measured with the Cumulative Illness Rating Scale – Geriatrics. Reference Miller, Paradis, Houck, Mazumdar, Stack and Rifai25
Participants who dropped out before week 12 (n = 31) were not significantly different from completers (n = 129) in age, gender, ethnicity, years of education, age at onset of GAD or rates of comorbid MDD. However, participants who dropped out before week 12 were significantly different from completers in terms of baseline severity on the Hamilton Rating Scale for Anxiety Reference Hamilton26 (HRSA; mean 25.1 (95% CI 22.9–27.2) v. 22.6 (95% CI 21.9–23.2, P = 0.003) and HRSD (mean 14.0 (95% CI 12.3–15.7) v. 11.6 (95% CI 11.0–12.2), P = 0.02), co-prescription of benzodiazepine (drop-out rate for benzodiazepine users 33.3%
Table 1 Participants: descriptive information
| Generalised anxiety disorder group (n = 160) | Comparison group (n = 37) | Test statistic | |||
|---|---|---|---|---|---|
| t (d.f) | χ2 (d.f) | P | |||
| Age, years: mean (s.d.)a | 71.6 (7.7) | 74.9 (6.2) | –2.53 (195) | 0.012 | |
| Female, % (n) | 68.8 (110) | 67.6 (25) | 0.02 (1) | 0.89 | |
| White, % (n) | 82.5 (132) | 91.9 (34) | 2.00 (1) | 0.16 | |
| Education, years: mean (s.d.)a | 13.9 (2.8) | 14.7 (3.0) | –1.48 (195) | 0.14 | |
| Cumulative Illness Rating Scale – Geriatrics, mean (s.d.) | |||||
| Total | 8.5 (3.7) | 7.9 (3.1) | 0.85 (195) | 0.40 | |
| Heart and vascular scales | 2.3 (1.7) | 2.1 (1.3) | 0.70 (195) | 0.48 | |
| Hamilton Rating Scale for Anxiety,a mean (s.d.), n | 22.8 (4.5) | 4.8 (3.7) 36 | 19.89 (40.4) | <0.0001b | |
| Penn State Worry Questionnaire,24 mean (s.d.), n | 56.3 (12.6) 158 | 28.1 (6.8) 30 | 17.71 (73.3) | <0.0001b | |
| Generalised anxiety disorder, mean (s.d.), n | |||||
| Onset, years | 39.6 (26.9) 159 | ||||
| Duration, years | 32.01 (26.00) | ||||
| Comorbid anxiety disorder, % (n) | 34.0 (53/156) | ||||
| Comorbid major depressive disorder, % (n) | 15.4 (24/156) | ||||
| Hamilton Rating Scale for Depression, mean (s.d.) | |||||
| Total | 11.93 (3.89) | 1.78 (2.20) | <0.0001 | ||
| Core depression items | 3.01 (1.85) | 0.11 (0.31) | <0.0001 | ||
| Prescribed lorazepam, mean (s.d.) | 22.5 | 2.7 | 772 (1) | 0.006 | |
| Mini-Mental State Exam, mean (s.d.), n | 28.14 (1.72) 159 | 29.03 (1.16) | 0.0015 | ||
a Square root (X) transformation used in the analyses. Means and standard deviations reported in the original units.
b Satterthwaite test used because of unequal variances.
(n = 9/27) and for non-users 16.0% (n = 24/150), P = 0.04) and ethnicity (drop-out rate for participants who were White 15.9% (n = 23/145) and for those who were Black 31.2% (n = 10/32), P = 0.04).
