Introduction
Prenatal environmental factors have been repeatedly associated with offspring cognitive, emotional, and behavioral development and with individual susceptibility for developing mental disorders over the life course (Gluckman & Hanson, Reference Gluckman, Hanson, Gluckman and Hanson2006; O’Donnell & Meaney, Reference O’Donnell and Meaney2017). During the prenatal period, the embryonic/fetal organism is developing rapidly which renders the organism vulnerable to environmental influences (Bock et al., Reference Bock, Wainstock, Braun and Segal2015). Following the theory of “developmental origins of health and disease,” prenatal environmental factors can have long-lasting programing effects on offspring health (O’Donnell & Meaney, Reference O’Donnell and Meaney2017). Several prenatal risk factors of offspring development have been established. These prenatal risk factors have been related to maternal health, maternal behavior and social environment, such as maternal psychological distress, physical diseases, mental disorders, and education (Entringer et al., Reference Entringer, Buss and Wadhwa2015; O’Donnell & Meaney, Reference O’Donnell and Meaney2017; Tearne et al., Reference Tearne, Allen, Herbison, Lawrence, Whitehouse, Sawyer and Robinson2015). Maternal psychological distress during pregnancy, for instance, has been associated with child behavioral and emotional problems (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019; Korja et al., Reference Korja, Nolvi, Grant and McMahon2017; O’Connor et al., Reference O’Connor, Heron, Golding, Glover and Team2003; O’Donnell et al., Reference O’Donnell, Glover, Barker and O’Connor2014) as well as with offspring anxiety and depressive disorders in adolescence and adulthood (Capron et al., Reference Capron, Glover, Pearson, Evans, O’Connor, Stein, Murphy and Ramchandani2015; Gentile, Reference Gentile2017). So far, most research has focused on the investigation of single prenatal risk factors. However, many families experience more than one risk factor given that risk factors often co-occur, partly due to shared background factors. As an example, mothers of lower education exhibit worse health habits (Härkönen et al., Reference Härkönen, Lindberg, Karlsson, Karlsson and Scheinin2018) as well as more anxiety and depression symptoms compared to mothers with higher education (Kotimäki et al., Reference Kotimäki, Härkönen, Karlsson, Karlsson and Scheinin2020). Thus, the investigation of cumulative adversity, by summing up present risk factors, might provide a more comprehensive picture of the risks to offspring compared to studies on single risk factors (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). However, thus far, few studies have addressed the association of cumulative prenatal adversity with offspring outcome. In these studies, different composite measures of prenatal adversity have been utilized, and prenatal adversity has consistently shown dose-dependent developmental, cognitive, and/or behavioral alterations in 1.5-year (Wade et al., Reference Wade, Madigan, Akbari and Jenkins2015), 3-year (Camerota & Willoughby, Reference Camerota and Willoughby2020; Garg et al., Reference Garg, Chen, Nguyen, Pokhvisneva, Chen, Unternaehrer, MacIsaac, McEwen, Mah, Gaudreau, Levitan, Moss, Sokolowski, Kennedy, Steiner, Meaney, Holbrook, Silveira, Karnani and O’Donnell2018), 4- and 5-year (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017), and 9-to-10-year old children (Roffman et al., Reference Roffman, Sipahi, Dowling, Hughes, Hopkinson, Lee, Eryilmaz, Cohen, Gilman, Doyle and Dunn2021). In some (Garg et al., Reference Garg, Chen, Nguyen, Pokhvisneva, Chen, Unternaehrer, MacIsaac, McEwen, Mah, Gaudreau, Levitan, Moss, Sokolowski, Kennedy, Steiner, Meaney, Holbrook, Silveira, Karnani and O’Donnell2018; Wade et al., Reference Wade, Madigan, Akbari and Jenkins2015), but not all of these studies (Camerota & Willoughby, Reference Camerota and Willoughby2020), moderating effects of the postnatal environment, assessed as infant attachment style, responsive parenting, and/or childcare quality, have additionally been observed. However, in all those studies that controlled for postnatal environment, cumulative prenatal adversity predicted child outcome above and beyond the quality of the postnatal environment (Camerota & Willoughby, Reference Camerota and Willoughby2020; Garg et al., Reference Garg, Chen, Nguyen, Pokhvisneva, Chen, Unternaehrer, MacIsaac, McEwen, Mah, Gaudreau, Levitan, Moss, Sokolowski, Kennedy, Steiner, Meaney, Holbrook, Silveira, Karnani and O’Donnell2018; Roffman et al., Reference Roffman, Sipahi, Dowling, Hughes, Hopkinson, Lee, Eryilmaz, Cohen, Gilman, Doyle and Dunn2021; Wade et al., Reference Wade, Madigan, Akbari and Jenkins2015).
Growing evidence suggests that the effects of prenatal adversity on offspring outcomes are moderated by offspring genotype (Acosta, Kantojärvi et al., Reference Acosta, Kantojärvi, Hashempour, Pelto, Scheinin, Lehtola, Lewis, Fonov, Collins, Evans, Parkkola, Lähdesmäki, Saunavaara, Karlsson, Merisaari, Paunio, Karlsson and Tuulari2020; O’Donnell & Meaney, Reference O’Donnell and Meaney2017; Qiu et al., Reference Qiu, Shen, Buss, Chong, Kwek, Saw, Gluckman, Wadhwa, Entringer, Styner, Karnani, Heim, O’Donnell, Holbrook, Fortier, Meaney and Group2017). To date, only one study on cumulative prenatal adversity took infant genotype into account. In that study, a coexpression polygenic risk score of the serotonin transporter gene (SLC6A4) was created from brain regions relevant for emotion processing (namely amygdala, hippocampus, and prefrontal cortex) and is – in its final form – based on the hippocampal-specific SLC6A4 gene network (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). Serotonin plays a key role in fetal brain development, and the serotonin transporter determines intra- and extracellular serotonin levels (Brummelte et al., Reference Brummelte, Mc Glanaghy, Bonnin and Oberlander2017; Whitaker-Azmitia, Reference Whitaker-Azmitia2001). Genetic variants in serotonergic pathways have been associated with child socioemotional development (Broekman et al., Reference Broekman, Chan, Goh, Fung, Gluckman, Saw and Meaney2011), autism (Lesch & Waider, Reference Lesch and Waider2012) and attention deficit hyperactivity disorder (Curran et al., Reference Curran, Purcell, Craig, Asherson and Sham2005; Purper-Ouakil et al., Reference Purper-Ouakil, Ramoz, Lepagnol-Bestel, Gorwood and Simonneau2011). There is also some evidence that genetic variations of serotonergic pathways might affect the individual’s susceptibility to prenatal environmental influences (Broekman et al., Reference Broekman, Chan, Goh, Fung, Gluckman, Saw and Meaney2011; Pluess et al., Reference Pluess, Velders, Belsky, van IJzendoorn, Bakermans-Kranenburg, Jaddoe, Hofman, Arp, Verhulst and Tiemeier2011). In support of this, Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) reported that a higher cumulative prenatal adversity score predicted more emotional and pervasive developmental problems in those 4- and 5-year-olds who also showed a higher coexpression polygenic risk score of the serotonin transporter gene. However, claims about an interaction between genetic variations in serotonergic pathways and environmental risk factors have been inconsistent in adult studies (Culverhouse et al., Reference Culverhouse, Saccone, Horton, Ma, Anstey, Banaschewski, Burmeister, Cohen-Woods, Etain, Fisher, Goldman, Guillaume, Horwood, Juhasz, Lester, Mandelli, Middeldorp, Olié, Villafuerte and Bierut2018; Risch et al., Reference Risch, Herrell and Lehner2009) and a further examination of these relations is warranted.
The biological mechanisms that underlie the association between prenatal adversity and offspring neurodevelopmental outcomes are not yet fully understood. They likely involve alterations in fetal brain development mediated by altered fetal exposure to glucocorticoids, inflammatory cytokines, and serotonin among other factors (Bock et al., Reference Bock, Wainstock, Braun and Segal2015; Dwyer et al., Reference Dwyer, Broide and Leslie2008; Entringer et al., Reference Entringer, Buss and Wadhwa2015; St-Pierre et al., Reference St-Pierre, Laurent, King and Vaillancourt2016; Yockey & Iwasaki, Reference Yockey and Iwasaki2018). Different types of prenatal adversity, depending on the timing and extent of the exposure, have been shown to be associated with offspring brain structures and functions implicated in cognition and emotion (Lautarescu et al., Reference Lautarescu, Craig and Glover2020). Prenatal maternal risk factors such as psychological distress (e.g., Lehtola et al., Reference Lehtola, Tuulari, Scheinin, Karlsson, Parkkola, Merisaari, Lewis, Fonov, Louis Collins, Evans, Saunavaara, Hashempour, Lähdesmäki, Acosta and Karlsson2020; Wen et al., Reference Wen, Poh, Ni, Chong, Chen, Kwek, Shek, Gluckman, Fortier, Meaney and Qiu2017), glucocorticoid levels (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012), immune activation (Graham et al., Reference Graham, Rasmussen, Rudolph, Heim, Gilmore, Styner, Potkin, Entringer, Wadhwa, Fair and Buss2018), and smoking (Ekblad et al., Reference Ekblad, Korkeila and Lehtonen2015) have been related to brain structural alterations. These alterations were most pronounced in amygdalar, hippocampal, and prefrontal areas, brain regions that are crucially involved in emotion and cognitive processing (Bock et al., Reference Bock, Wainstock, Braun and Segal2015). In some of these studies, structural brain alterations mediated the effects of prenatal adversity on child self-regulation, cognitive performance, as well as affective and behavioral problems (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019; Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; Davis et al., Reference Davis, Head, Buss and Sandman2017; Graham et al., Reference Graham, Rasmussen, Rudolph, Heim, Gilmore, Styner, Potkin, Entringer, Wadhwa, Fair and Buss2018; Moog et al., Reference Moog, Nolvi, Kleih, Styner, Gilmore, Rasmussen, Heim, Entringer, Wadhwa and Buss2021; Rifkin-Graboi et al., Reference Rifkin-Graboi, Meaney, Chen, Bai, Hameed, Tint, Broekman, Chong, Gluckman, Fortier and Qiu2015; Sandman et al., Reference Sandman, Buss, Head and Davis2015). In addition, sex-specific associations between early adversity and offspring brain structures have been observed (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019; Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; Lehtola et al., Reference Lehtola, Tuulari, Scheinin, Karlsson, Parkkola, Merisaari, Lewis, Fonov, Louis Collins, Evans, Saunavaara, Hashempour, Lähdesmäki, Acosta and Karlsson2020; Wen et al., Reference Wen, Poh, Ni, Chong, Chen, Kwek, Shek, Gluckman, Fortier, Meaney and Qiu2017). These findings are paralleled by sexually dimorphic associations between single prenatal risk factors and child behavior (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019; Braithwaite et al., Reference Braithwaite, Pickles, Sharp, Glover, O’Donnell, Tibu and Hill2017). To our knowledge, the association of offspring brain structure with cumulative prenatal adversity has not yet been targeted by studies. Evidence of such an association could lend further support to the notion that fetal brain structural alterations mediate effects of prenatal adversity on offspring behavior.
