Introduction
From the onset to the peak of the Roundup Ready® (glyphosate-resistant crops) era, the use of soil-residual herbicides decreased, because postemergence-topical applications of glyphosate effectively controlled most weed species (Faircloth et al. Reference Faircloth, Patterson, Monks and Goodman2001; Young Reference Young2006). The increased use of glyphosate without other modes of action and tillage provided the selection pressure for glyphosate-resistant weeds. Glyphosate-resistant weeds began appearing in 2001 in Tennessee when horseweed [Conyza canadensis (L.) Cronq.] was confirmed as the first glyphosate-resistant weed in cotton (Gossypium hirsutum L.) (Heap Reference Heap2021; Steckel and Gwathmey Reference Steckel and Gwathmey2009). Soil-active herbicides are important tools for cotton growers because of their broad-spectrum efficacy and ability to control herbicide-resistant Palmer amaranth plants before they become troublesome (Price et al. Reference Price, Koger, Wilcut, Miller and van Santen2008).
There are 502 unique cases of herbicide-resistant weeds in the United States (Heap Reference Heap2021). The most recent herbicide class of chemistry developed were the p-hydroxyphenylpyruvate dioxygenase inhibitors, also known as HPPD inhibitors, which were first patented in the United States in the 1980s (Michaely and Kratz Reference Michaely and Kratz1988). HPPD inhibitors are part of a larger group of carotenoid biosynthesis inhibitors. Herbicides in this group deplete plastoquinone, an essential electron acceptor in the carotenoid-biosynthetic pathway (Norris et al. Reference Norris, Barrette and DellaPenna1995; Pallett et al. Reference Pallett, Little, Sheekey and Veerasekaran1998). Carotenoids are essential for plant life, as they protect chlorophyll molecules from photooxidation. This generates an abundance of singlet oxygen in the absence of carotenoids (Beaudegnies et al. Reference Beaudegnies, Edmunds, Fraser, Hall, Hawkes, Mitchell, Schaetzer, Wendeborn and Wibley2009). Once the carotenoid biosynthesis pathway is blocked and the formation of new carotenoids stopped, all new plant growth exhibits symptomology that resembles “bleaching” or albino colored meristematic tissue (Lee et al. Reference Lee, Prisbylla, Cromartie, Dagarin, Howard, Provan, Ellis, Fraser and Mutter1997).
Isoxaflutole received Federal 3 label status in the United States in 1998 and has been an effective herbicide at controlling a number of annual grass and broadleaf weed species in field corn (Zea mays L.) (Environmental Protection Agency 1998; Grichar et al. Reference Grichar, Besler and Palrang2005; Stephenson and Bond Reference Stephenson and Bond2012). When used as part of a preemergence herbicide program in soybean, isoxaflutole provided up to 95% Palmer amaranth control for 3 wk in bare-ground experiments (Meyer et al. Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2016). Johnson et al. (Reference Johnson, Chahal and Regehr2012) also observed a similar response in corn, with 87% to 99% Palmer amaranth control for 8 wk. Mixtures of other residual herbicides with isoxaflutole have the potential to broaden the spectrum of weeds controlled based on experiments with other HPPD inhibitors (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Woodyard et al. Reference Woodyard, Bollero and Riechers2009), extend the length of residual weed control, and lessen the potential for resistance development––especially when considering that two Amaranthus species have been confirmed to mount resistance to HPPD inhibitors (Diggle et al. Reference Diggle, Neve and Smith2003; Duke Reference Duke2011; Heap Reference Heap2021; Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001).