Neuropsychological functioning of GAD and comparison groups at baseline
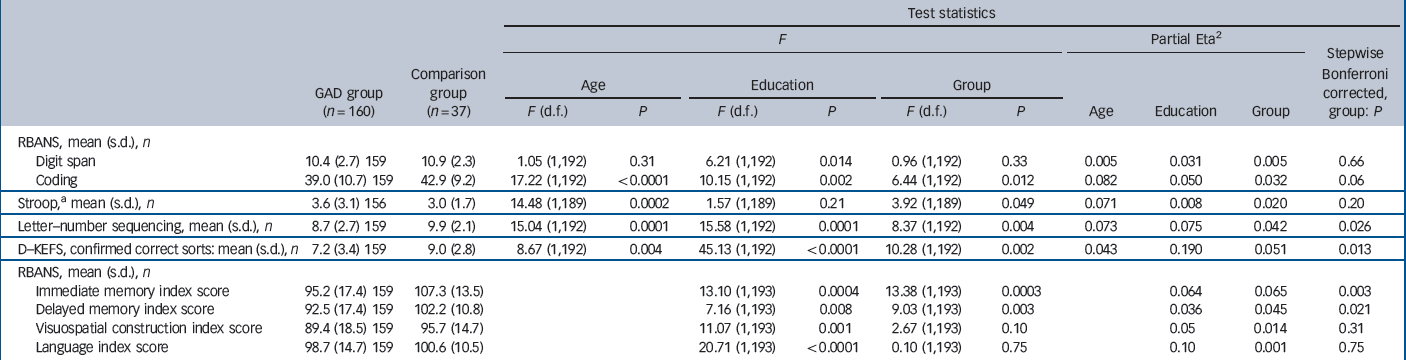
Among the main outcome measures (Table 2 and Fig. 1), after controlling for age and education, those in the GAD group performed significantly worse than those in the comparison group on RBANS coding (F(1,192) = 6.44, P = 0.012), the Stroop test (F(1,189) = 3.92, P = 0.049), letter–number sequencing (F(1,192) = 8.37, P = 0.004), the D–KEFS sorting test (F(1,192) = 10.28, P = 0.002) and both RBANS immediate memory (F(1,193) = 13.38, P = 0.0003) and delayed memory (F(1,193) = 9.03, P = 0.003) indexes. There was no difference between the groups on the RBANS digit span (F(1,192) = 0.96, P = 0.33) or on the other neuropsychological domains assessed, language (F(1,193) = 0.10, P = 0.75) and visuospatial construction (F(1,193) = 2.67, P = 0.10). When the comparisons were corrected using the stepwise Bonferroni method, all of the findings remained except that the two groups performed similarly on the Stroop and RBANS coding (Table 2). Size and strength of the results did not change when excluding participants who met criteria for MDD or using MDD as a covariate. We also re-ran the analyses using lorazepam co-prescription as a covariate and the results did not significantly change from the previous results (results not reported).
Functional correlates of neuropsychological performance at baseline
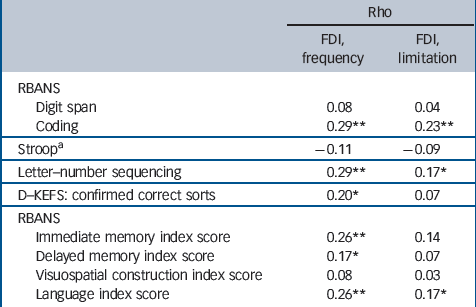
Table 3 displays correlations between the two FDI subscales and nine neuropsychological variables. Of the 18 correlations, 9 were statistically significant at P<0.05 suggesting that in late-life GAD, neuropsychological impairments are correlated with functional disability and lending credence to the importance of
Table 2 Neuropsychological measures
| Test statistics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Partial Eta2 | Stepwise Bonferroni corrected, group: P | ||||||||||
| GAD group (n = 160) | Comparison group (n = 37) | Age | Education | Group | ||||||||
| F (d.f.) | P | F (d.f.) | P | F (d.f.) | P | Age | Education | Group | ||||
| RBANS, mean (s.d.), n | ||||||||||||
| Digit span | 10.4 (2.7) 159 | 10.9 (2.3) | 1.05 (1,192) | 0.31 | 6.21 (1,192) | 0.014 | 0.96 (1,192) | 0.33 | 0.005 | 0.031 | 0.005 | 0.66 |
| Coding | 39.0 (10.7) 159 | 42.9 (9.2) | 17.22 (1,192) | < 0.0001 | 10.15 (1,192) | 0.002 | 6.44 (1,192) | 0.012 | 0.082 | 0.050 | 0.032 | 0.06 |
| Stroop,a mean (s.d.), n | 3.6 (3.1) 156 | 3.0 (1.7) | 14.48 (1,189) | 0.0002 | 1.57 (1,189) | 0.21 | 3.92 (1,189) | 0.049 | 0.071 | 0.008 | 0.020 | 0.20 |
| Letter–number sequencing, mean (s.d.), n | 8.7 (2.7) 159 | 9.9 (2.1) | 15.04 (1,192) | 0.0001 | 15.58 (1,192) | 0.0001 | 8.37 (1,192) | 0.004 | 0.073 | 0.075 | 0.042 | 0.026 |
| D–KEFS, confirmed correct sorts: mean (s.d.), n | 7.2 (3.4) 159 | 9.0 (2.8) | 8.67 (1,192) | 0.004 | 45.13 (1,192) | < 0.0001 | 10.28 (1,192) | 0.002 | 0.043 | 0.190 | 0.051 | 0.013 |
| RBANS, mean (s.d.), n | ||||||||||||
| Immediate memory index score | 95.2 (17.4) 159 | 107.3 (13.5) | 13.10 (1,193) | 0.0004 | 13.38 (1,193) | 0.0003 | 0.064 | 0.065 | 0.003 | |||
| Delayed memory index score | 92.5 (17.4) 159 | 102.2 (10.8) | 7.16 (1,193) | 0.008 | 9.03 (1,193) | 0.003 | 0.036 | 0.045 | 0.021 | |||
| Visuospatial construction index score | 89.4 (18.5) 159 | 95.7 (14.7) | 11.07 (1,193) | 0.001 | 2.67 (1,193) | 0.10 | 0.05 | 0.014 | 0.31 | |||
| Language index score | 98.7 (14.7) 159 | 100.6 (10.5) | 20.71 (1,193) | < 0.0001 | 0.10 (1,193) | 0.75 | 0.10 | 0.001 | 0.75 | |||
GAD, generalised anxiety disorder; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; D–KEFS, Delis–Kaplan Executive Function System.