With this study, we first aimed at examining the claim that cumulative prenatal adversity is associated with child problem behavior at 4 and 5 years of age and is moderated by a hippocampal-specific coexpression polygenic risk score based on the serotonin transporter (SLC6A4) gene (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) in a large Finnish sample. We expected that greater cumulative prenatal adversity is associated with more problematic child behavior (Garg et al., Reference Garg, Chen, Nguyen, Pokhvisneva, Chen, Unternaehrer, MacIsaac, McEwen, Mah, Gaudreau, Levitan, Moss, Sokolowski, Kennedy, Steiner, Meaney, Holbrook, Silveira, Karnani and O’Donnell2018; Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017), independent of the postnatal environment, and that children with a higher polygenic risk score show more emotional problems with increasing cumulative prenatal adversity according to the MAVAN study (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). Given the sexually dimorphic associations between single prenatal risk factors and child behavioral outcomes (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019; Braithwaite et al., Reference Braithwaite, Pickles, Sharp, Glover, O’Donnell, Tibu and Hill2017), we also explored sex differences.
Second, we aimed at probing whether cumulative prenatal adversity is linked to infant subcortical structure volumes and whether subcortical structure volume alterations might mediate the effects of cumulative prenatal adversity on child problem behavior. We chose amygdalar and hippocampal volumes as regions of interest, as they are crucially involved in emotion processing (Kim et al., Reference Kim, Song, Kim, Song and Kosten2006; Phelps, Reference Phelps2004; Yilmazer-Hanke, Reference Yilmazer-Hanke, Mai and Paxinos2012). Amygdala and hippocampus have also been shown to be affected by single prenatal risk factors in animal and human studies (Acosta, Tuulari et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2020; Bock et al., Reference Bock, Wainstock, Braun and Segal2015; Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; Graham et al., Reference Graham, Rasmussen, Entringer, Ben Ward, Rudolph, Gilmore, Styner, Wadhwa, Fair and Buss2019; Lehtola et al., Reference Lehtola, Tuulari, Scheinin, Karlsson, Parkkola, Merisaari, Lewis, Fonov, Louis Collins, Evans, Saunavaara, Hashempour, Lähdesmäki, Acosta and Karlsson2020) and were used in the creation of the coexpression polygenic risk score by Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). We also investigated sex-specific associations between cumulative prenatal adversity and amygdalar and hippocampal volumes.
Finally, we wanted to explore whether infant genotype is related to infant amygdalar and hippocampal volumes and whether it moderates the association of cumulative prenatal adversity with these volumes.
Methods
Subjects
Participants were mother–infant dyads recruited from the FinnBrain Birth Cohort Study [www.finnbrain.fi] (Karlsson et al., Reference Karlsson, Tolvanen, Scheinin, Uusitupa, Korja, Ekholm, Tuulari, Pajulo, Huotilainen, Paunio, Karlsson and Group2018).
Child behavior sample
The Strengths and Difficulties Questionnaire (SDQ) was returned by 1632 mothers at two time points, that is, when their children were 4 and 5 years old. 1568 mothers (i.e., 96.1% of the participating mothers; children: female 45.3%) provided sufficient SDQ data for inclusion in this study (for details see below). For a subset of 1161 children (female: 46.0%), genetic data were available.
Neuroimaging sample
Neuroimaging data were collected from 189 infants at the age of 1–8 weeks after birth. Inclusion criteria for neuroimaging were gestational age at birth ≥ 35 weeks and birth weight > 1500 g. Exclusion criteria were previously diagnosed CNS anomalies or abnormal findings in a previous MRI scan. Of the 189 participants, 64 were excluded due to failed MRI scanning or motion artifacts in the MR images. Of the remaining 125 infants, three more subjects were excluded due to missing maternal questionnaire and/or sociodemographic data as described below. In the final neuroimaging sample, we included 122 mother–infant dyads (infants: female 43.4%, age after birth [days]: M = 26.2, SD = 7.7, range = 11–54; mothers’ age (at term) [years]: M = 29.8, SD = 4.4, range = 19–41). For a subset of 77 children (female: 49.4%), SDQ data were available, for a subset of 104 children (female: 41.4%) genetic data were available, and for a subset of 66 children (female: 50.0%) both SDQ and genetic data were available.
Written informed consent was obtained from all parent(s). The study was conducted according to the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of the Hospital District of Southwest Finland.
Measures and procedures
The prenatal adversity sum score
The prenatal adversity sum (PRE-AS) score was created similarly to the one described in Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). We included the variables as depicted in Table 1. We added maternal prenatal general anxiety to the PRE-AS score, given that it has been established as a prenatal risk factor (e.g., O’Connor et al., Reference O’Connor, Heron, Golding, Glover and Team2003; O’Donnell & Meaney, Reference O’Donnell and Meaney2017). Presence of each risk factor (i.e., each bullet point, total number of categories = 11) yielded one point. The summation of the points amounted to the prenatal adversity sum score. Hence, the PRE-AS score ranged from 0 to 11. Mothers who did not provide any data for ≥ 4 risk factors were excluded from the study (one risk factor missing: N = 163, two risk factors missing: N = 65, three risk factors missing: N = 25, excluded mothers, i.e., ≥4 risk factors missing: N = 24).
Table 1. The variables selected for the cumulative prenatal adversity sum (PRE-AS) score of this study and their cutoffs (if applicable) are listed on the left
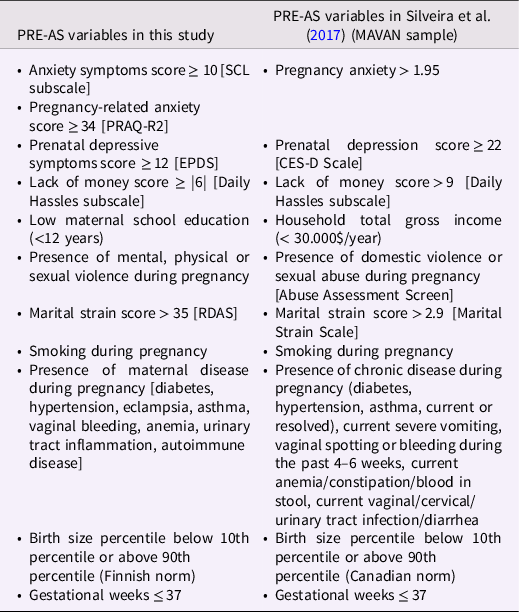
Note. On the right the variables as chosen in the MAVAN study (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) are depicted. CES-D Scale = Center of Epidemiologic Studies Depression Scale; EPDS = Edinburgh Postnatal Depression Scale; PRAQ-R2 = Maternal Pregnancy-Related Anxiety Questionnaire; RDAS = Revised Dyadic Adjustment Scale.
The following maternal variables were assessed via mothers’ self-report at gwk 14 and/or 34: maternal education, prenatal nicotine consumption, maternal age, prenatal maternal medication, alcohol and illicit drug consumption. Obstetric data were retrieved from the Finnish Medical Birth Register of the National Institute for Health and Welfare (http://www.thl.fi) and included maternal diseases, infant birth weight, length of pregnancy (gestational weeks) and infant Apgar scores (Apgar = appearance, pulse, grimace, activity, and respiration). Education was dichotomized [low: high school or vocational education (<12 years), middle/high: (career) college (12–15 years) or university (+15 years)]. In the final sample, 143 infants (9.1%) had a record of mild asphyxia (missings: N = 22).
We administered the anxiety subscale of the revised Symptom Checklist 90 (SCL-90-R) (Derogatis, Reference Derogatis1983; Holi et al., Reference Holi, Sammallahti and Aalberg1998) and the Finnish version of the Edinburgh Postnatal Depression Scale (Cox et al., Reference Cox, Holden and Sagovsky1987) at gestational weeks (gwk) 14, 24, and 34 to assess general maternal anxiety (SCL) and maternal depressive symptoms (EPDS) during pregnancy. Missing values (at maximum three items per time point) of EPDS and SCL were imputed with the mean value of the existing ones. Maternal pregnancy-related anxiety (PRAQ-R2) was assessed with the PRAQ-R2 questionnaire (Huizink et al., Reference Huizink, Delforterie, Scheinin, Tolvanen, Karlsson and Karlsson2016) at gwk 24 and 34. The PRAQ-R2 consists of 10 items rated from 1 to 5, and is a revised version of an earlier measure (PRAQ-R) of pregnancy-related anxiety (Huizink et al., Reference Huizink, Mulder, Robles De Medina, Visser and Buitelaar2004, Reference Huizink, Delforterie, Scheinin, Tolvanen, Karlsson and Karlsson2016). Missing items (one item per time point at maximum) of PRAQ-R2 were imputed with the mean value of the existing ones. Cut-off values of these questionnaires have been chosen according to Karlsson et al. (Reference Karlsson, Tolvanen, Scheinin, Uusitupa, Korja, Ekholm, Tuulari, Pajulo, Huotilainen, Paunio, Karlsson and Group2018): ≥ 12 for EPDS, ≥ 10 for SCL and ≥ 34 for PRAQ-R2 (for more details see SI). A value above the cut-off value of at least one of the three assessment points (gwk 14, 24, 34) was counted with 1 point for the PRE-AS (1 point at maximum for each questionnaire). For this purpose, the variables EPDS-pre, SCL-pre and PRAQ were computed as follows: subjects with questionnaire values below the cut-off for all three assessment points (→ 0); subjects with at least one value above the cut-off point (→ 1).
The marital strain of mothers was assessed at gwk 34 with the Finnish version of the Revised Dyadic Adjustment Scale (Busby et al., Reference Busby, Christensen, Crane and Larson1995). The scale comprises 14 items of which 13 are rated from 1 to 6 and 1 item from 1 to 5 (Finnish version). A sum score of > 35 indicates marital distress. Furthermore, mothers rated their experienced lack of money (LoM) at gwk 14, 24, and 34 by use of the Daily Hassles subscale which consists of 1 item rated from 0 to −3 (Kanner et al., Reference Kanner, Coyne, Schaefer and Lazarus1981; Korpela et al., Reference Korpela, Ylén, Tyrväinen and Silvennoinen2008). The LoM sum scores of each assessment point (gwk 14, 24, 34) were summed up to create a LoM Sum score for the second and third trimester of pregnancy (LoM Sum (i) = LoM gwk14 (i) + LoM gwk24 (i) + LoM gwk34 (i)]). The 15th percentile of LoM Sum (see Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) was assessed in the child behavior sample and used as a cutoff value.
