Menopausal women have a higher risk of uterine fibroids, fibrocystic breast disease, breast or uterine cancer, CHD and obesity(Reference Pike, Pearce and Wu1, Reference Carr2). The most prevalent disease in postmenopausal women is osteoporosis, a disorder of excess skeletal fragility caused partly by changes in bone microstructure(Reference Quereda, Mendoza, Olalla, Baro and Duran3, Reference Ito4). Hormone-replacement therapy (HRT) is currently used to treat osteoporosis or to reduce the symptoms of the menopause, even though HRT has a risk of adverse side effects including breast cancer and endometrial adenocarcinoma(Reference Yadava and Verma5).
Concerns about the adverse side effects of HRT have led to interest in phyto-oestrogens, which are natural alternatives to HRT. Treatment with phyto-oestrogens such as equol, genistein and daidzein has shown favourable effects on bone metabolism, lipid metabolism, obesity and inhibition of menopausal symptoms(Reference Fujioka, Uehara, Wu, Adlercreutz, Suzuki, Kanazawa, Takeda, Yamada and Ishimi6–Reference Mathey, Mardon and Fokialakis8). However, they are also associated with uterotrophic effects, which may increase the risk of endometrial cancer(Reference Rachon, Vortherms, Seidlova-Wuttke and Wuttke7, Reference Weiderpass, Adami, Baron, Magnusson, Bergstrom, Lindgren, Correia and Persson9). Therefore, the development of new phyto-oestrogens with minimal uterotrophic effects is an urgent research focus.
Psoralea corylifolia L. is a widely used medicinal plant in Asia and India(Reference Yadava and Verma5, Reference Miura, Nishida and Iinuma10). The seeds of P. corylifolia L. exert antioxidative, antimicrobial and anti-inflammatory activities(Reference Haraguchi, Inoue, Tamura and Mizutani11–Reference Ferrándiz, Gil, Sanz, Ubeda, Erazo, González, Negrete, Pacheco, Payá and Alcaraz13). Recent research suggests that P. corylifolia has potent oestrogenic effects and that its seeds may be a useful remedy for bone fractures, osteomalacia and osteoporosis(Reference Miura, Nishida and Iinuma10, Reference Zhang, Wang, Zhang, Chen and Liang14). Components derived from P. corylifolia, including bakuchiol, corylifolia, corylin, psoralidin and isobavachin, have strong antioxidant activities(Reference Haraguchi, Inoue, Tamura and Mizutani11), and corylin and bavachin have been shown to stimulate osteoblastic proliferation(Reference Wang, Li and Jiang15). However, little information is available concerning the oestrogenic characteristics of P. corylifolia L. in animal models.
In the present study, we screened the most oestrogenic component derived from P. corylifolia L. by two different in vitro assays, and identified the active component by instrumental analyses such as HPLC and LC/MS, and then compared binding affinities of the component for oestrogen receptor (ER) α and ERβ. We also evaluated the effects of P. corylifolia extracts and its active component on body-weight gain, uterine weight and bone loss in ovariectomised rats.
Experimental methods
Materials and sample preparation
P. corylifolia L. seeds were purchased from a commercial supplier of herbs and pharmaceuticals (Seongnam, Republic of Korea) and were identified by Professor Y. M. Park, Department of Life Science, Cheongju University (Cheonju, Republic of Korea). Voucher specimens (KFRI-PM03220) were preserved in the Korea Food Research Institute. Psoralen and bavachinin were purchased from Sigma Co. (St Louis, MO, USA), and bakuchiol, bavachalcone, isobavachalcone, bavachromene and isobavachromene isolated from P. corylifolia L. were obtained from the Korea Research Institute of Chemical Technology (Daejeon, Republic of Korea) as standard compounds for instrumental analyses (purities > 98 % by HPLC, LC/MS, IR and 1H and 13C NMR spectroscopy). Catechin and 17β-oestradiol (E2) were purchased from Sigma Co. All other chemicals were of analytical grade (Fisher, Springfield, NJ, USA).