a Natural Log (X) transformation used In the analyses. Means and standard deviations reported In the original units.
neuropsychological functioning in late-life GAD. Significant correlations included: the FDI frequency subscale with RBANS coding, letter–number sequencing, D–KEFS sorting, and RBANS immediate and delayed memory index scores as well as the language index score; and the FDI limitations subscale with RBANS coding and letter–number sequencing and the RBANS language index score.
Comparing neuropsychological functioning pre- and post-treatment in GAD participants
Low cognitive scorers
Sixty-six participants scored below the group median score (total index score ≤94) on RBANS (30 in the active and 36 in the placebo treatment conditions) and thus were considered low cognitive scorers for the purpose of these analyses. The scores of the 66 participants who performed below the median RBANS total score ranged from the low end of the average range (RBANS digit span and the RBANS language index score) to mildly impaired (approximately 1 standard deviation below the age-corrected mean: letter–number sequencing, RBANS immediate and delayed memory index scores) to moderately impaired (1 to 2 standard deviations below the age-corrected mean: D–KEFS sorting, RBANS coding task, RBANS visuospatial construction index), to moderately to severely impaired (2 to 3 standard deviations below the age-correlated mean: the Stroop test). There was no difference between the lower and upper halves in age at onset of GAD (39 years for both groups; P = 0.99) or per cent who had comorbid MDD (20 v. 9.7, P = 0.10), but the lower half did have slightly higher anxiety as measured by the HRSA (23.42 v. 21.37, P = 0.05).
The repeated measures ANOVA revealed no significant main effects for treatment group, time, or treatment×time interactions for the Stroop test, RBANS digit span, coding or immediate memory or language index scores. There were significant main effects for time but not for treatment group or treatment×time interactions on letter–number sequencing (F(1,64) = 4.82, P = 0.032) and RBANS delayed memory (F(1,64) = 6.65, P = 0.01) and visuospatial construction index scores, suggesting that working memory, delayed memory and visuospatial ability improved in both treatment conditions over the course of the study. There was a significant treatment×time interaction on the D–KEFS sorting task (F(1,62) = 4.06, P = 0.048) indicating that the group receiving escitalopram improved in problem-solving, conceptual ability and mental flexibility more than did the group receiving placebo.
Reported improvement in anxiety
Forty-two participants reported ‘improvement’ in anxiety (CGI score ≤2) over the course of the study. These participants experienced significant improvement on the Stroop (change score 0.60 (s.d. = 5.38), P = 0.0063) and both RBANS immediate (change score 3.74 (s.d. = 10.08), P = 0.0114) and delayed memory (change score 5.41 (s.d. = 10.08), P = 0.0019), indicating an association between improved anxiety and improved ability to engage in inhibition and episodic recall in both treatment conditions over the course of the study.
Discussion
Main findings
To our knowledge, this is the first large-scale study to comprehensively evaluate neuropsychological function in late-life GAD and its response to treatment in a systematic manner. We found broad based, but not global impairments. Older participants with

Fig. 1 Boxplots depicting performance of generalised anxiety disorder (GAD) and comparison groups on the neuropsychological variables. (a) Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) digital span; (b) RBANS coding; (c) Stroop; (d) letter–number sequencing; (e) Delis–Kaplan Executive Function System (D–KEFS) confirmed correct sorts; (f) RBANS immediate memory index score; (g) RBANS delayed memory index score.