Our aim was to investigate the associations of the cumulative prenatal adversity score. The correlations of each risk factor with the outcome have been presented in Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). For comparison, we assessed the regression weights of each risk factor in multiple regression analyses, including all single risk factors as predictors, and the results are presented in Table SI-6.
The Strengths and Difficulties Questionnaire
The SDQ Version P4–16 (Goodman et al., Reference Goodman, Ford, Simmons, Gatward and Meltzer2000; Goodman, Reference Goodman2001) was administered to the mothers when their children were 4 and 5 years old. The SDQ is a brief behavioral screening instrument for children aged 4–16 years and measures emotional and behavioral difficulties and prosocial behavior. Comparing the SDQ to the longer established screening questionnaire, the Child Behavior Checklist (CBCL), the scores of both instruments highly correlated with another and showed equal discriminatory qualities (Goodman & Scott, Reference Goodman and Scott1999; Warnick et al., Reference Warnick, Bracken and Kasl2008). The SDQ scales’ criterion validity has been rated superior to that of the CBCL, with a statistically significant difference for inattention / hyperactivity, and the SDQ is considered the more time-effective instrument due to its brevity (Goodman & Scott, Reference Goodman and Scott1999). The SDQ questionnaire contains 25 three-point items which are divided between 5 subscales: “emotional symptoms” (F1), “conduct problems” (F2), “hyperactivity/inattention” (F3), “peer relationship problems” (F4), and “prosocial behavior” (F5). The first four subscales that measure a child’s difficulties are summed up to generate a total difficulties score (SDQ Sum). In this study, we used both the first four subscales as well as their sum score of each time point (4 years, 5 years). Mothers who did not provide sufficient data for at least one subscale at one of the two time points were excluded from the study (N = 64).
The postnatal distress sum score
Maternal postnatal depressive symptoms were assessed by use of the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., Reference Cox, Holden and Sagovsky1987), and maternal marital strain was assessed by use of the Revised Dyadic Adjustment Scale (Busby et al., Reference Busby, Christensen, Crane and Larson1995), each at 3 and 6 months, and at 1, 2, and 4 years postpartum. Maternal postnatal anxiety was measured using the anxiety subscale of the revised Symptom Checklist 90 (SCL-90-R) (Derogatis, Reference Derogatis1983; Holi et al., Reference Holi, Sammallahti and Aalberg1998) at 3 and 6 months, and at 2 and 4 years postpartum. We created a postnatal distress sum score (POST-DS), using the same cutoff values for each scale as described for PRE-AS. Values above the cutoff point were counted with 1 point. We considered at maximum 1 point for the first year, 1 point for the second year and 1 point for the fourth year of assessment for each scale (i.e., EPDS, SCL, RDAS), resulting in a value range of 0 to 3 for the variables EPDS-post, SCL-post and RDAS-post. We additionally included dichotomized maternal education into the POST-DS, resulting in a range of POST-DS from 0 to 10.
Data imputation
In order to deal with our missing data, we imputed our questionnaire and obstetric data. The analysis with nonimputed data, that is, listwise deletion analysis, has been critizised as it “may lead to biased, underpowered, or unreliable parameter estimates” (Lodder, Reference Lodder2013), especially if the data are not missing randomly. In our study, missing data of (sub)scales and other maternal and infant variables were imputed by a nonparametric approach, the MissForest method (Stekhoven & Bühlmann, Reference Stekhoven and Bühlmann2012). Table SI-1 gives an overview on the number of imputed missings for the child behavior sample and the neuroimaging sample. As a control, we also analyzed the complete cases (nonimputed data) and results are reported in the SI.
MRI acquisition
A detailed description of the MRI acquisition protocol is provided in a previous publication by the same research team (Lehtola et al., Reference Lehtola, Tuulari, Karlsson, Parkkola, Merisaari, Saunavaara, Lähdesmäki, Scheinin and Karlsson2019). Participants were scanned with a Siemens Magnetom Verio 3T scanner (Siemens Medical Solutions, Erlangen, Germany) during natural sleep. The 40-min imaging protocol included an axial PD-T2 TSE (Dual-Echo Turbo Spin Echo) sequence (repetition time (TR): 12070 ms, effective echo times (TE): 13 ms and 102 ms) and a sagittal 3D-T1 MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) sequence (TR: 1900 ms, TE: 3.26 ms, inversion time: 900 ms) with whole brain coverage and isotropic voxels of 1.0 mm3 for both sequences. All brain images were assessed for incidental findings by a pediatric neuroradiologist.
Assessment of subcortical structure volumes
The volumes of the left and right amygdalae and hippocampi were assessed for each subject via label fusion based methods. These methods depend on achieving good registrations between the subjects and the entries in a library of templates. This is increasingly difficult to achieve the further the templates are from the subjects in terms of similarity. Thus, we constructed a template library based on the subjects in this study. We first constructed a population specific base infant template (Fonov et al., Reference Fonov, Evans, Botteron, Almli, McKinstry and Collins2011). Then we warped that template to the 21 subjects that best represented the morphological variation in the sample, and manually labeled the structures of interest in each, based on the methods established by Hashempour et al. (Reference Hashempour, Tuulari, Merissari, Lidauer, Luukkonen, Saunavaara, Parkkola, Lähdesmäki, Lehtola, Keskinen, Lewis, Scheinin, Karlsson and Karlsson2019). Then, from these 21 manual segmentations, we created consensus segmentation labels on the base infant template via voxel-wise majority vote. We then constructed a library of warped versions of the labeled template, such that the library best represented the morphological variation in the sample for amygdalae and hippocampi. That library was then used to label the individual brains via label fusion based labeling methods (Coupé et al., Reference Coupé, Manjón, Fonov, Pruessner, Robles and Collins2011; Lewis et al., Reference Lewis, Fonov, Collins, Evans and Tohka2019; Weier et al., Reference Weier, Fonov, Lavoie, Doyon and Louis Collins2014). Finally, we calculated the volume of each structure from its label. The details of this approach are described in Acosta, Kantojärvi et al. (Reference Acosta, Kantojärvi, Hashempour, Pelto, Scheinin, Lehtola, Lewis, Fonov, Collins, Evans, Parkkola, Lähdesmäki, Saunavaara, Karlsson, Merisaari, Paunio, Karlsson and Tuulari2020).
Genetic analyses
An umbilical cord blood sample was drawn from each newborn at birth. DNA samples were extracted according to standard procedures at the National Institute for Health and Welfare and genotyped with Illumina Infinium PsychArray and Illumina Infinium Global Screening Array at the Estonian Genome Centre. Quality control (QC) was performed with PLINK 1.9 (www.cog-genomics.org/plink/1.9/) (Chang et al., Reference Chang, Chow, Tellier, Vattikuti, Purcell and Lee2015). Markers were removed for missingness (>5%) and Hardy−Weinberg equilibrium (p-value < 1 × 10−6). Individuals were checked for missing genotypes (>5%), relatedness (identical by descent calculation, PI_HAT > 0.2) and population stratification (multidimensional scaling). Genotyped data was pre-phased with Eagle v2.4 (Loh et al., Reference Loh, Danecek, Palamara, Fuchsberger, Reshef, Finucane, Schoenherr, Forer, McCarthy, Abecasis, Durbin and Price2016) and imputed with Beagle v4.1 (Browning & Browning, Reference Browning and Browning2016) using the population-specific SISu v2 whole-genome sequencing data as imputation reference panel.
In our sample, we computed a coexpression polygenic risk score (ePRS) as described by Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). In brief, Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) have created this ePRS based on a transcriptional coexpression matrix with SLC6A4 in the amygdala, hippocampus, and prefrontal cortex in mice. From this list, they have selected transcripts with a prenatal enrichment within the human brain and identified 29 genes. After quality control and exclusion of genes on the X chromosome, the final list consisted of 22 genes. Derived from the selected genes, 206 functional independent SNPs (personal communication by P. Silveira, 11/09/18) have been identified using GTEx (https://www.gtexportal.org/home/) and linkage disequilibrium clumping. The number of alleles at a given SNP, weighted by the slope coefficient from a regression model predicting gene expression by SNPs in cis (cis = in the gene’s own regulatory regions), have been counted for the subjects. We used the list of SNPs and the weights used to calculate the ePRS as provided from P. Silveira (personal communication, 11/09/18). In our study, the ePRS consisted of 192 selected SNPs (due to imputation the numbers of selected SNPs differ from those reported in Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) and was standardized to a mean of zero (N = 1161).
In summary, the ePRS is based on a prenatal “hippocampal-specific SLC6A4 gene network” (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) consisting of 22 genes whose expression is either highly positively or negatively correlated with the SLC6A4 expression. Some of these genes have been implicated in epigenetic modifications and dopaminergic neuron differentiation among others (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). The nature of the associations in this gene network is unknown and does not necessarily imply causality, though it is assumed that coexpressed genes are functionally related, with functional ties being stronger if gene coexpression is conserved through evolution (Oti et al., Reference Oti, Van Reeuwijk, Huynen and Brunner2008). Notwithstanding these considerations, the ePRS does not allow assumptions on the expression of SLC6A4 itself, but on the expression of genes associated with the SLC6A4 expression. A higher individual ePRS score can be interpreted as indicative of a preponderance of genetic variants that are associated with a stronger expression of genes that are positively correlated with the SLC6A4 expression. On the other hand, a more negative ePRS can be understood as indicative of a relatively higher proportion of genetic variants that are associated with a higher expression of genes that are negatively correlated with the SLC6A4 expression. Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) interpreted a higher ePRS as a marker of higher vulnerability to prenatal adversity.
Of note, weaker transcriptional activity of SLC6A4 has originally been considered as conferring sensitivity to environmental influences, but this view has been challenged by large meta-analyses (Culverhouse et al., Reference Culverhouse, Saccone, Horton, Ma, Anstey, Banaschewski, Burmeister, Cohen-Woods, Etain, Fisher, Goldman, Guillaume, Horwood, Juhasz, Lester, Mandelli, Middeldorp, Olié, Villafuerte and Bierut2018; Heils et al., Reference Heils, Teufel, Petri, Stöber, Riederer, Bengel and Lesch1996; Risch et al., Reference Risch, Herrell and Lehner2009).
Statistical analyses
Statistical analyses were performed using R 3.6.3/4.2.0 (R Core Team, 2016) (http://www.r-project.org/). First, we probed the association of cumulative prenatal adversity (PRE-AS) with SDQ scores in multiple regression analyses, controlling for sex, in the large sample of 1568 children, separately for 4 and 5 years. We further investigated the interaction of PRE-AS with sex on the SDQ values.