Dried seeds (300 g) were washed and boiled in 3 litres of 80 % ethanol at 95°C for 4 h, and the ethanol extract of P. corylifolia L. (PCE) was filtered through no. 2 filter paper (Whatman International Ltd, Maidstone, Kent, UK). A portion of PCE (1 ml) was used for fractionation, and the remainder was dried using a rotary evaporator (Rotavapor R-200; Buchi, Postfach, Switzerland) and stored at 4°C until it was used to prepare the diets.
Screening of oestrogenicity and identification of active component
A quantity of 1 ml of PCE (25 °Bx) was diluted to 15 °Bx using 80 % ethanol, and ninety fractions (each 5 ml) were obtained by gel chromatography (1·5 × 120 cm, 1 ml/min; Lipophilic Sephadex LH-20; Sigma Co.) eluting with 80 % ethanol. The total polyphenol content was analysed using the Folin–Ciocalteu protocol(Reference Singleton and Rossi16). We used the in vitro yeast transactivation assay(Reference Gaido, Leonard, Lovell, Gould, Babai, Portier and McDonnell17) and E-screen assay(Reference Soto, Sonnenschein, Chung, Fernandez, Olea and Serrano18) for the screening of oestrogenicity. For the yeast transactivation assay, Saccharomyces cerevisiae ER+LYS 8127 was obtained from Dr K. S. Kang (Seoul National University, Republic of Korea) and stored in 20 % glycerol at − 70°C. For the E-screen assay, MCF-7 cells were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea) and grown in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10 % fetal bovine serum, penicillin (100 U/ml) and insulin (10 μg/ml). Identification of the active component was performed by HPLC (PU-2089; Jasco, Tokyo, Japan) and LC/MS (Hewlett Packard Co., Palo Alto, CA, USA). The ER binding assay measures the capacity of competitor chemical to displace a high binding affinity fluorescent ligand. To determine the relative binding affinity (RBA) of the main active component for ERα and ERβ, we used an ERα competitor screening kit (Wako code no. 295-56 301; Wako Pure Chemical Industries Ltd, Osaka, Japan) and an ERβ competitor assay kit (part no. 2700; Invitrogen, Carlsbad, CA, USA). RBA were calculated by dividing the half maximal inhibitory concentration (IC50) of unlabelled E2 by the IC50 of a competitor and then multiplying the value by 100.
Animal study: animals and diets
Seventy female Sprague–Dawley rats, aged 5 weeks, were purchased from Orient Bio Inc. (Seongnam, Republic of Korea). The animals were housed under controlled temperature (20 ± 1°C), relative humidity (50–80 %) and illumination (12 h light–dark) conditions and acclimatised for 1 week. The animals were either ovariectomised (six groups, ten rats in each group) or sham-operated (sham; one group of ten rats). After a 1-week recovery period, the animals were fed the following experimental diet based on AIN-93M (Dyets Inc., Bethlehem, PA, USA) for 6 weeks: two ovariectomised (OVX) groups were supplied with a PCE-containing diet (PCE at a low dose of 0·25 % (PL) or at a high dose of 0·5 % (PH)); three OVX groups were fed the control diet and injected subcutaneously with bakuchiol (bakuchiol at a low dose of 15 mg/kg per d (BL) or at a high dose of 30 mg/kg per d (BH)) or E2 (20 μg E2/kg per d; OE group), respectively. The sham and OVX groups were fed the control diet and were treated with the vehicle. Body weight and feed intake of all experimental animals were measured every week. The feed efficiency ratio was calculated as the weight gain (g) divided by feed intake (g).
Serum and tissue collection
Experimental animals were fasted for 24 h before dissection. At necropsy, the uterus, livers, kidneys, blood and femurs were collected. Blood was centrifuged for 10 min at 3000 rpm to separate the serum. The uterus was weighed after the fat was trimmed and again after the connective tissue was trimmed, drained of intra-uterine fluid, and dried at 50°C overnight. The livers and kidneys were also trimmed, weighed, and immediately stored at − 70°C. Femurs were trimmed and stored at − 70°C.