Adding a new mode of action to a weed management program such as isoxaflutole, a Group 27 herbicide (Herbicide Resistance Action Committee 2020), will help delay the development of herbicide-resistant weeds against the new auxinic-resistant systems recently developed in cotton (Gould Reference Gould1995; Orson Reference Orson1999; Peever and Milgroom Reference Peever and Milgroom1995). Although current cotton varieties do not tolerate HPPD inhibitors, BASF Corp. has developed HPPD-resistant cotton that will allow growers to use isoxaflutole in future weed management programs. Growers are currently able to use isoxaflutole in both corn and HPPD-resistant soybean weed management programs. According to the WSSA’s 2019 survey of most common and troublesome weeds in broadleaf crops, Palmer amaranth and morningglory (Ipomoea spp.) ranked number 1 and 2, respectively, in cotton production (Van Wychen Reference Van Wychen2019). Other weeds on those lists include horseweed, crabgrass (Digitaria spp.), and barnyardgrass [Echinochloa crus-galli (L.) Beauv]. Isoxaflutole has been studied alone and in mixture with many corn and soybean herbicides (Grichar et al. Reference Grichar, Besler and Palrang2005; Steckel et al. Reference Steckel, Simmons and Sprague2003; Stephenson and Bond Reference Stephenson and Bond2012; Wicks et al. Reference Wicks, Knezevic, Bernards, Wilson, Klein and Martin2007), but not with cotton herbicides. Therefore, the objective of this study was to determine the most effective tank-mix partners with isoxaflutole to enhance and extend soil-residual control of the most common and troublesome weeds across the Cotton Belt.
Materials and Methods
Field Studies
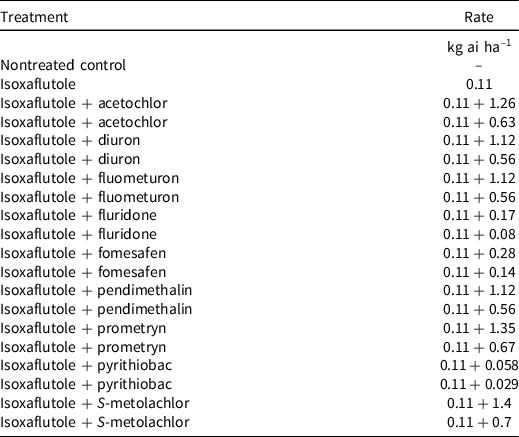
Non-crop field experiments were conducted at 10 locations in 2019 and 9 locations in 2020 across the Cotton Belt; site information (pH, organic matter, etc.) is provided in Table 1. Applications were made to bare-ground plots with a CO2-pressurized backpack sprayer, and all locations received at least 6 mm rainfall or sprinkler irrigations for activation within 6 d of application, except for Stillwater, OK, 2020 (8 d) and San Angelo, TX, 2020 (13 d) (Table 2). All sites were conventionally tilled, except for the Jackson, TN, location, where no-till practices are implemented. Treatments included isoxaflutole applied alone and in tank mix with high and low rates of commonly used preemergence herbicides labeled for use in cotton (Table 3). Treatments were arranged in a randomized complete block design with four replications at each location.
Table 1. Location, year, GPS coordinates, altitude, average rainfall, soil type, pH, organic matter content, and application date of isoxaflutole tank-mix partner field experiments in 2019 and 2020.
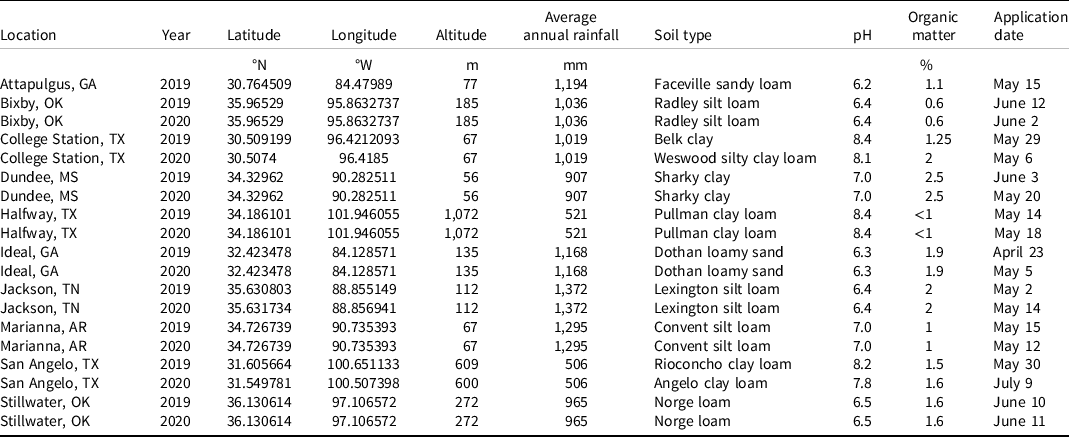
Table 2. Location, year, spray volume, nozzles, pressure, speed, plot size, and precipitation for field experiments in 2019 and 2020. a

a Abbreviations: DAT, days after treatment; DG, drift guard; TTI, Turbo TeeJet Induction; TT, Turbo TeeJet; XR, extended-range.