To control for postnatal distress, we included POST-DS as a control variable in additional analyses. To test how SDQ scores of children with no prenatal adversity (PRE-AS = 0) differed from those with prenatal adversity (PRE-AS > 0), given POST-DS = 0 or POST-DS > 0, we dichotomized PRE-AS (=0: N = 403; >0: 1165) and POST-DS (=0: N = 621; > 0: N = 947) and combined them into a new factor (PRE-POST) with four groups [PRE-AS = 0/POST-DS = 0 (N = 276), PRE-AS > 0/POST-DS = 0 (N = 345), PRE-AS = 0/POST-DS > 0 (N = 127), PRE-AS > 0/POST-DS > 0 (N = 820)]. We explored the associations of PRE–POST with SDQ scores in multiple regression analyses, using the group without pre- and postnatal distress as baseline. Details on further control analyses, their results, and statistical tests of the assumptions of the multiple regression analyses are provided in the Supplement (SI).
Second, we analyzed the association of PRE-AS and of PRE-AS-by-sex with infant amygdalar and hippocampal volumes in the neuroimaging sample, running standard multiple linear regression analyses. Infant age after birth at MRI scan time, gestational weeks at birth, total brain volume and infant sex were included as control variables in all our analyses of subcortical structure volumes (if not included as predictor). Mediation analyses for the relationship between PRE-AS, regional brain volumes and SDQ values were performed by use of the “mediate” function of the R package “psych” (version 2.2.5), separately for boys and girls. The sample sizes for the mediation analyses were small (N = 39 for boys, N = 38 for girls). We estimated the necessary sample size to achieve a power of 0.8 by using the observed a and b paths’ sizes of z-standardized variables according to Fritz and MacKinnon (Fritz & MacKinnon, Reference Fritz and MacKinnon2007).
Finally, we investigated (1) the main effect of ePRS genotype, (2) the interaction of ePRS with sex, (3) the interaction of ePRS with PRE-AS, and (4) the interaction of ePRS with sex and PRE-AS on following outcomes separately: (a) SDQ values, controlling for sex, and (b) infant subcortical structure volumes in the respective subsamples. Control analyses were performed as described above and in SI.
The significance threshold was set to p < .05. Effect sizes are reported as r or phi values. In order to control for the error rate related to multiple comparisons, we additionally report a false-discovery rate (FDR) correction that was used for the outcome measures (1. SDQ subscales 1 to 4; 2. left/right amygdalae and left/right hippocampi) (“p.adjust” function in R).
Results
Demographic overview
Table SI-1 gives an overview on demographic variables, containing imputed data, and the number of missing values (mis) in both samples. Comparing the neuroimaging and the child behavior samples (Table SI-1), no significant differences were found with regard to PRE-AS (p = .185), but significant differences between the two samples were observed for several subscales of PRE-AS and for POST-DS. More details are given in the SI.
Table S1 presents an overview on the relations between the single risk factors and the cumulative risk scores PRE-AS (M = 1.53, SD = 1.43, range 0–9) and POST-DS (M = 1.65, SD = 1.89, range 0–10) in the child behavior sample. The prenatal risk factors EPDS and SCL were strongly associated, and low education showed a medium association with smoking. Moreover, we observed high correlations between pre- and postnatal adversity.
Association of PRE-AS with child problem behavior
We probed the association of cumulative prenatal adversity (PRE-AS) with problem behavior of 4- and 5-year-olds, assessed with the SDQ, in multiple regression analyses controlling for sex. We also analyzed interactions of PRE-AS with sex. Table 2 provides an overview on the results.
Table 2. Association between PRE-AS and SDQ
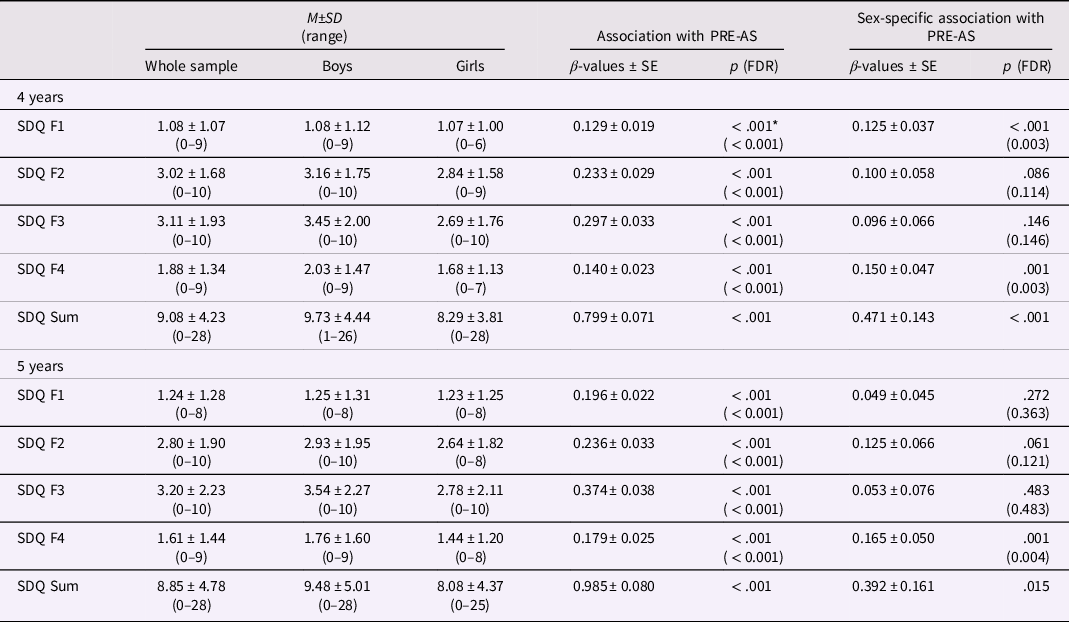
Note. On the right, the associations of SDQ scores at 4 and 5 years of age with PRE-AS and the PRE-AS-by-sex interaction are presented. On the left, mean values and standard deviations of SDQ scores in the whole sample (N = 1568), and for boys (N = 858) and girls (N = 710) separately are listed. SDQ values were significantly higher in boys than in girls (all p < .003) except for emotional symptoms (SDQ F1) at 4 and 5 years (both p > .78). *p > .05 after controlling for POST-DS.
Higher PRE-AS was significantly associated with higher scores of all SDQ subscales and of the SDQ Sum score of the 4- and 5-year-olds (Table 2, Figure 1). PRE-AS explained between 2% and 9% of the observed variance. Additionally, we found sex differences: PRE-AS was significantly more positively associated with emotional symptoms at 4 years, as well as peer relationship problems and SDQ Sum scores at 4 and 5 years in boys compared to girls. The control analyses supported the results, except for SDQ Sum at 5 years using nonimputed data (see SI, Table SI-2).
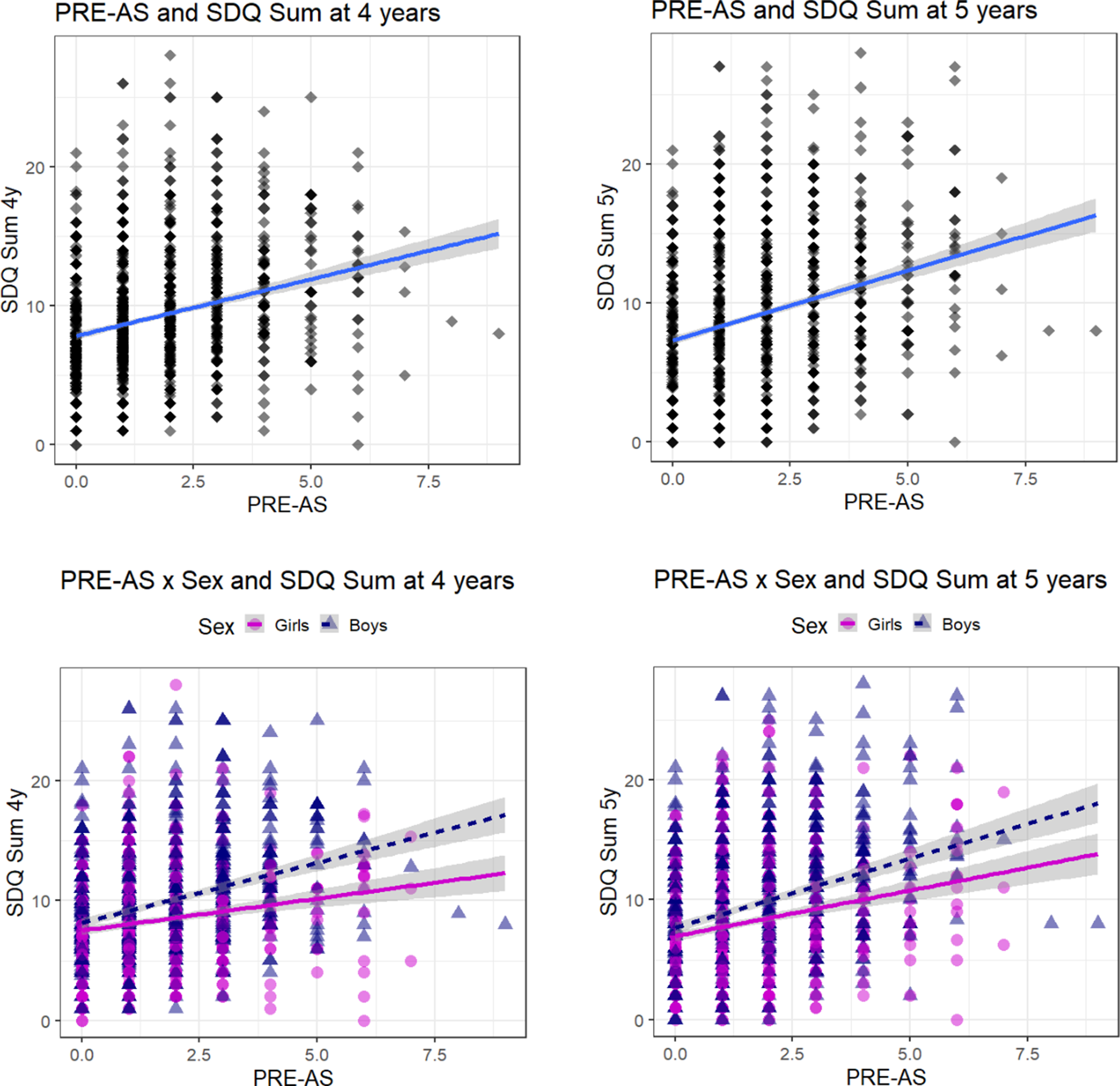
Figure 1. Association between PRE-AS and SDQ Sum. The association of cumulative prenatal adversity (PRE-AS) with the SDQ Sum scores of 4- and 5-year old children is shown, for the whole sample (top row; N = 1568; Table 2), and for boys (N = 858; post hoc analyses: SDQ Sum 4y: β ± SE: 1.00 ± 0.10, p < 0.001; SDQ Sum 5y: β ± SE: 1.15 ± 0.11, p < 0.001) and girls (N = 710; post hoc analyses: SDQ Sum 4y: β ± SE: 0.53 ± 0.10, p < 0.001, SDQ Sum 5y: β ± SE: 0.76 ± 0.11, p < 0.001) separately (bottom row).