Analysis of alkaline phosphatase, inorganic-phosphorus, calcium and 17β-oestradiol concentrations in serum
Alkaline phosphatase (ALP), inorganic-phosphorus (IP) and Ca concentrations were measured using commercial reagent kits (Bayer Inc., West Haven, CT, USA) and read in an ADVIA 1650 Auto-analyser (Bayer Inc., Tokyo, Japan). Serum E2 concentration was measured using an E2 Enzyme Immunoassay test kit (BC-1111; BioCheck, Inc., Foster City, CA, USA) and read at 450 nm in a UV spectrophotometer (V-530; Jasco, Tokyo, Japan). The E2 concentration of the specimens and controls run concurrently with the standards were calculated from the standard curve.
Analysis of femoral bone mineral density
The bone mineral density (BMD) of the trimmed femurs was analysed using peripheral dual-energy X-ray absorptiometry (models pDEXA Forearm X-Ray Bone Densitometer and pDEXA Sabre; Cooper Surgical Co., Fort Atkinson, WI, USA). BMD values were calculated using the same cross-section of proximal femurs.
Statistical analysis
All data are presented as mean values with their standard errors. Statistical analysis was performed using ANOVA followed by Duncan's multiple-range test (P = 0·05) using a SAS program (SAS Institute Inc., Cary, NC, USA). The IC50 values of the compound binding affinity to ERα and ERβ were calculated with Sigmaplot 9.0 software (Systat Software Inc., Richmond, CA, USA).
Results
Identification of oestrogenic component from ethanol extract of Psoralea corylifolia L
PCE was fractionated using Sephadex LH-20, and the total phenolic content of each fraction was measured (Appendix 1) and the oestrogenic activities of the main fractions were measured by an in vitro yeast transactivation assay and an E-screen assay. In the yeast transactivation assay, only the no. 40 fraction (1 μg/ml) resulted in 58 % β-galactosidase activity compared with 10− 9m-E2, whose concentration was shown as having the highest oestrogenicity. The other fractions did not exhibit any oestrogenic activity (Appendix 2 (A)). In the E-screen assay, the no. 40 fraction also showed oestrogenic activity in the range of 0·1–1 μg/ml. MCF-7 cells treated with the no. 40 fraction (0·1 μg/ml) showed proliferation at approximately two times higher than that induced by the control (Appendix 2 (B)). The no. 44 and no. 50 fractions showed no significant difference at 0·1 μg/ml compared with the control, while they increased cell growth by 120 and 150 % at 1·0 μg/ml, respectively. Taken together, these results indicate that the no. 40 fraction induced the highest effect on MCF-7 proliferation.
To identify the active component from the no. 40 fraction, we performed HPLC and LC/MS analyses. We separated only one peak by HPLC (Appendix 3 (A)), and its major fragmentation ion was m/z 257·3 in LC/MS (Appendix 3 (B)), which matched the analysis pattern of standard bakuchiol (m/z 256·4). Subsequently we identified the active component of no. 40 as bakuchiol.
To determine ER selectivity, the binding affinities of bakuchiol for ERα and ERβ were measured by an in vitro ER binding assay. The IC50 value for ERα was 1·34 × 10− 6m, while the IC50 value for ERβ was 1·20 × 10− 6m. These values were converted to RBA compared with positive controls (E2). The affinity of bakuchiol for ERα was three times higher than for ERβ (RBAERα = 1·51 and RBAERβ = 0·44), indicating that bakuchiol has higher selectivity for ERα.
Effects of ethanol extract of Psoralea corylifolia L. and bakuchiol treatments in ovariectomised rats
Body and uterus weight
After 6 weeks, the final body weight of the OVX group was 21·6 % higher than that of the sham group (P < 0·01). The body weight was lower in the OE (78·4 %), PL (81·4 %) and PH (74·0 %) groups than in the OVX group. The amount of weight gained by the PL and PH groups differed significantly from the OVX group; however, the weight gain by the BL and BH groups did not differ from the OVX group (Table 1). The feed efficiency ratios were about 20 % lower in the OE, PL and PH groups than in the OVX group (P < 0·001) despite no significant differences in feed intake. This indicates that PCE supplementation prevented body-weight gain in ovariectomised rats. The weight gain of the uterus was measured to evaluate the uterotrophic activity of bakuchiol and PCE treatments for 6 weeks. The absolute and relative wet uterine weights (weight per 100 g body weight) decreased by 87 and 90 %, respectively, after the ovariectomy operation (Table 2). The uterine weight was 4·7 times higher in the OE group than in the OVX group, although this difference was not significant for either bakuchiol or PCE treatment (P>0·05). Relative liver and absolute kidney weights showed slight changes after PCE treatments (Table 2).