Table 3. Preemergence treatments and herbicide rates used in weed control experiments across the Cotton Belt in 2019 and 2020.
Evaluations
Weed control by species was evaluated on a 0 to 100% scale (0 being no control and 100% being no presence of the target weed) (Frans et al. Reference Frans, Talbert, Marx, Crowley and Camper1986) every 7 d starting 14 d after treatment (DAT) and concluding 49 DAT. Palmer amaranth was present at the following sites: Halfway, TX, Marianna, AR, Bixby, OK, College Station, TX, Ideal, GA, Jackson, TN, and Dundee, MS; large crabgrass (Digitaria sanguinalis L.) was present at Marianna, AR, Ideal, GA, and Bixby, OK; and morningglory was present at Bixby, OK [ivyleaf morningglory (Ipomoea hederacea Jacq.)] and Marianna, AR, College Station, TX, and Jackson, TN [pitted morningglory (Ipomoea lacunosa L.)]. Weed density by species was recorded in two 0.5-m quadrats, except at Ideal, GA, where the entire 17-m2 plot was counted, in each plot 28 to 35 DAT at six locations. Data from 28 and 42 DAT evaluations are presented.
Data Analysis
Data were separated by location, but year was considered a random effect to broaden the inference and account for environmental variability; therefore, data were pooled across years (Blouin et al. Reference Blouin, Webster and Bond2011; Carmer et al. Reference Carmer, Nyquist and Walker1989; Moore and Dixon Reference Moore and Dixon2014). Data were analyzed using the GLIMMIX procedure (2014 Version 9.4, SAS Institute Inc., Cary, NC) for ANOVA and Tukey’s HSD at α = 0.05. Single degree-of-freedom contrast statements were conducted to examine the difference in control between the high and low rates of tank-mix partners.
Results and Discussion
Palmer Amaranth
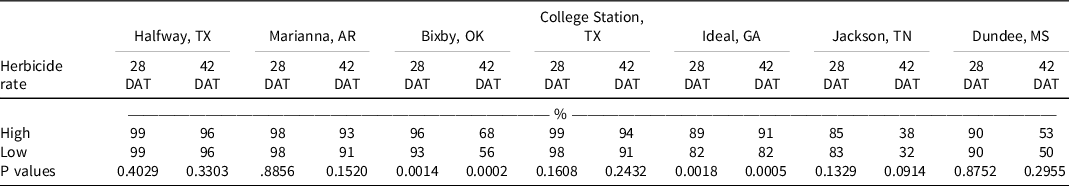
At Bixby, OK, at 28 DAT, isoxaflutole provided 82% control and all tank-mix partners improved control by at least 12% except for pyrithiobac and the low rate of pendimethalin (Table 4). By 42 DAT, isoxaflutole provided 31% control, and the addition of fluometuron, pendimethalin, or prometryn at the low rate or pyrithiobac at either rate were the only tank-mix partners not improving control. Mixtures including the high rate of prometryn, diuron, or acetochlor provided ≥75% control. This site had a large population of Palmer amaranth that is resistant to acetolactate synthase–inhibiting herbicides (Heap Reference Heap2021).