We noted that mean values of SDQ F1 and F4 scores were rather low at both time points, and residuals were quite skewed. Homoscedasticity was not given for F1 at 4 years and F4 at 5 years. Hence, we performed additional control analyses, creating a factor for the SDQ subscales F1 and F4 with two levels, that is, containing the lowest 20% and the highest 20% of the distribution, for both 4- and 5-year-olds. With this dichotomous factor, we yielded the same results as for the continuous variable, except for the PRE-AS-by-sex interaction on SDQ F4 scores at 5 years which was no longer significant (p = .143).
Association of PRE-AS with child problem behavior controlling for POST-DS
Including POST-DS as control variable into the regression analyses for the main and interaction effects, all associations between PRE-AS and SDQ scores were weakened, but remained significant (with and without correction for multiple comparisons) except for the main effect of PRE-AS on emotional symptoms at 4 years (Table 2).
In exploratory multiple regression analyses, we analyzed how SDQ scores of children with prenatal adversity (PRE-AS > 0) differed from those with no prenatal adversity (PRE-AS = 0) given no postnatal adversity (POST-DS = 0).
In the group of children with prenatal adversity, but no postnatal adversity, we observed significantly higher hyperactivity/inattention (SDQ F3) and SDQ Sum scores at 4 and 5 years (4-year: F3: p = .001, Sum: p = .011; 5-year: F3: p = .001, Sum: p = .003) and higher conduct problems (SDQ F2) at 5 years (p = .044) compared to the group with neither pre- nor postnatal adversity (Figure SI-1). All other associations were insignificant (all p > .09).
We do not report results for the group with PRE-AS = 0 & POST-DS > 0, as the focus of this study lies on the effects of prenatal adversity.
Of note though, in the group with both pre- and postnatal adversity, PRE-AS scores were significantly higher compared to the group with only prenatal adversity (p < .001). Accordingly, highest SDQ scores were observed for children with both pre- and postnatal adversity exposure (PRE-AS > 0 & POST-DS > 0; all p < .001).
Association of PRE-AS with infant amygdalar and hippocampal volumes
We investigated the association of PRE-AS with offspring amygdalar and hippocampal volumes in 122 infants, controlling for infant sex, age at scan time, gestational weeks, and total brain volumes. We observed no main effects of PRE-AS, but a significant interaction of PRE-AS with sex on bilateral amygdalar volumes which survived the correction for multiple comparisons (Table 3, Figure 2). Amygdalar volumes were significantly more positively associated with PRE-AS in girls compared to boys. However, these associations were reduced to nonsignificance for left amygdalar volumes and partly for right amygdalar volumes in our control analyses, but stayed significant in the analyses with the nonimputed data (see SI, Table SI-3).
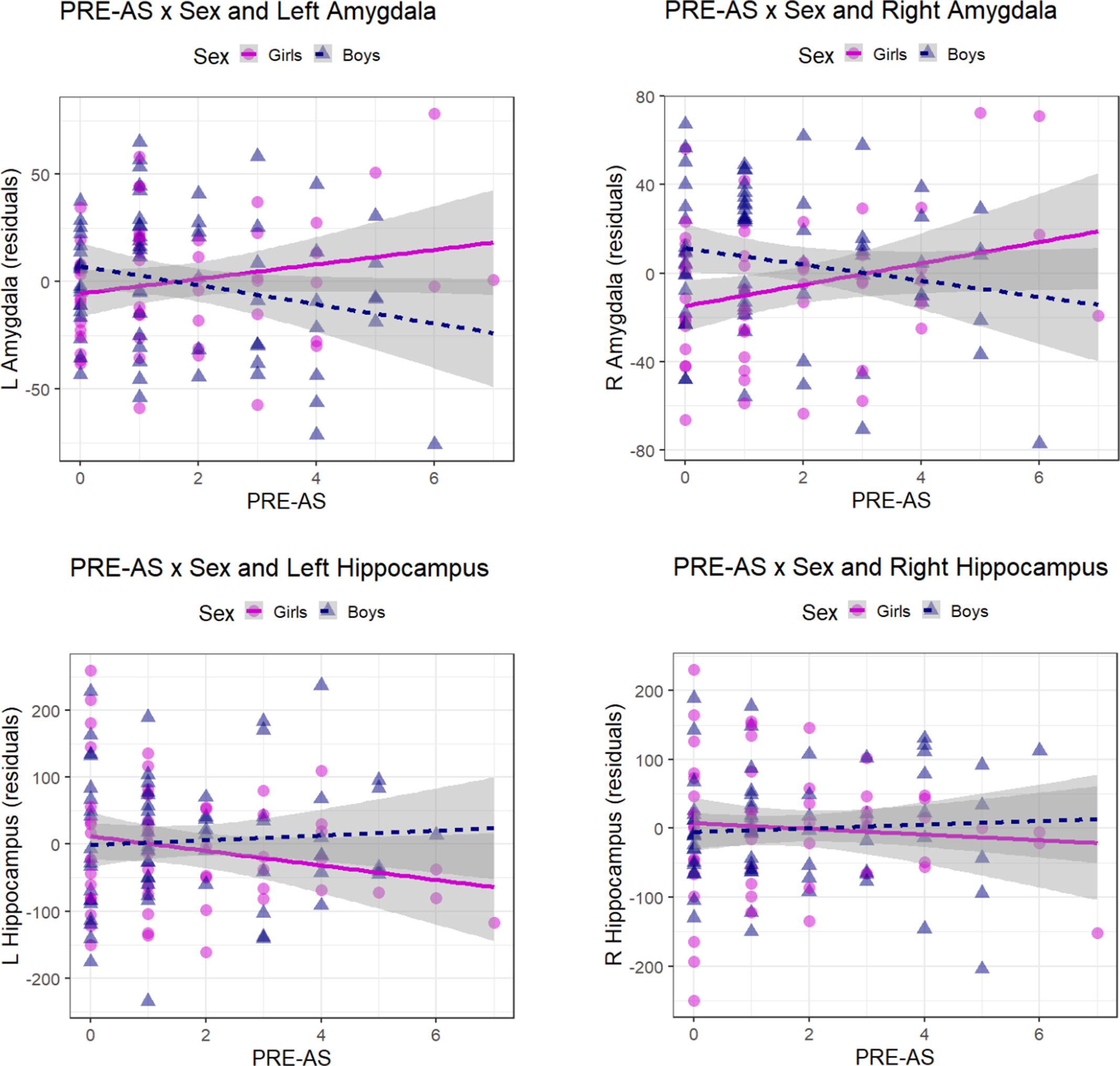
Figure 2. Sex-specific association between PRE-AS and amygdalar / hippocampal volumes. The sex-specific association between PRE-AS and bilateral amygdalar (top row) and hippocampal volumes (bottom row) is shown (residuals, controlling for infant age after birth at MRI scan time, gestational weeks at birth and total brain volume). The interaction effects were significant for bilateral amygdalar volumes and nonsignificant for bilateral hippocampal volumes (Table 3; post hoc analyses for girls: N = 53; left amygdala: β ± SE: 3.02 ± 2.20, p = 0.177; right amygdala: β ± SE: 4.48 ± 2.39, p = 0.068; left hippocampus: β ± SE: −12.12 ± 7.17, p = 0.097; right hippocampus: β ± SE: −4.87 ± 7.58, p = 0.523; for boys: N = 69; left amygdala: β ± SE: −3.96 ± 2.35, p = 0.097; right amygdala: β ± SE: −3.74 ± 2.41, p = 0.126; left hippocampus: β ± SE: 4.94 ± 7.07, p = 0.487; right hippocampus: β ± SE: 3.90 ± 5.98, p = 0.517).
Table 3. PRE-AS and amygdalar/hippocampal volumes
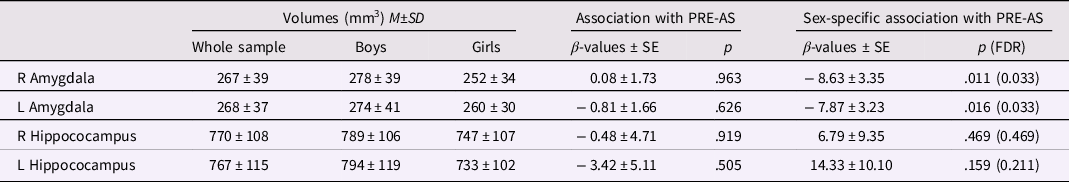
Note. On the right, the associations of infant amygdalar and hippocampal brain volumes with PRE-AS and the PRE-AS-by-sex interaction are listed. On the left, descriptives of the brain volumes for the whole sample (N = 122), and for boys (N = 69) and girls (N = 53) separately are shown.
Both neuroimaging and SDQ data were available in a subsample of 77 children. In this subsample, we tested whether amygdalar volumes mediate the effects of PRE-AS on SDQ scores, in girls and boys separately. Our data revealed a significant mediation effect (indirect effect “ab”) of right amygdalar volumes in girls (N = 38) on hyperactivity/inattention (SDQ F3) at 4 years of age (Figures 3 and SI-2, “ab” effect estimate through mediators: 0.19, SD = 0.11, CI 95%: 0.004, 0.420). However, according to Fritz & MacKinnon (Reference Fritz and MacKinnon2007) we would have needed a sample size of approx. 110 to achieve 0.8 power taking into account the observed path sizes (a z = 0.32, b z = 0.39).