GAD performed worse than those in the comparison group on measures of information processing speed, working memory, inhibition and problem-solving (including concept formation and mental flexibility), as well as immediate and delayed memory. These findings are consistent with published literature describing decrements in memory and executive functions in young and middle-aged adults Reference Airaksinen, Larsson and Forsell27 and memory and working memory in older adults with GAD. Reference Mantella, Butters, Dew, Mulsant, Begley and Tracey28,Reference Caudle, Senior, Wetherell, Rhoades, Beck and Kunik29 The impairments of highest magnitude were in memory, as immediate and delayed memory in the GAD group were about one standard deviation below that of the comparison group. This finding fits well with the literature review of Beaudreau & O’Hara, Reference Beaudreau and O'Hara30 who noted that memory impairments tended to be the most consistent cognitive deficits in late-life anxiety. Our findings also suggest that worry may compete for cognitive resources thereby interfering with execution of some especially vulnerable cognitive functions, including
Table 3 Functional correlates of neuropsychological performance at baseline (n = 155)
| Rho | ||
|---|---|---|
| FDI, frequency | FDI, limitation | |
| RBANS | ||
| Digit span | 0.08 | 0.04 |
| Coding | 0.29** | 0.23** |
| Stroopa | –0.11 | –0.09 |
| Letter–number sequencing | 0.29** | 0.17* |
| D–KEFS: confirmed correct sorts | 0.20* | 0.07 |
| RBANS | ||
| Immediate memory index score | 0.26** | 0.14 |
| Delayed memory index score | 0.17* | 0.07 |
| Visuospatial construction index score | 0.08 | 0.03 |
| Language index score | 0.26** | 0.17* |
FDI, Function and Disability Instrument; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; D–KEFS, Delis–Kaplan Executive Function System.
a Natural Log (X) transformation used in the analyses.
* P < 0.05
** P < 0.01.
processing speed, some aspects of executive function and episodic memory.
We also found that neuropsychological functioning was significantly, but relatively weakly correlated with everyday functioning, consistent with the hypothesis that impaired cognitive function plays a significant role in the functional disability associated with late-life GAD. Reference Wetherell, Le Roux and Gatz31
Finally, we found that among participants with GAD with poor cognitive performance at baseline, multiple domains showed improvement during the course of the clinical trial, suggesting that either clinical improvement in anxiety led to improvements in poor cognitive performance or clinical improvement in poor cognitive performance led to improvements in anxiety. Further, those who received escitalopram had greater improvements compared with placebo on the D–KEFS sorting task, which measures problem-solving, concept formation and mental flexibility. In addition, those participants with GAD who reported improved anxiety over the course of the trial experienced significant improvement in inhibition and both immediate and delayed recall.
Findings from other studies
Most studies of cognitive function and anxiety in older adults examine community-based populations with symptomatic measures of anxiety and report that higher levels of anxiety symptoms are associated with poorer fluid intelligence, Reference Cohen, Eisdorfer, Vitaliano and Bloom32 complex visuospatial skills, Reference Wetherell, Reynolds, Gatz and Pedersen33 learning and memory, Reference Wetherell, Reynolds, Gatz and Pedersen33–Reference Paterniti, Dufouil, Bisserbe and Alperovitch36 information processing speed Reference Beaudreau and O'Hara30,Reference Bierman, Comijs, Jonker and Beekman34,Reference Hogan37 and executive functions Reference Bierman, Comijs, Jonker and Beekman34,Reference Paterniti, Dufouil, Bisserbe and Alperovitch36 including inhibition. Reference Beaudreau and O'Hara30 By contrast, some community-based studies report no association between anxiety symptoms and cognitive function in late life. Reference Biringer, Mykletun, Dahl, Smith, Engedal and Nygaard38,Reference Lindesay, Briggs and Murphy39 Three clinic-based studies have examined cognitive functioning in older adults who met specific clinical criteria for GAD. We previously reported that relative to a comparison group, individuals with late-life GAD exhibited impairment on measures of immediate and delayed recall as well as mental set shifting. Reference Mantella, Butters, Dew, Mulsant, Begley and Tracey28 Caudle and colleagues Reference Caudle, Senior, Wetherell, Rhoades, Beck and Kunik29 found that greater GAD symptom severity was associated with poorer working memory and Price & Mohlman Reference Price and Mohlman40 found an association with better inhibitory control (as measured with the Stroop).