Table 4. Palmer amaranth control as affected by herbicide combination and rate 28 and 42 DAT at seven locations in 2019 and 2020. a,b
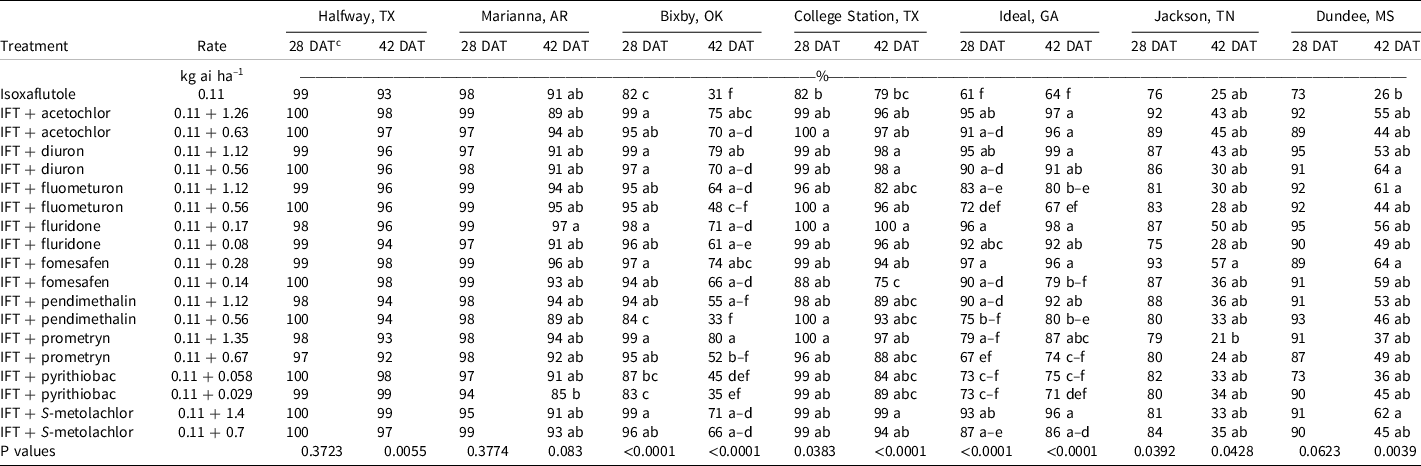
a Abbreviations: DAT, d after treatment; IFT, isoxaflutole.
b Palmer amaranth control was combined across 2019 and 2020 at all locations.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s HSD test at α = 0.05.
At College Station, TX, the only treatments that failed to control Palmer amaranth >90% at 28 DAT were isoxaflutole and isoxaflutole plus the low rate of fomesafen (Table 4). By 42 DAT, isoxaflutole provided 79% control, whereas mixtures with the high rate of fluridone or S-metolachlor or either rate of diuron improved control to ≥98%. Density in the nontreated control was 346,900 plants ha–1 at 28 DAT, and all herbicide treatments reduced the population similarly by at least 92% (Table 5).
Table 5. Palmer amaranth density as affected by herbicide combination and rate 28 and 35 DAT at six locations in 2019 and 2020. a,b
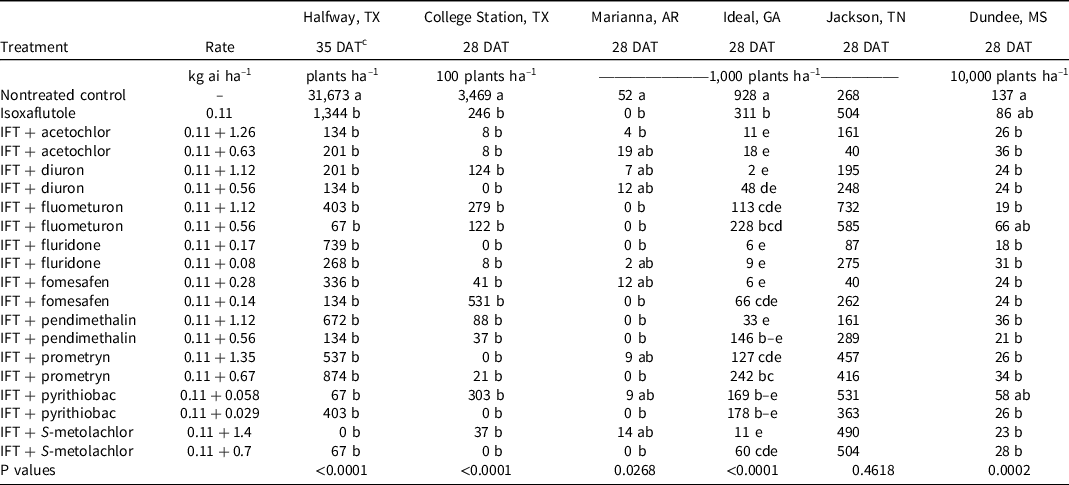
a Abbreviations: DAT, days after treatment; IFT, isoxaflutole.
b Palmer amaranth density was combined across 2019 and 2020 at all locations except for Marianna, AR, where density was only recorded in 2019.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s HSD test at α = 0.05.