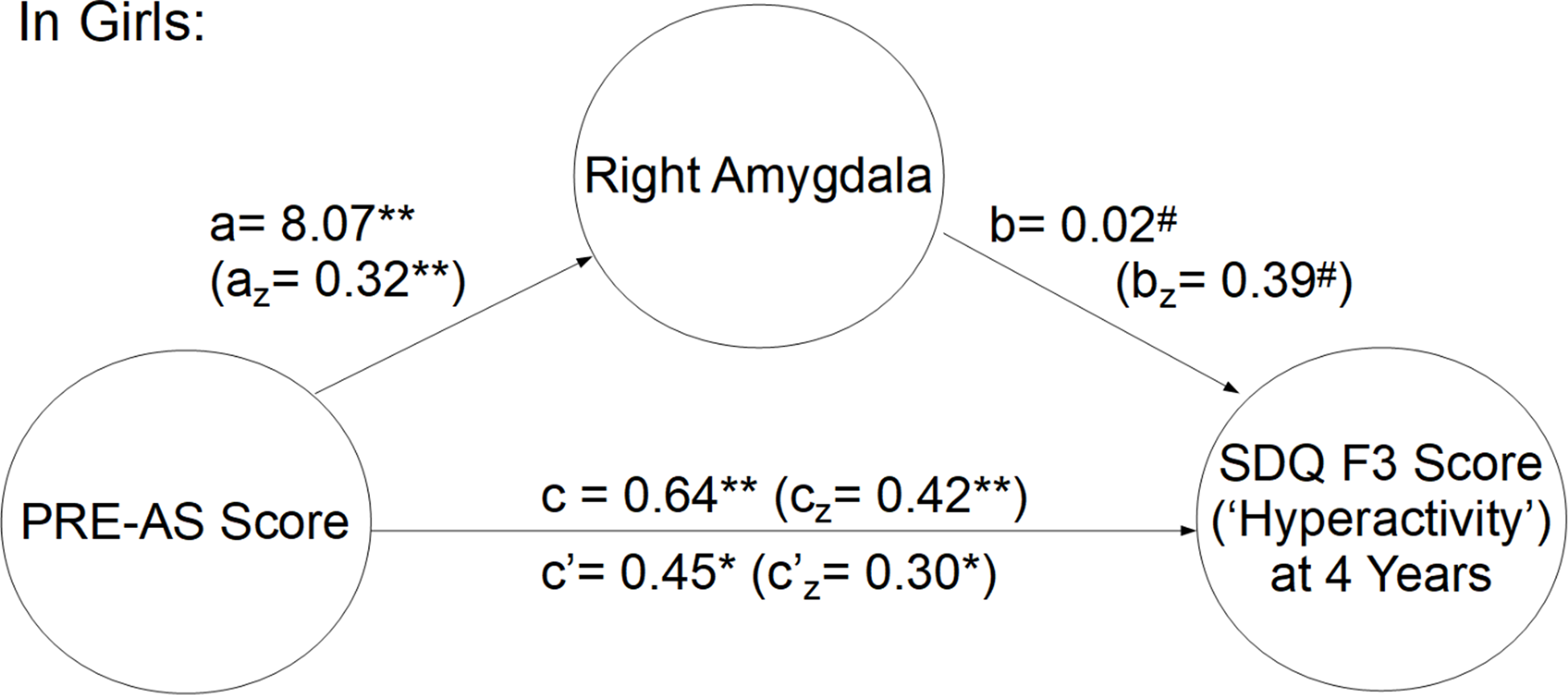
Figure 3. The mediation of right amygdalar volume of PRE-AS on hyperactivity/inattention (SDQ F3 scores) in 4-year-old girls (N = 38). The z-standardized values are reported in brackets. P-values: #= .05, *<.05, **<.01.
With regard to SDQ F3, no other mediation effects were observed, neither in 4-year old boys (“ab” effect estimate through mediators: −0.001, SD = 0.04, CI 95%: −0.070, 0.084), nor in 5-year-old girls (“ab” effect estimate through mediators: 0.22, SD = 0.12, CI 95%: −0.001, 0.460), and no other mediation effects of right of left amygdalar volumes on SDQ scores were found.
Associations of ePRS
Main and sex-specific interaction effects of ePRS on SDQ scores
Offspring ePRS was not significantly related to PRE-AS or POST-DS in either sample (all p > .1) and no sex (p ≥ .08) or sample differences (p = .108) were observed, but interestingly, significant associations were yielded for the risk factors “lack of money” (p = .043 uncorr.) and “prenatal EPDS” (p = .044 uncorr.) in the child behavior sample, showing higher offspring ePRS in the mother-offspring dyads with these prenatal risk factors compared to the mother–offspring dyads without these prenatal risk factors. Maternal ePRS was available for a small subsample (N = 247), and we probed whether maternal genotype might be related to pre- or postnatal risk factors. However, no significant associations were observed (all p > .13).
We investigated the main effects of offspring ePRS on SDQ scores in multiple regression analyses controlling for sex. Further, we probed sex-specific associations with ePRS. The results are presented in Table 4.
Table 4. ePRS, SDQ, and PRE-AS
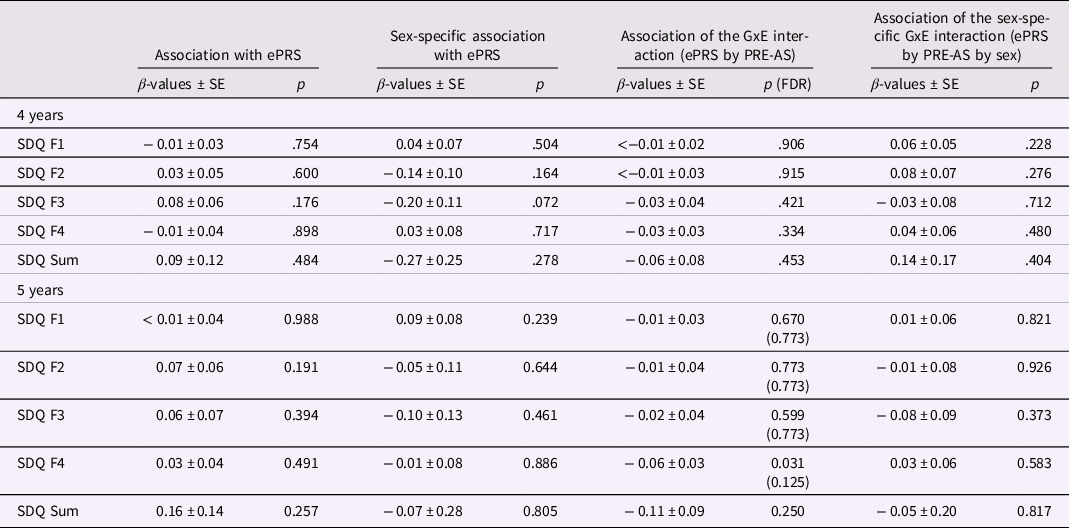
Note. The association of child SDQ scores with ePRS genotype and its interaction with sex and/or PRE-AS is shown (whole sample: N = 1161; boys: N = 627; girls = 534). Beta estimates, standard errors, and p-values are presented. For significant results, FDR-corrected p-values are additionally listed.
No significant results for the association of ePRS or the ePRS-by-sex interaction with SDQ scores were observed. Neither the control analyses in the subsamples, nor the additional control analyses with the dichotomous SDQ F1 and F4 variables (see 3.2 refers to the second chapter in the result section and SI) changed the results. Also, exploring the interaction between ePRS and the PRE–POST factor did not show significant results (all p > .075).
However, after controlling for POST-DS, a significant sex-specific association of ePRS with hyperactivity/inattention (SDQ F3) at 4 years was detected (p = .0496). This sex-specific association was supported by the analyses with nonimputed data (GxExSex: N = 817, β ± SE: −0.32 ± 0.15, p = .032). Post hoc analyses revealed a significant association in 4-year-old girls with and without controlling for POST-DS (controlling for POST-DS: β ± SE: 0.19 ± 0.07, p = .011), but not in boys (β ± SE: −0.02 ± 0.08, p = .795). Including both genotype and PRE-AS into the model for SDQ F3 of 4-year-old girls, both predictors stayed significant (ePRS: β ± SE: 0.19 ± 0.08, p = .012; PRE-AS: β ± SE: 0.26 ± 0.05, p < .001; controlling for POST-DS: ePRS: β ± SE: 0.19 ± 0.07, p = .010; PRE-AS: β ± SE: 0.14 ± 0.06, p = .019).
ePRS – by – environment interaction effects on SDQ
Next, we probed the interaction between ePRS and PRE-AS (GxE interaction) on SDQ scores. Results are presented in Table 4 on the right. A significant ePRS-by-PRE-AS interaction on peer relationship problems (SDQ F4 scores) at 5 years was found. This interaction effect stayed significant in all but one of the control analyses (see SI), but did not survive correction for multiple comparisons nor the analyses with nonimputed data (Table SI-4). Post hoc analyses, splitting the sample into two groups according to the ePRS (≤0 />0), indicated that the association between PRE-AS and SDQ F4 was more positive in children with low ePRS (β ± SE: 0.27 ± 0.04, p < .001) compared to those with high ePRS (β ± SE: 0.11 ± 0.04, p = .007).
In the control analyses with the dichotomous SDQ F1 factor, we additionally found a significant GxE interaction effect for SDQ F1 scores at 5 years (β ± SE: −0.16 ± 0.06, p = .011), with higher SDQ F1 scores in 5-year-old children with low compared to high ePRS.
ePRS – main and sex-specific interaction effects on amygdalar and hippocampal volumes
We observed no significant associations of ePRS with amygdalar or hippocampal volumes (Table 5). Further, no sex-specific associations were found. In the control analyses, no significant results were observed.
Table 5. ePRS, amygdalar/hippocampal volumes, and PRE-AS
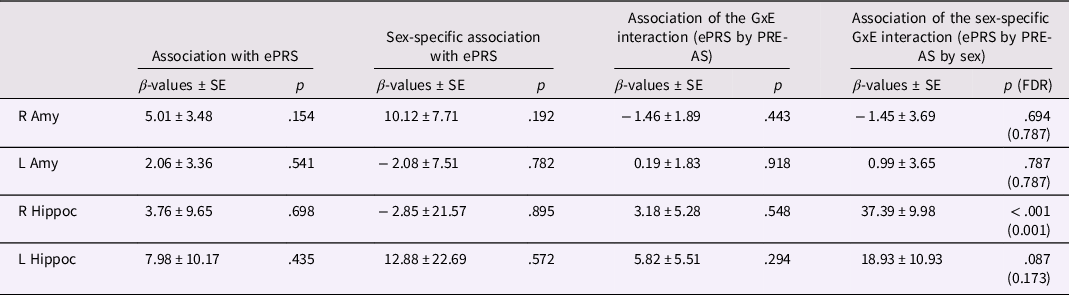
Note. The association of infant amygdalar and hippocampal volumes with ePRS genotype and its interaction with sex and/or PRE-AS (whole sample: N = 104; boys: N = 61; girls: N = 43) is shown. Beta estimates, standard errors, and p-values are presented. For significant results, FDR-corrected p-values are additionally listed.
ePRS – by – environment interaction effects on amygdalar and hippocampal volumes
Probing the ePRS-by-PRE-AS interaction (i.e., GxE interaction) on amygdalar and hippocampal volumes, no significant results were found. However, a significant sex-specific interaction effect was observed on right hippocampal volumes (Table 5). Post hoc analyses, performed separately in boys and girls, yielded a significant interaction effect in boys (β ± SE: 23.80 ± 6.65, p < .001), but not in girls (β ± SE: −13.75 ± 8.07, p = .097). In boys with low ePRS, PRE-AS was significantly negatively associated with hippocampal volumes (β ± SE: −20.43 ± 9.33, p = .036), while a significant positive association was found in boys with high ePRS (β ± SE: 22.78 ± 9.28, p = .025). No significant associations were observed in girls (p > .4). The GxExSex interaction effect stayed significant with correction for multiple comparisons and in all but one of the control analyses (see SI, Table SI-5). In subsequent mediation analyses, performed in boys and girls separately, right hippocampal volumes did not mediate the effects of the GxE interaction on SDQ F4 or F1 values at 5 years and were not significantly related to these SDQ scores (all p > .075). Given the small sample size for the three-way interaction analysis, both the GxExSex multiple regression analyses (N = 104) and the mediation analyses (N = 33) have to be considered as very exploratory and the respective results as tentative.
