Increased consumption of wholegrain foods has been associated with reduced risk of several chronic diseases such as CVD, type 2 diabetes and certain cancers(Reference Schatzkin, Park and Leitzmann1–Reference Larsson, Giovannucci and Bergkvist7). Beneficial health effects are suggested to originate from several constituents of whole grains, including fibre, fermentable carbohydrates, antioxidants, minerals and phytochemicals(Reference Slavin8). Among these phytochemicals, alkylresorcinols are almost exclusively found in rye and wheat, being located in the intermediate layer, between the testa and pericarp(Reference Landberg, Kamal-Eldin and Salmenkallio-Marttila9) and therefore they have been suggested to serve as objective measures for the intake (biomarkers) of these two cereals(Reference Ross, Shepherd and Schupphaus10). Biomarkers enable the evaluation of intake of certain foods even if there are problems concerning the identification and documentation of these food items(Reference Westerterp and Goris11–Reference Potischman and Freudenheim14); alkylresorcinols or their metabolites may fill this role for wholegrain wheat and rye. According to Ross et al. (Reference Ross, Becker and Chen15) Finns have the highest alkylresorcinol intake among several populations studied and we have previously shown that in Finnish women, the urinary alkylresorcinol metabolites reflect cereal fibre (wheat + rye) intake(Reference Aubertin-Leheudre, Koskela and Marjamaa16). Alkylresorcinols (1,3-dihydroxy-5-alkylbenzene homologues) are members of a large group of phenolic lipids, which, in rye and wheat, occur mainly bound to saturated odd-numbered hydrocarbon side-chains(Reference Ross, Kamal-Eldin and Åman17, Reference Kozubek and Tyman18). In addition to having potential as biomarkers, they eventually possess a large variety of bioactivities in vitro, such as antimicrobial, antioxidative and antimutagenic activities, as well as interaction with some proteins(Reference Ross, Kamal-Eldin and Åman17, Reference Kozubek and Tyman18). Alkylresorcinols are reported to be stable during food processing and baking(Reference Ross, Shepherd and Schupphaus10), and after extremely high intake, minute amounts of intact, albeit conjugated, alkylresorcinols have been identified in urine(Reference Ross, Åman and Kamal-Eldin19). However, typically after ingestion, these lipophilic compounds are converted to water-soluble metabolites in excretable form, similar to tocopherols (vitamin E)(Reference Ross, Kamal-Eldin and Åman17, Reference Ross, Åman and Kamal-Eldin19, Reference Soderholm, Koskela and Lundin20). The two urinary alkyresorcinol metabolites 3-(3,5-dihydroxyphenyl)-1-propanoic acid (DHPPA) and 3,5-dihydroxybenzoic acid (DHBA) were first described by Ross et al. (Reference Ross, Åman and Kamal-Eldin19); these too have been proposed to serve as biomarkers for wholegrain rye and wheat intake(Reference Landberg, Aman and Friberg21, Reference Guyman, Adlercreutz and Koskela22) like their intact precursors, plasma alkylresorcinols(Reference Ross, Kamal-Eldin and Lundin23, Reference Linko, Juntunen and Mykkanen24), and the more recently determined plasma metabolites DHPPA and DHBA(Reference Soderholm, Koskela and Lundin20, Reference Koskela, Samaletdin and Aubertin-Leheudre25). In order to evaluate the suitability of these compounds to serve as biomarkers, pharmacokinetic data are helpful. The kinetics of plasma alkylresorcinols(Reference Landberg, Linko and Kamal-Eldin26) and plasma DHBA and DHPPA(Reference Soderholm, Koskela and Lundin20) have been described, but to our knowledge the kinetics of urinary DHBA and DHPPA have not been determined previously. In our present study we aimed to determine the pharmacokinetics of urinary DHBA and DHPPA in healthy subjects after a single dose of rye bread.
Subjects and methods
Chemicals and materials
Acetonitrile and methanol were obtained from Rathburn Chemicals Ltd (Walkenburg, Scotland, UK). Ortho-phosphoric acid was purchased from Riedel-de Haën (Seelze, Germany). Acetic acid, potassium dihydrogenphosphate and sodium acetate were purchased from Merck GmbH (Darmstadt, Germany). β-Glucuronidase was obtained from Roche Diagnostics GmbH (Mannheim, Germany), and sulfatase and syringic acid from Sigma-Aldrich Co. (St Louis, MO, USA). DHBA was obtained from Aldrich (Steinheim, Germany) and DHPPA from IsoSep AB (Tollinge, Sweden). The sourdough-processed, yeast-leavened rye bread, consisting of (by dry weight) wholegrain rye (68·3 %), added rye bran (8·3 %), refined wheat flour (20 %), barley and rye malt extract, mineral salt, magnesium sulfate, potassium chloride and yeast (altogether 3·3 %), was specially baked for our studies by Fazer Bakeries (Lahti, Finland). For nutrient content, see Table 1.