At Ideal, GA, Palmer amaranth control and density were recorded in 2019 and 2020, but control at 42 DAT was evaluated only in 2019. Visible control exceeded 99% by all treatments at 14 DAT (data not shown); however, isoxaflutole controlled Palmer amaranth 61% by 28 DAT. The addition of diuron, fluridone, fomesafen, S-metolachlor, or acetochlor at either rate or the high rate of pendimethalin improved control to at least 90%. By 42 DAT in 2019, isoxaflutole plus the high rate of fluometuron (80%), prometryn (87%), fomesafen (96%), and both rates of diuron (91% to 99%), fluridone (92% to 98%), pendimethalin (80% to 92%), S-metolachlor (86% to 96%), and acetochlor (96% to 97%) controlled Palmer amaranth better than isoxaflutole alone (64%). Density in the control consisted of 928,000 plants ha–1 at 28 DAT. Isoxaflutole alone reduced the population by 67%. Tank-mix partners reduced the population by 86% to 99% compared to the control except for the low rate of fluometuron, pendimethalin, or prometryn and both rates of pyrithiobac. Palmer amaranth densities were <10,000 plants ha–1 with isoxaflutole plus the high rate of diuron or fomesafen and with either rate of fluridone.
At Halfway, TX, and Marianna, AR, Palmer amaranth control and density was evaluated in 2019 and 2020. All treatments provided ≥93% at 28 DAT (Table 4). By 42 DAT, isoxaflutole alone provided 91% to 93% control, which was similar to that observed with all tank mixtures (85% to 99%). Plant density recorded in the control exceeded 31,000 plants ha–1 in Halfway wherein isoxaflutole alone or in mixtures reduced the population similarly by >95% (Table 5). In Marianna, AR, plant density was 52,000 plants ha–1 in the control, and treatments reduced the population similarly and at least 64%.
Palmer amaranth control with isoxaflutole in Jackson, TN, was 76% and 25% at 28 and 42 DAT, respectively. At Dundee, MS, Palmer amaranth control with isoxaflutole was 73% and 26% at 28 DAT and 42 DAT, respectively. The addition of tank-mix partners did not improve control during any evaluation date, nor did they influence plant population. At Attapulgus, GA, control was 100% in treated plots, regardless of herbicide treatment (data not shown).
At 28 DAT, Palmer amaranth control improved at three locations with the addition of the high rate of diuron or fluridone and the low rate of acetochlor. By 42 DAT, the addition of both rates of diuron and the high rates of fluometuron, fomesafen, and fluridone improved control at three locations, and the addition of the high rate of S-metolachlor improved control at four locations. Contrast statements comparing high vs low rates of tank-mix partners indicated no differences at 28 or 42 DAT at all locations except for Bixby, OK, and Ideal, GA, where tank-mixing high rates of another residual herbicide with isoxaflutole increased Palmer amaranth control (Table 6). Although the metabolite of isoxaflutole alone is active on Palmer amaranth, control increased when tank-mixing isoxaflutole with another soil-residual herbicide. These results are similar to those reported by Stephenson and Bond (Reference Stephenson and Bond2012), where isoxaflutole alone controlled Palmer amaranth 83% at the end of the season and tank-mixing isoxaflutole with thiencarbazone-methyl and/or atrazine increased Palmer amaranth control to 92%.
Table 6. Contrast statements comparing high vs low rates of tank-mix partners on Palmer amaranth control. a,b
a Single degree-of-freedom contrast statements were conducted to examine the difference in control between the high and low rates of tank-mix partners.
b Abbreviations: DAT, d after treatment.