The causal relationship between generalised anxiety and cognitive impairment is unclear. One prevalent model posits that worry is a cognitive process designed to avoid the anxiogenic images that induce somatic activation through increased noradrenergic discharge. Reference Borkovec and Inz41 However, it is not clear whether the affective (increased anxiety) or cognitive (avoidance) component of GAD plays a more prominent role in the interplay between GAD and cognitive impairment. Affective interference (e.g. selective processing of anxious information at the expense of other cognitive tasks Reference Ingram, Kendall, Smith, Donnell and Ronan42 ) seems to be related to a particular aspect of anxiety, namely ruminative worry. Rumination might mitigate the ability to shift resources between emotional and cognitive tasks, thus reducing task performance. Reference Whalen, Bush, McNally, Wilhelm, McInerney and Jenike43 In a recent report, our group has shown that older adults with depression and high comorbid anxiety have elevated activation of several brain areas involved in cognitive performance, including dorsal prefrontal cortex and dorsal anterior cingulate cortex. Reference Andreescu, Butters, Lenze, Venkatraman, Nable and Reynolds44
Generalised anxiety disorder may be particularly detrimental to cognitive function in older adults, Reference Paterniti, Dufouil, Bisserbe and Alperovitch36 who have less cognitive reserve against central nervous system insults than do younger adults. Cognitive reserve refers to the degree to which an individual is able to maintain cognitive function in the face of mounting neuropathology. Moreover, worry may compete for the cognitive resources necessary for working memory thereby interfering with execution of some cognitive functions. Reference Beaudreau and O'Hara30 Additionally, ageing increases vulnerability to cognitive impairment because homeostatic mechanisms that prevent an excessive biological stress response are diminished. Reference Wetherell, Le Roux and Gatz31,Reference Hogan37 Consequently, some deleterious effects of excessive stress response – such as neurotoxic hypercortisolemia – worsen with age. Despite these threats to cognition, few studies have examined neuropsychological functioning in late-life anxiety disorders, and there are very few reports on late-life GAD in particular.
Whereas observational studies provide some support for a cross-sectional association between anxiety and cognitive impairment in older adults, better-designed cross-sectional and longitudinal studies may be the more appropriate approach. Experimental research designs that involve manipulating anxiety levels, rather than simply assessing them repeatedly, could also provide informative data about the possible causal impact of anxiety on cognitive change.
Limitations
Our study has some limitations. Although the RBANS has parallel forms to reduce practice effects, improved performance over time may be attributable to repetition rather than true treatment effects. Also, the clinical trial was only 12 weeks long; longer protocolised evaluations may be more robust for finding differences in the course of cognitive decline and its amelioration with effective treatment. Comparing individuals with late-life GAD with non-psychiatric controls leaves open the possibility that the impairments that were identified could be the result of having a psychiatric disorder in general and may not be specific to late-life GAD. Although future studies should employ this approach, in the case of GAD, the most appropriate psychiatric control group is unclear. It is possible that IQ differences could explain our findings. However, all of our participants were recruited from the same sources and the average years of education was similar in both the GAD and comparison groups, minimising the likelihood that there were substantial IQ differences. We did not measure sleep quality or quantity, and it is possible that sleep disturbance may account for some or all of the effects on memory. Finally, our understanding of the neurobiology of late-life GAD is lacking, and better understanding of the structural or functional changes leading to cognitive impairment in this disorder may yield more informative neuropsychological hypotheses and, eventually, better treatments. The greatest strength of this study is that it examines the relationship between anxiety and cognition in the context of an experimental manipulation (i.e. treatment). In the context of a 12-week clinical trial, it is more likely that reductions in anxiety were driving improvements in cognition rather than the reverse.
Implications
In conclusion, GAD in older adults is associated with widespread neuropsychological impairments, including information processing speed, working memory, inhibition, problem-solving and both immediate and delayed memory. Although there was specific improvement in problem-solving, concept formation and mental flexibility attributable to SSRI treatment, many of these cognitive domains showed improvement during the course of treatment regardless of treatment assignment. Although medication-specific benefits are minimal in this analysis, we found that improvement in anxiety symptoms was associated with neuropsychological improvements. Such findings are not a treatment effect per se but could be interpreted as showing that the clinical fluctuation of anxiety symptoms also affects cognitive performance. Future treatment research might usefully focus on providing long-term remission stability, both clinically and cognitively and should examine whether such improvements translate to meaningful gains in function and quality of life. These findings underscore the importance of cognitive functioning as a potential treatment target to reduce the impairment associated with anxiety disorders in older adults.
Funding
This research was supported by United States Public Health Service grants , and , as well as a 2009 NARSAD Young Investigator Award to C.A.
Acknowledgements
We would like to thank Michelle Zmuda, Program Coordinator of the Geriatric Neuropsychology Research Program for overseeing the neuropsychological assessments.







eLetters
No eLetters have been published for this article.