Table 1 Nutrient content of the rye bread* per 100 g fresh weight and per test dose
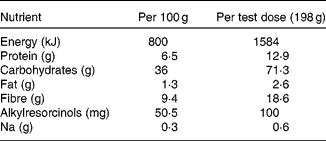
* Fazer Bakeries, Lahti, Finland.
Subjects and study design
The basic characteristics of the study participants are shown in Table 2. A total of fifteen healthy volunteers participated in the study after completing an eligibility questionnaire and attending a screening blood test.
Table 2 Basic characteristics of the participants
(Mean values and standard deviations)

The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee at the Helsinki University Central Hospital, Helsinki, Finland. Written informed consent was obtained from all subjects.
The participants were asked to avoid wholegrain rye and wheat products for 2 d before the study to minimise alkylresorcinol intake. After overnight fasting, the baseline urine sample was collected. Thereafter the participants ingested a single dose (198 g) of rye bread (Table 1) containing 100 mg (258 μmol) alkylresorcinols together with 21 g butter. Fat was considered an important supplement to ensure the absorption of the highly lipophilic alkylresorcinols. However, vegetable margarine was not served for the concern of long-chain TAG potentially retarding the intestinal absorption of alkylresorcinols similarly to that of vitamin E(Reference Borel27). The participants were allowed 25 min for the consumption of the rye bread and butter. Standardised meals and drinks not containing wholegrain cereals were served throughout the study (ham and potato casserole, chicken soup, refined wheat bread, cucumber, lettuce, yogurt, coffee, tea, milk, water), and the participants were advised to avoid consuming any other foods.
After the ingestion of rye bread and butter, all urine was collected for the following 25 h at twelve time points 3, 4, 5, 6, 7, 8, 10, 12, 14 and 16 h. During the night-time (between 16 and 24 h) participants collected the urine in a container. The collection was completed on the following morning at 25 h.
Analytical methods
Rye bread was analysed for alkylresorcinols using the method of Ross et al. (Reference Ross, Shepherd and Schupphaus10). The volume of the urine sample was measured and 10 ml of each sample was stored at − 20°C until the analysis of DHBA and DHPPA by the method of Koskela et al. (Reference Koskela, Linko-Parvinen and Hiisivuori28). In detail, the procedure was as follows: a 25 μl urine sample was hydrolysed overnight at 37°C with an equal volume (25 μl) of hydrolysis solution (0·1 mm-sodium acetate buffer (pH 5), β-glucuronidase (0·2 U/ml) and sulfatase (2 U/ml)). After incubation, 318·5 ng of the internal standard syringic acid was added in 50 μl methanol and 650 μl HPLC mobile phase (20 % phase B–80 % phase A; for compositions, see below) and 10 μl was analysed with HPLC (ESA Biosciences, Inc., Chelmsford, MA, USA) equipped with a model 540 autosampler, two model 580 solvent pumps, and a model 5600 coulometric electrode array detector (CEAD) with eight electrode pairs. The analytes were separated using mobile phases consisting of 50 mm-phosphate buffer (pH 2·3)–methanol (90:10, v/v) (phase A) and 50 mm-phosphate buffer (pH 2·3)–methanol–acetonitrile (40:40:20, by vol.) (phase B), with a 25-min linear gradient from 0 to 100 % phase B, thereafter 12 min 100 % phase B, and the column was re-equilibrated with 0 % B for 15 min, total flow was 0·3 ml/min. The analytical column was an Inertsil ODS-3 (GL Sciences Inc., Tokyo, Japan), 3 × 150 mm with particle size 3 μm, connected to a Quick Release RP-18 (Upchurch Scientific Inc., Oak Harbor, WA, USA) 3 × 10 mm guard column. DHBA was quantified at 670 mV, DHPPA at 570 mV and syringic acid at 380 mV.
Pharmacokinetic analysis
The highest excretion rate of DHBA and DHPPA between baseline (0 h) and 25 h after the rye bread intake is defined as the maximum excretion rate (ERmax; μmol/h), with tmax being the time at which ERmax is reached. The half-life (t1/2) is defined as the time at which the urinary excretion rate has decreased to half of ERmax. The area under the curve (AUC) as well as other pharmacokinetic parameters were calculated using STATA software (version 10.0; StataCorp, College Station, TX, USA).