Large Crabgrass
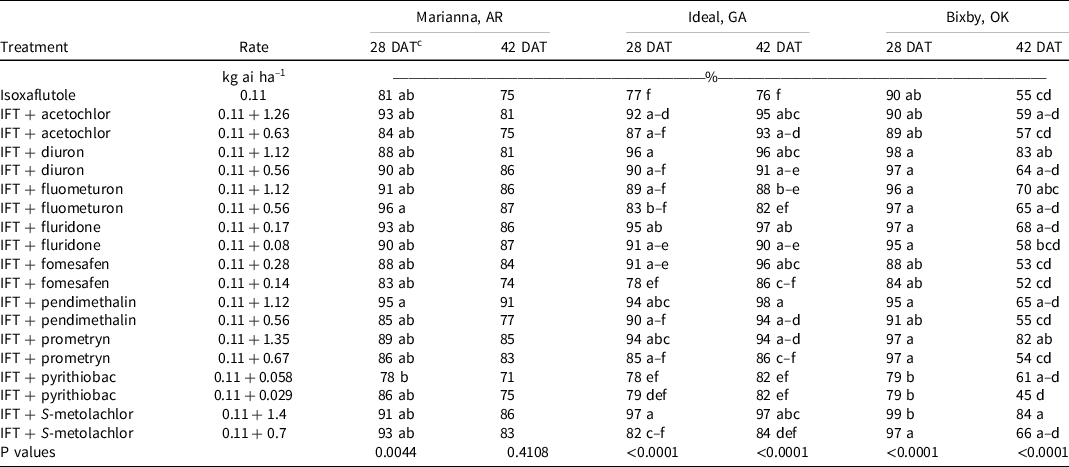
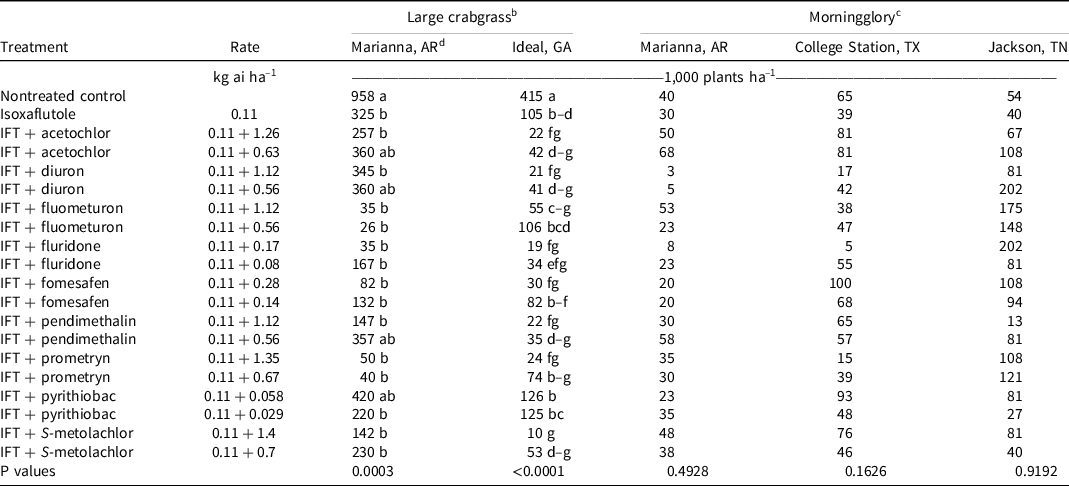
Large crabgrass was present at Marianna, AR, in 2019. At 28 and 42 DAT, isoxaflutole provided 75% to 81% control, and no tank-mix partner improved control. However, >90% control was observed at both evaluation dates when isoxaflutole was mixed with the high rate of pendimethalin and both rates of S-metolachlor (Table 7). At 28 DAT, large crabgrass density in the nontreated control was 958,000 plants ha–1 (Table 8). All treatments decreased large crabgrass density at least 66% except for isoxaflutole plus the low rate of diuron, pendimethalin, or acetochlor, and the high rate of pyrithiobac.
Table 7. Large crabgrass control as affected by herbicide combination and rate 28 and 42 DAT at three locations in 2019 and 2020. a,b
a Abbreviations: DAT, d after treatment; IFT, isoxaflutole.
b Large crabgrass control was combined across 2019 and 2020 at all locations except for Marianna, AR, where large crabgrass was only present in 2019.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s HSD test at α = 0.05.
Table 8. Large crabgrass and morningglory density as affected by herbicide combination and rate 28 DAT at two and three locations, respectively, in 2019 and 2020. a,b
a Abbreviations: DAT, d after treatment; IFT, isoxaflutole.
b Large crabgrass density was combined across 2019 and 2020 at all locations except for Marianna, AR, where large crabgrass was only present in 2019.
c Morningglory density was only recorded in 2020 at all locations.
d Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s HSD test at α = 0.05.