Discussion
With this study, the association of cumulative prenatal adversity (PRE-AS) with child problem behavior at 4 and 5 years, assessed with the SDQ, and with infant subcortical structure volumes (bilateral amygdalae and hippocampi) was probed in Finnish mother–offspring dyads. The PRE-AS score encompassed a broad range of prenatal adversities, that is, maternal distress (depression, anxiety, marital distress and worries about lack of money, violence exposure), maternal diseases, smoking, education, infant birth weight, and gestational age. We investigated if the associations of PRE-AS were moderated by a polygenic risk score (ePRS), which was based on the prenatal hippocampal-specific coexpression of the serotonin transporter solute carrier family C6, member 4 (SLC6A4), gene (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017).
Our data showed that higher PRE-AS was linked to more problematic child behavior in every assessed domain at both time points. Partly stronger positive associations between PRE-AS and problem behavior were observed in boys than girls. Sexually dimorphic associations of PRE-AS were also found for amygdalar volumes: PRE-AS was more positively associated with bilateral infant amygdalar volumes in girls compared to boys, while no associations of PRE-AS were yielded for hippocampal volumes. Hereby, our study is the first to demonstrate a dose-dependent sexually dimorphic relationship between cumulative prenatal adversity and infant amygdalar volumes.
Right infant amygdalar volumes partly mediated the effects of PRE-AS on hyperactivity of 4-year-old girls, but not boys. Interestingly, child hyperactivity/inattention and overall problem behavior (SDQ Sum score) at 4 and 5 years were positively associated with PRE-AS also in those mother–offspring dyads that reported low postnatal distress. These results suggest that the association of PRE-AS with child overall problem behavior and hyperactivity/inattention was at least partly independent of postnatal adversity, a claim that was further supported by the partial mediation effect of infant amygdalar volumes in our study. Furthermore, our data revealed that higher hyperactivity/inattention of 4-year-old girls was also linked to a higher ePRS, independent of PRE-AS. In sum, hyperactivity/inattention in 4-year old girls was related to genetic factors and prenatal adversity, the latter partly mediated by right amygdalar volumes. However, contrary to our expectations, we did not detect higher child problem behavior with increasing PRE-AS in children with high ePRS. By contrast, a more positive association of PRE-AS with 5-year-olds’ emotional and peer relationship problems emerged in children with low ePRS compared to those with high ePRS. A similar pattern was observed for subcortical volumes in male infants: Higher PRE-AS was related to smaller right hippocampal volumes in male infants with low ePRS, but to larger ones in male infants with high ePRS. No association between right hippocampal volumes and SDQ scores in boys was found.
With our data, we were able to replicate findings from other studies (Garg et al., Reference Garg, Chen, Nguyen, Pokhvisneva, Chen, Unternaehrer, MacIsaac, McEwen, Mah, Gaudreau, Levitan, Moss, Sokolowski, Kennedy, Steiner, Meaney, Holbrook, Silveira, Karnani and O’Donnell2018; Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) showing that higher cumulative prenatal adversity is related to greater affective and behavioral problems in 4- and 5-year-olds. Furthermore, we were able to demonstrate that the associations of PRE-AS with overall child problem behavior, affective problems and peer relationship problems are greater in boys compared to girls. This finding suggests a higher vulnerability of boys than girls, regarding the selected outcome phenotypes in the given child ages. To date, both a higher and a lower vulnerability to prenatal adversity have been observed in male compared to female offspring. These inconsistencies in the direction of sex-bias presumably depend on several factors such as the timing of prenatal stress and the investigated outcome (Bock et al., Reference Bock, Wainstock, Braun and Segal2015). Alternatively, boys’ behavioral problems might be experienced as more disruptive by mothers compared to those of girls, and thereby lead to a bias in maternal reports.
Interestingly, on a neurobiological level, a more complex picture emerged in our study. PRE-AS was associated with larger bilateral amygdalar volumes in girls compared to boys. This finding dovetails with studies on single prenatal risk factors in newborns and children. In female compared to male newborns, relatively larger bilateral amygdalar volumes have been related to maternal psychological distress (Lehtola et al., Reference Lehtola, Tuulari, Scheinin, Karlsson, Parkkola, Merisaari, Lewis, Fonov, Louis Collins, Evans, Saunavaara, Hashempour, Lähdesmäki, Acosta and Karlsson2020). In girls compared to boys, larger amygdalar volumes have been linked to maternal cortisol levels in the first trimester (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012), to maternal pregnancy-related anxiety in the second trimester (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019) and to maternal depressive symptoms in the second or third trimester (Acosta, Tuulari et al., Reference Acosta, Kantojärvi, Hashempour, Pelto, Scheinin, Lehtola, Lewis, Fonov, Collins, Evans, Parkkola, Lähdesmäki, Saunavaara, Karlsson, Merisaari, Paunio, Karlsson and Tuulari2020; Wen et al., Reference Wen, Poh, Ni, Chong, Chen, Kwek, Shek, Gluckman, Fortier, Meaney and Qiu2017). In the study of Buss et al. (Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012) right amygdalar volumes partially mediated the associations between maternal cortisol concentrations and 6-to-9-year old girls’ emotional problems. However, on the whole, neuroimaging studies have reported partly inconsistent associations of amygdalar volume alterations with child neurodevelopmental outcomes. Larger right amygdalar volumes have been linked to a lower impulse control in toddlers (Graham et al., Reference Graham, Rasmussen, Rudolph, Heim, Gilmore, Styner, Potkin, Entringer, Wadhwa, Fair and Buss2018), to a higher fearfulness in girls (van der Plas et al., Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010), but also to less peer relationship problems, the latter more pronounced in boys (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019). Larger left amygdalar volumes have predicted less emotional symptoms, more pronounced in boys (Acosta et al., Reference Acosta, Tuulari, Scheinin, Hashempour, Rajasilta, Lavonius, Pelto, Saunavaara, Parkkola, Lähdesmäki, Karlsson and Karlsson2019), a better cognitive mental state inference in 4-year-olds (Rice et al., Reference Rice, Viscomi, Riggins and Redcay2014), a higher probability to disengage from fearful facial expressions in 8-month-olds (Tuulari et al., Reference Tuulari, Kataja, Leppänen, Lewis, Nolvi, Häikiö, Lehtola, Hashempour, Saunavaara, Scheinin, Korja, Karlsson and Karlsson2020), but also to poorer working memory in 2.5-year-old girls (Nolvi et al., Reference Nolvi, Tuulari, Pelto, Bridgett, Eskola, Lehtola, Hashempour, Korja, Kataja, Saunavaara, Parkkola, Lähdesmäki, Scheinin, Fernandes, Karlsson, Lewis, Fonov, Collins and Karlsson2021) and more anxiety in 7–9-year old children (Qin et al., Reference Qin, Young, Duan, Chen, Supekar and Menon2014).
In our study, neonatal right amygdalar volumes partly mediated the effect of PRE-AS on hyperactivity/inattention in 4-year-old girls. Hyperactivity and/or inattention are characteristic symptoms of attention deficit hyperactivity disorder (ADHD). Interestingly, in females diagnosed with ADHD, internalizing disorders (e.g., anxiety, depression) are more prevalent while boys with ADHD manifest higher rates of externalizing disorders (Skogli et al., Reference Skogli, Teicher, Andersen, Hovik and Øie2013; Young et al., Reference Young, Adamo, Ásgeirsdóttir, Branney, Beckett, Colley, Cubbin, Deeley, Farrag, Gudjonsson, Hill, Hollingdale, Kilic, Lloyd, Mason, Paliokosta, Perecherla, Sedgwick, Skirrow and Woodhouse2020). Emotional lability and emotion dysregulation appear to be more common and/or more severe in females with ADHD than in their male counterparts (Young et al., Reference Young, Adamo, Ásgeirsdóttir, Branney, Beckett, Colley, Cubbin, Deeley, Farrag, Gudjonsson, Hill, Hollingdale, Kilic, Lloyd, Mason, Paliokosta, Perecherla, Sedgwick, Skirrow and Woodhouse2020). Alternatively, female anxiety could present as hyperactivity and restlessness, considering that phenotypes are not that clear at this young age. As mentioned above, right amygdalar volumes have been associated both with impulse control in toddlers (Graham et al., Reference Graham, Rasmussen, Rudolph, Heim, Gilmore, Styner, Potkin, Entringer, Wadhwa, Fair and Buss2018) and with internalizing problems in girls (Buss et al., Reference Buss, Davis, Shahbaba, Pruessner, Head and Sandman2012; van der Plas et al., Reference van der Plas, Boes, Wemmie, Tranel and Nopoulos2010). We propose that the comorbidity of hyperactivity/inattention symptoms and internalizing problems in girls, but not boys, might partly be conveyed by sex-specific right amygdalar volume alterations in association with cumulative prenatal adversity. However, given the small sample size of our mediation analysis, our results and their interpretation are tentative. Future studies with larger sample sizes are warranted to verify our findings.
Environmental factors account for 10% to 40% of the variance associated with ADHD (Sciberras et al., Reference Sciberras, Mulraney, Silva and Coghill2017). Nevertheless, ADHD has an estimated high heritability of 76% (Khan & Faraone, Reference Khan and Faraone2006), and a complex relationship of environmental factors with genetic liability for ADHD has been reported (Li et al., Reference Li, Franke, AriasVasquez and Mota2021). Several prenatal risk factors have been shown to be associated with ADHD, such as maternal smoking, substance abuse, exposure to chemical toxins, maternal distress, and birth complications. However, studies controlling for genetic and/or familial confounding factors reported smaller or no associations. Overall, findings have been rated as inconclusive in recent meta-analyses and no causal links between prenatal risk factors and ADHD have been established yet (Kian et al., Reference Kian, Samieefar and Rezaei2022; Manzari et al., Reference Manzari, Matvienko-Sikar, Baldoni, O’Keeffe and Khashan2019; Sciberras et al., Reference Sciberras, Mulraney, Silva and Coghill2017). However, an in vitro fertilization study has revealed a gene-by-environment interaction for the occurrence of offspring ADHD symptoms: Higher prenatal maternal stress has been correlated with more offspring ADHD symptoms only in biologically related, but not in biologically unrelated mother-offspring dyads (Rice et al., Reference Rice, Harold, Boivin, Van Den Bree, Hay and Thapar2010). Consequently, we assume that genetic factors might be implicated in the association between PRE-AS, amygdalar volumes and hyperactivity/inattention in girls in our study. However, we were not able to find a gene-by-environment interaction on hyperactivity/inattention. Instead, we observed a main genetic effect of ePRS on hyperactivity/inattention in girls. In sum, we put forward that our data add to the evidence that serotonergic pathways are involved in the pathogenesis of ADHD (Khan & Faraone, Reference Khan and Faraone2006; Sharp et al., Reference Sharp, McQuillin and Gurling2009), by showing an association between a hippocampal-specific ePRS, based on the SLC6A4 gene network, and hyperactivity/inattention in girls. However, our data also indicate that cumulative prenatal adversity does not interact with the ePRS, based on the hippocampal-specific SLC6A4 gene network, on child hyperactivity/inattention. This latter finding is consistent with the results as reported by Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) who did not observe a GxE interaction on child hyperactivity/inattention. Presumably, other genetic variations than those tested in our study might be involved in the relationship between prenatal adversity, amygdalar volumes and hyperactivity/inattention symptoms, such as genetic variations in dopaminergic pathways or in the synaptosomal-associated protein of 25 kDa gene (SNAP25) (Khan & Faraone, Reference Khan and Faraone2006; Sharp et al., Reference Sharp, McQuillin and Gurling2009).