Statistical analysis
Data on DHPPA and DHBA excretion rates are presented as means and standard deviations. The pharmacokinetic parameters ERmax, tmax, t1/2 and AUC for DHPPA and DHBA were statistically analysed using unpaired t tests (STATA 10.0; StataCorp LP, College Station, TX, USA) to clarify whether there are significant differences between these two metabolites. The groups of females and males were compared using unpaired t tests to determine potential differences in the pharmacokinetic parameters between the sexes. P < 0·05 was considered significant.
Results
All the participants consumed the test dose within the given 25 min; thus all had an intake of 100 mg rye alkylresorcinols, of which the relative homologue composition was C15 : 0 (1 %), C17 : 0 (27 %), C19 : 0 (28 %), C21 : 0 (22 %), C23 : 0 (12 %) and C25 : 0 (10 %).
The mean urine DHPPA and DHBA excretion rates at each time point for the whole group (n 15) are presented in Fig. 1, and the pharmacokinetic data in Table 3. Maximum concentration (μmol/ml urine) values are not used in the present study because of vast variation in urine volumes, both between individuals and also collection time points. Instead, the excretion rates of DHBA and DHPPA for each 1 h are calculated from the samples by first correcting for the volume of the sample and then dividing by the amount of time (in h) that had passed since previous collection time point. Therefore the use of creatinine as a corrector for urinary volume was not needed. The mean baseline (0 h) urinary excretion rates for DHPPA and DHBA were low; 0·737 (sd 0·46) and 0·386 (sd 0·36) μmol/h, respectively, indicating that participants had avoided alkylresorcinol-containing foods before the study. At 3 h after consuming the bread dose, urinary excretion rates rose rapidly and every participant reached ERmax fairly simultaneously for both metabolites (data not shown); thirteen of the fifteen participants reached ERmax for DHPPA and DHBA between 5 and 6 h, while one reached ERmax at 3 h and another at 10 h. DHPPA was the major metabolite in all participants, reaching higher ERmax and AUC than DHBA (P < 0·0001 for both). Excretion rates declined gradually after ERmax and the mean t1/2 for DHPPA and DHBA was reached at 11·9 and 9·9 h, respectively (P = 0·142) (n 12). In three participants there were multiple peaks and hence the t1/2 could not be determined for them (data not shown). The final (25 h) urinary excretion rates for DHPPA and DHBA (1·68 (sd 0·96) and 1·01 (sd 0·57) μmol/h, respectively) were significantly different from baseline (P = 0·001 for both). The mean total urinary recovery of the two metabolites at 25 h was 43·4 % calculated from the ingested alkylresorcinols. There were no significant differences between females and males in any of the pharmacokinetic parameters (P>0·168 for all; Table 3).

Fig. 1 Urinary pharmacokinetics of alkylresorcinol metabolites between baseline (0 h) and 25 h after consumption of a single dose of rye bread containing 258 μmol alkylresorcinols (n 15). Values are means, with standard errors of the mean represented by vertical bars. The time point of 24 h represents the overnight urine collection between 16 and 24 h; other time points represent spot urine samples. (–○–), 3-(3,5-Dihydroxyphenyl)-1-propanoic acid; (–●–), 3,5-dihydroxybenzoic acid.
Table 3 Pharmacokinetic parameters of alkylresorcinol metabolites in human urine collected between baseline (0 h) and 25 h after administration of a single dose of rye bread containing 258 μmol alkylresorcinols
(Mean values and standard deviations)

ERmax, maximum excretion rate; tmax, time to reach maximum excretion rate; AUC, area under the curve; t1/2, half-life; DHPPA, 3-(3,5-dihydroxyphenyl)-1-propanoic acid; DHBA, 3,5-dihydroxybenzoic acid.
* For t1/2: all subjects, n 12; females, n 6; males, n 6.