At Ideal, GA, large crabgrass control and density were evaluated in 2019 and 2020. At 28 DAT, isoxaflutole plus the high rate of prometryn (94%), diuron (96%), fomesafen (91%), pendimethalin (94%), S-metolachlor (97%), acetochlor (92%), and both rates of fluridone (91% to 95%) improved large crabgrass control compared to isoxaflutole alone (77%) (Table 7). Plant density in the nontreated control was 415,000 plants ha–1, and all treatments lowered populations by 70% to 98%. The addition of acetochlor, diuron, fomesafen, pendimethalin, and S-metolachlor at the high rate and fluridone at either rate with isoxaflutole reduced densities beyond that noted with isoxaflutole applied alone. By 42 DAT, isoxaflutole provided only 76% control, and all tank-mix partners controlled large crabgrass ≥90% 42 DAT except the low rate of prometryn (86%), fomesafen (86%), or S-metolachlor (84%), and both rates of pyrithiobac (82%) or fluometuron (82% to 88%).
Large crabgrass control was evaluated at Bixby, OK, in 2019 and 2020. Density was not measured at this site. At 28 DAT, no tank-mix partner improved control when compared to isoxaflutole applied alone. By 42 DAT, only isoxaflutole plus the high rate of diuron (83%), S-metolachlor (84%), and prometryn (82%) controlled large crabgrass better than isoxaflutole alone (55%).
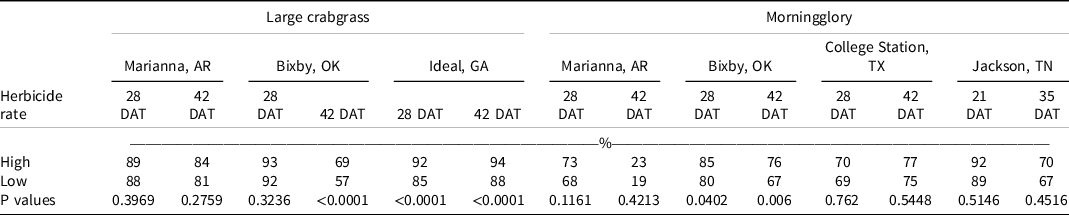
At 28 DAT, isoxaflutole plus the high rate of diuron, fluridone, pendimethalin, or S-metolachlor, and isoxaflutole plus the low rate of fluometuron controlled large crabgrass ≥95% in two of three locations. Additionally, in two of three locations, isoxaflutole plus the high rate of pendimethalin or S-metolachlor improved large crabgrass control at 42 DAT when compared with isoxaflutole alone. Contrast statements comparing high and low rates of tank-mix partners on large crabgrass control indicated no differences at Marianna, AR, regardless of evaluation timing, and at Bixby, OK, at 28 DAT (Table 9). Tank-mixing isoxaflutole with the high rate of another residual herbicide compared to the low rate increased large crabgrass control at 42 DAT at Bixby, OK, and at 28 and 42 DAT at Ideal, GA. Brown and Masiunas (Reference Brown and Masiunas2002) also observed that large crabgrass was controlled 95% following isoxaflutole 21 DAT. Combinations of isoxaflutole plus metribuzin controlled large crabgrass 97% to 100% in studies conducted by Smith et al. (Reference Smith, Soltani, Kaastra, Hooker, Robinson and Sikkema2019).
Table 9. Contrast statements comparing high vs low rates of tank-mix partners on large crabgrass and morningglory control. a,b
a Single degree-of-freedom contrast statements were conducted to examine the difference in control between the high and low rates of tank-mix partners.
b Abbreviations: DAT, d after treatment.
Morningglory
At Marianna, AR, morningglory control and density were recorded in 2020. At 28 DAT, isoxaflutole alone controlled morningglory 50%, and only the addition of the high rate of diuron (88%) or fluridone (85%) improved control (Table 10). Plant density in the control was 40,000 plants ha–1, and there were no detectable differences in density between treatments. By 42 DAT, control was <50% with all treatments.