By contrast, Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) have reported a GxE effect, involving the ePRS used in our study and cumulative prenatal adversity, on emotional and pervasive developmental problems in 4- and 5-year-olds. In their study, a higher ePRS conferred a higher sensitivity to cumulative prenatal adversity. Contrary to this finding, our data yielded that a lower ePRS conveyed a higher sensitivity for cumulative prenatal adversity to develop emotional and peer relationship problems. Hence, our data showed the opposite GxE interaction effect compared to those reported by Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). Similar to our results, a lower ePRS, based on the amygdala serotonin gene network (de Lima et al., Reference de Lima, Barth, Arcego, de Mendonça Filho, Clappison, Patel, Wang, Pokhvisneva, Sassi, Hall, Kobor, O’Donnell, Bittencourt, Meaney, Dalmaz and Silveira2020), which included mostly different genes than the hippocampal-specific one (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017), has been associated with higher child hyperactivity/inattention, as postnatal adversity increased.
Analyzing GxE effects on infant subcortical structure volumes, we only observed a sex-specific GxE effect on right hippocampal volumes. In rats, sex differences in gene expression in the hippocampus have been reported (Yagi & Galea, Reference Yagi and Galea2019), and prenatal stress has been shown to affect the serotonergic network in the animal hippocampus, likely independent of sex (Soti et al., Reference Soti, Ranjbar, Kohlmeier and Shabani2022). As our GxExSex analyses lacked power due to the small sample size, our results have to be considered as very tentative and future studies with larger samples are warranted for replication.
So far, large meta-analyses have not supported interactions of functional SLC6A4 polymorphisms with stressful life events on adult psychopathology (Culverhouse et al., Reference Culverhouse, Saccone, Horton, Ma, Anstey, Banaschewski, Burmeister, Cohen-Woods, Etain, Fisher, Goldman, Guillaume, Horwood, Juhasz, Lester, Mandelli, Middeldorp, Olié, Villafuerte and Bierut2018; Risch et al., Reference Risch, Herrell and Lehner2009). We suggest that our unsuccessful effort to find similar GxE effects in our study compared to those reported by Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) reinforces the doubts on the validity of GxE effects of the serotonergic network on mental health.
Finally, the effect of postnatal environmental risk factors presents a challenge for most longitudinal studies on prenatal stress in humans. Our study has shown that pre- and postnatal adversity are highly correlated. Furthermore, our data consistently showed that child problem behavior was lowest with low cumulative pre- and postnatal adversity, but highest given both pre- and postnatal adversity. To disentangle the effects of pre- and postnatal adversity, we did not only use a cumulative postnatal distress score as a control variable, but additionally, we split our sample in mother–offspring dyads with a cumulative postnatal distress score of POST-DS = 0 and POST-DS > 0. The results of our analyses provided some evidence that cumulative prenatal adversity, independent of postnatal adversity, contributes to higher overall child problem behavior at 4 and 5 years, most pronounced for child hyperactivity/inattention at 4 years. However, considering our study design, we cannot rule out that underlying genetic risk factors are associated with both PRE-AS and child problem behavior. Indeed, our genetic data, limited to the prenatal hippocampal-specific serotonin network, suggest a genetic association with both prenatal risk factors in the whole sample, that is, lack of money and maternal depressive symptoms, and with child outcome, that is, hyperactivity/inattention in 4-year-old girls.
While the underlying genetic and biological mechanisms of prenatal adversity on offspring outcome have to be further addressed in future studies, our study provides evidence that child emotional and behavioral problems, in particular ADHD-related symptoms, can partly be related to cumulative prenatal risk. ADHD-related symptoms, for instance, have a direct negative effect on social, academic, and occupational functioning and lead to a substantial burden for the individual and their family over the lifespan (e.g., Caci et al., Reference Caci, Asherson, Donfrancesco, Faraone, Hervas, Fitzgerald and Döpfner2015). Hence, our study highlights the clinical importance of paying attention to cumulative adversity, as early as in the prenatal period. A broad assessment of maternal adversity in the prenatal period, spanning from maternal emotional symptoms, physical disorders and health-related behaviors to economic factors and relational aspects, could inform prevention efforts and improve the early identification of families in need for interventions, in order to reduce the burden for offspring and their family.
Limitations
A strength of this study has been the large sample size of the behavioral data. However, the neuroimaging sample was rather small compared to the behavioral sample and with regard to the complex statistical analyses. This was due to the challenges of scanning neonates in an MRI setting. In addition, for the assessment of child emotional and behavioral problems, we used maternal reports which could to some extent be biased, for example by maternal stress at the assessment time point.
We used nonparametric missing value imputation to obtain unbiased and reliable parameter estimates. We also analyzed the complete cases (nonimputed data). Interestingly, the results gained with the imputed data were predominantly paralleled by those obtained with the nonimputed data. Thus, the missing data seem not to considerably bias our results.
In our study we aimed at following the MAVAN cohort study protocol (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017) as closely as possible, but we have to acknowledge some methodological differences in the creation of the PRE-AS score: Marital strain, pregnancy-related anxiety and maternal depression have been assessed with different questionnaires and we added general anxiety to the PRE-AS score given its relevance for prenatal stress. Our cutoff values for these questionnaires were informed by the literature and were not determined by use of the 85th percentile like in the original study: The 85th percentiles in our study were lower than most recommended clinical cutoff values. However, additional analyses with the 85th percentiles as cutoff values (data not shown) confirmed our results. Furthermore, we assessed mostly overlapping, but not entirely identical maternal diseases during pregnancy. Finally, we used the level of education as an indicator of the socioeconomic status rather than household total gross income. We also determined the outcome with a different instrument, the SDQ, that highly correlates with the MAVAN cohort study’s measure and shows a higher criterion validity for inattention/hyperactivity. For the ePRS calculation in our (southern) Finnish sample, we applied the SNP list that has been derived from the 22 selected genes by linkage disequilibrium (LD) clumping in a Canadian sample (Silveira et al., Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017). LD patterns can vary between populations depending on the genomic region (Teo et al., Reference Teo, Fry, Bhattacharya, Small, Kwiatkowski and Clark2009). However, on average, only small and insignificant differences in LD patterns have been observed among European populations (Nelis et al., Reference Nelis, Esko, Mägi, Zimprich, Toncheva, Karachanak, Piskáčková, Balaščák, Peltonen, Jakkula, Rehnström, Lathrop, Heath, Galan, Schreiber, Meitinger, Pfeufer, Wichmann, Melegh and Metspalu2009). The Canadian population is not exclusively, but predominantly of European ancestry (Boyd & Norris, Reference Boyd and Norris2001). Nevertheless, we cannot rule out that differences in LD between the samples affect the power of the ePRS in our study. While we successfully replicated the association of PRE-AS with child emotional and behavioral problems, we did not find similar gene-by-environment interactions compared to Silveira et al. (Reference Silveira, Pokhvisneva, Parent, Cai, Rema, Broekman, Rifkin-Graboi, Pluess, O’Donnell and Meaney2017), and we cannot preclude that the genetic associations are less robust to methodological variations.
While we included a novel coexpression polygenic risk score, based on SLC6A4, in our analyses, our study design did not allow to take into account further genetic factors. A more genetically informed design, such as an in vitro fertilization study, would be warranted as a next step to elucidate the complex relationship between genetic liability, cumulative prenatal adversity, child brain development and child neurodevelopmental outcome. Further, a longer follow-up interval would shed light on the long-term outcomes of cumulative prenatal adversity on offspring brain and behavioral development.
Conclusions
Altogether, our data extend findings of single risk factor studies by showing that cumulative prenatal adversity displays a dose-dependent relationship not only with child emotional and behavioral problems, but also with infant amygdalar volumes, the latter in a sex-specific way. Amygdalar volumes partially mediated the effects of PRE-AS on hyperactivity/inattention symptoms of 4-year-old girls. While our data provided some evidence that postnatal adversity, assessed as cumulative risk score, does not account for the observed associations of child problem behavior with cumulative prenatal adversity, we cannot rule out underlying shared genetic factors. In fact, we observed that genetic variations in the serotonergic network are linked to both prenatal risk factors as well as to hyperactivity/inattention symptoms in girls. Altogether, our study supports the clinical relevance of a broad cumulative assessment of prenatal maternal risk factors in order to enhance prevention efforts and the early identification of families in need for interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423000275.
Acknowledgment
We thank the participating families.
Funding statement
This work was supported by the Jane and Aatos Erkko Foundation (to HA and HK), the Academy of Finland (grant numbers 264363, 253270, 134950 to HK; grant numbers 1350941 and 253346 to TP; grant number 308176 and 325292/Profi 5 to LK, grant number 26080983 to HM), the Hospital District of Southwest Finland State Research Grants (grant number P3006 to JT, grant number P3003 to NMS, grant number P3498 to HK, grant number P3654 to LK, K3562 to RP), the Signe and Ane Gyllenberg Foundation (to HK, NMS, SN and LK), the Yrjö Jahnsson Foundation (grant number 6847 to LK), the Alfred Kordelin Foundation (to JT), the Turku University Foundation (to JT), the Emil Aaltonen Foundation (to JT), the Maire Taponen Foundation (to SL), the Juho Vainio Foundation (to SL), the Sigrid Jusélius Foundation (to JT), the NARSAD Brain and Behavior Research Foundation (grant number 1956 to LK), the Foundation for Pediatric Research (to RP), the Azrieli Neurodevelopmental Research Program (grant number ANRP-MIRI13-3388 to JL and AE), the Brain Canada Multi-Investigator Research Initiative (to AE), the Canadian Institutes of Health Research (to VF and LC) and the Natural Sciences and Engineering Research Council of Canada (to LC). The research also benefited from computational resources provided by Compute Canada (www.computecanada.ca) and Calcul Quebec (www.calculquebec.ca).
Conflicts of interest
None.