Discussion
The present study reports the pharmacokinetics of urinary DHPPA and DHBA, which to our knowledge have not been determined before. The present study strengthens the suggestions that these compounds are useful biomarkers for the intake of wholegrain wheat and rye(Reference Landberg, Aman and Friberg21, Reference Guyman, Adlercreutz and Koskela22). Wholegrain cereals are of increasing interest for their health-promoting effects and the present data might be helpful when analysing existing samples from previous trials or databanks without dietary documentation. The wash-out period needed before the present study as well as the time points for sampling were determined after conducting a pilot study. According to that data we were expecting a very low rate of alkylresorcinol metabolites after 25 h and therefore a 25 h collection period was regarded sufficient, and a 2 d wash-out period before the study was considered appropriate. In the present study we observed very low baseline values, indicating that this wash-out period was successful. After consumption of rye bread the excretion rate for DHPPA and DHBA increased rapidly, reaching maximum values between 5 and 6 h. Conversely, the decrease in the excretion rate was quite slow for both metabolites. The lowest excretion rates were detected during the overnight collection (16–24 h), indicating a slight slowdown of alkylresorcinol metabolism during the sleeping hours. At 25 h the final excretion rates for both metabolites were still more than double compared with baseline, but, on the other hand, the rates during the last 1 h accounted only for about 2·5 % of the AUC0–25 h. Despite this, the total recovery of the two alkylresorcinol metabolites in urine at 25 h accounted only for 43 % of the ingested alkylresorcinols, being similar to values reported by Landberg et al. (Reference Landberg, Aman and Friberg21). The low urinary recovery is in concordance with recent findings. Firstly, Ross et al. (Reference Ross, Kamal-Eldin and Lundin23) described the absorption of alkylresorcinols to be about 60 % and in another study on pigs(Reference Ross, Shepherd and Bach Knudsen29) they suggested a decrease in absorption with higher levels of intake. Furthermore, Landberg et al. (Reference Landberg, Aman and Friberg21) reported the urinary recovery of alkylresorcinols to decrease with increasing dose from 90 % to approximately 45 %. Thus, one could speculate that a lower dose of alkylresorcinols with a higher rate of absorption as well as urinary recovery would probably give quite similar pharmacokinetic curves as observed in the present study. Second, it is possible that from the absorbed amount of alkylresorcinols, a proportion may still be stored or delayed in the body. This is supported by our recently reported plasma pharmacokinetic data of DHPPA and DHBA which indicated that at the time point of 25 h after consumption of the same rye bread, significant amounts of the alkylresorcinol metabolites remained in the circulation(Reference Soderholm, Koskela and Lundin20). Besides alkylresorcinols, also alkenylresorcinols with unsaturated hydrocarbon side-chains are known to exist in wholegrain wheat and rye. For the rye bread used in the present study, alkenylresorcinols accounted for about 20 % of the total amount of alkylresorcinols (data not shown). The metabolism of alkenylresorcinols has not been studied yet but if they too are precursors of DHBA and DHPPA, the urinary recovery of these metabolites as calculated above is overestimated.
Nevertheless, the present pharmacokinetic data suggest that even a single spot urine sample, collected after overnight fasting, potentially can act as a qualitative or semi-quantitative biomarker for the intake of wholegrain wheat or rye during the previous day and indeed distinguish between the consumers and non-consumers of wholegrain wheat and rye. However, to clarify this, a larger set of subjects would be needed for the comparison of the 24 h urine collections with spot urine samples.
The urinary pharmacokinetics of alkylresorcinol metabolites appear to closely reflect those reported for plasma(Reference Soderholm, Koskela and Lundin20). However, in contrast to the findings in plasma, urinary DHPPA achieved higher concentrations than DHBA, possibly explaining why the opposite(Reference Soderholm, Koskela and Lundin20) was observed in plasma. The formation of DHPPA from alkylresorcinols requires fewer metabolic steps – at least one less – compared with DHBA and it may therefore be excreted more rapidly.
The relatively small number of study participants and the large variation between individuals, which might be explained by differences in intestinal microbiota and absorption as well as the extent of metabolism in the liver and enterohepatic circulation, can be considered as limitations of the present study. Further studies are needed to understand the metabolism and excretion of alkylresorcinols in human subjects.
Taken together, our data suggest that urinary DHPPA and DHBA can be utilised as biomarkers for wholegrain wheat and rye intake, which is particularly helpful in the absence of dietary data. In addition, even a spot urine sample may be used to find out whether or not wholegrain wheat or rye is a daily dietary component.
Acknowledgements
We thank the participants for making the study possible, Päivi Ihamuotila, Paula Kokko, Merja Lahtinen and Päivi Ruha for skilful sample treatment and Adile Samaletdin for analysing the rye bread used in the study. The present study was supported by the Sigrid Jusélius Foundation, Helsinki, Finland, Samfundet Folkhälsan, Helsinki, Finland and Fazer Bakeries, Vantaa and Lahti, Finland who also provided the test bread used in the study. The authors' contributions were as follows: P. P. S., A. H. K., M. J. T. and H. C. A. designed the study; P. P. S. conducted most of the experimental work; A. H. K. carried out the laboratory analyses; J. E. L. conducted statistical and pharmacokinetic analyses; M. J. T. and H. C. A. supervised the work; P. P. S. wrote the paper; P. P. S., M. J. T. and H. C. A. had primary responsibility for the final content. All of the authors read and approved the manuscript.
M. J. T. received a grant from Fazer Bakeries (Finland) in support of the dietary study. The other authors declare no conflicts of interest.