Table 10. Morningglory control as affected by herbicide combination and rate 21, 28, 35, and 42 DAT at four locations in 2019 and 2020. a,b
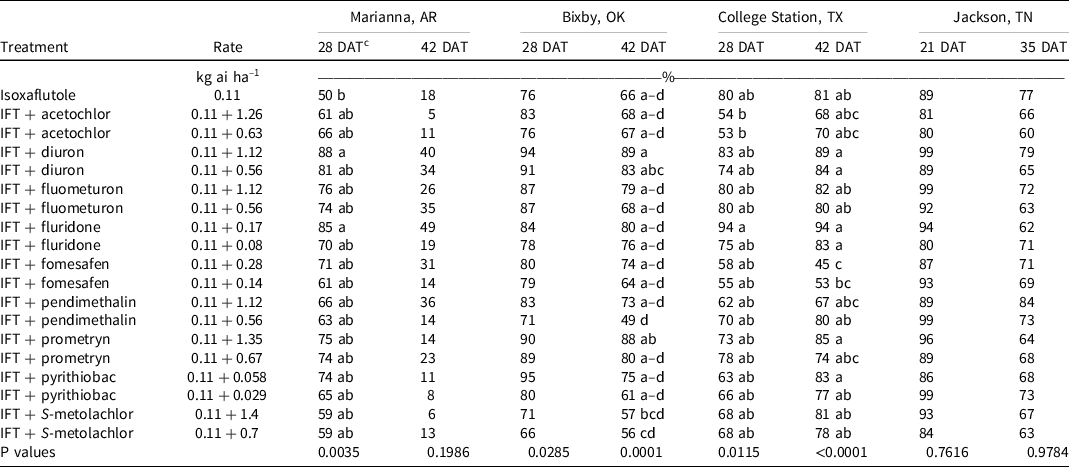
a Abbreviations: DAT, d after treatment; IFT, isoxaflutole.
b Morningglory control was combined across 2019 and 2020 at all locations except for Marianna, AR, and Jackson, TN, where morningglory was only present in 2020.
c Treatment means within a column followed by the same or no letter do not statistically differ according to Tukey’s HSD test at α = 0.05. 0.05.
At Bixby, OK, morningglory control was evaluated in 2019 and 2020. Density was not measured at this site. At 28 DAT, control was 53% to 94% and did not differ among treatments. All tank-mix partners provided similar morningglory control when compared to isoxaflutole alone (66%) at 42 DAT.
At College Station, TX, morningglory control was evaluated in 2019 and 2020, and densities were recorded in 2019. At 28 DAT, no treatment control differed from isoxaflutole (80%). In 2019, morningglory density with isoxaflutole was 39,000 plants ha–1 28 DAT, and no tank-mix partner decreased density. By 42 DAT, morningglory control ranged from 45% to 94%, and only isoxaflutole plus the high rate of fomesafen decreased control compared to isoxaflutole alone.
At Jackson, TN, morningglory control and density were recorded in 2020. Isoxaflutole provided 89% and 77% control at 21 and 35 DAT, respectively. The addition of tank-mix partners did not influence the level of control or densities observed. No differences were observed between the high and low rates of tank-mix partners on morningglory control except at Bixby, OK, where tank-mixing the high rate of another residual herbicide with isoxaflutole increased morningglory control compared to the low rate (Table 9).
Summary
Herbicides that consistently performed well with isoxaflutole across multiple locations and weed species were diuron, fluridone, and S-metolachlor. For broadleaf weed species, diuron, S-metolachlor, and fluridone were most effective when tank-mixed with isoxaflutole, whereas tank-mixing isoxaflutole with pendimethalin or S-metolachlor was most effective on grass species. Fluridone is an HRAC Group 27 herbicide and shares isoxaflutole’s mode of action, although it binds to a different enzyme (phytoene desaturase); therefore, tank-mixing with this active ingredient would be considered similar to other herbicide modes of action and would help slow the development of weed resistance to HPPD-inhibiting herbicides (Sandmann et al. Reference Sandmann, Schmidt, Linden and Boger1991). Diuron and S-metolachlor offer modes of action different from isoxaflutole, and tank-mixing with these two herbicides could help slow the spread of resistant weeds. Overall, weed control decreased more rapidly in environments that received higher amounts of average annual rainfall (National Weather Service 2021) (Table 1). Tank-mix partner recommendations will probably depend on several factors, such as environment, soil type, target weed species, and rotational crops. The opportunity to use isoxaflutole in cotton weed management systems not only will improve season-long control of a number of troublesome weeds but also will add a novel site of action for cotton growers, diversifying weed control programs.
Acknowledgments
This research was partially supported by Cotton Incorporated, the Texas State Support Committee, the Georgia Cotton Commission, and BASF. No other conflicts of interest are noted.