Table 1 Body-weight gain and feed efficiency ratio of ovariectomised rats fed the experimental diets for 4 weeks
(Mean values with their standard errors)

OVX, ovariectomised vehicle-treated control group; OE, 17β-oestradiol-administered group (+20 μg 17β-oestradiol/kg per d); BL, bakuchiol-administered group with low dose (+15 mg/kg per d); BH, bakuchiol-administered group with high dose (+30 mg/kg per d); PL, ethanol extract of Psoralea corylifolia L. (PCE)-supplemented group with low dose (+0·25 % PCE); PH, PCE-supplemented group with high dose (+0·5 % PCE).
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05; Duncan's multiple-range test).
* P < 0·05, ** P < 0·01, *** P < 0·001 (ANOVA).
† Weight gain (g)/feed intake (g).
Table 2 Effect of ethanol extract of Psoralea corylifolia L. (PCE) and bakuchiol treatments on the organ weight of ovariectomised rats fed the diets experimental for 4 weeks
(Mean values with their standard errors)

OVX, ovariectomised vehicle-treated control group; OE, 17β-oestradiol-administered group (+20 μg 17β-oestradiol/kg per d); BL, bakuchiol-administered group with low dose (+15 mg/kg per d); BH, bakuchiol-administered group with high dose (+30 mg/kg per d); PL, PCE-supplemented group with low dose (+0·25 % PCE); PH, PCE-supplemented group with high dose (+0·5 % PCE).
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05; Duncan's multiple-range test).
* P < 0·05, *** P < 0·001 (ANOVA).
Alkaline phosphatase, calcium, inorganic-phosphorus and 17β-oestradiol concentrations in serum
ALP, Ca and IP are key factors for bone calcification. ALP and IP concentrations increased by 13 and 23 % in the OVX group compared with the sham group. However, the ALP and IP values in PCE-treated animals were 17 and 10 % lower in the OVX group (P < 0·01) and were 24 and 5 % lower in bakuchiol-treated animals in the OVX group (P < 0·01; Table 3). Ca concentration was 3 % lower in the OVX group than in the sham group. PCE and bakuchiol treatment increased Ca levels above 2 % compared with the OVX group (P < 0·01). These data suggest that both PCE supplements and bakuchiol administration suppressed ALP increases in bone turnover of post-ovariectomised rats.
Table 3 Effect of ethanol extract of Psoralea corylifolia L. (PCE) and bakuchiol treatments on serum alkaline phosphatase (ALP), calcium and inorganic phosphorus (IP) concentrations of ovariectomised rats fed the experimental diets for 4 weeks
(Mean values with their standard errors)

OVX, ovariectomised vehicle-treated control group; OE, 17β-oestradiol-administered group (+20 μg 17β-oestradiol/kg per d); BL, bakuchiol-administered group with low dose (+15 mg/kg per d); BH, bakuchiol-administered group with high dose (+30 mg/kg per d); PL, PCE-supplemented group with low dose (+0·25 % PCE); PH, PCE-supplemented group with high dose (+0·5 % PCE).
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05; Duncan's multiple-range test).
*** P < 0·001 (ANOVA).
The E2 concentration in serum was reduced by the ovariectomy. The E2 concentration was 16·9 pg/ml in the sham group and 4 pg/ml in the OVX group (Fig. 1 (A)). The E2 concentration was 6·25 times higher in the OE group than in the OVX group. Compared with the OVX group, the E2 concentrations were 3·25 times higher in the BL group, 5·25 times higher in the BH group, 2·75 higher times in the PL group and 4·5 times higher in the PH group. These data suggest that bakuchiol was more effective than PCE in increasing the E2 concentration in blood after ovariectomy.
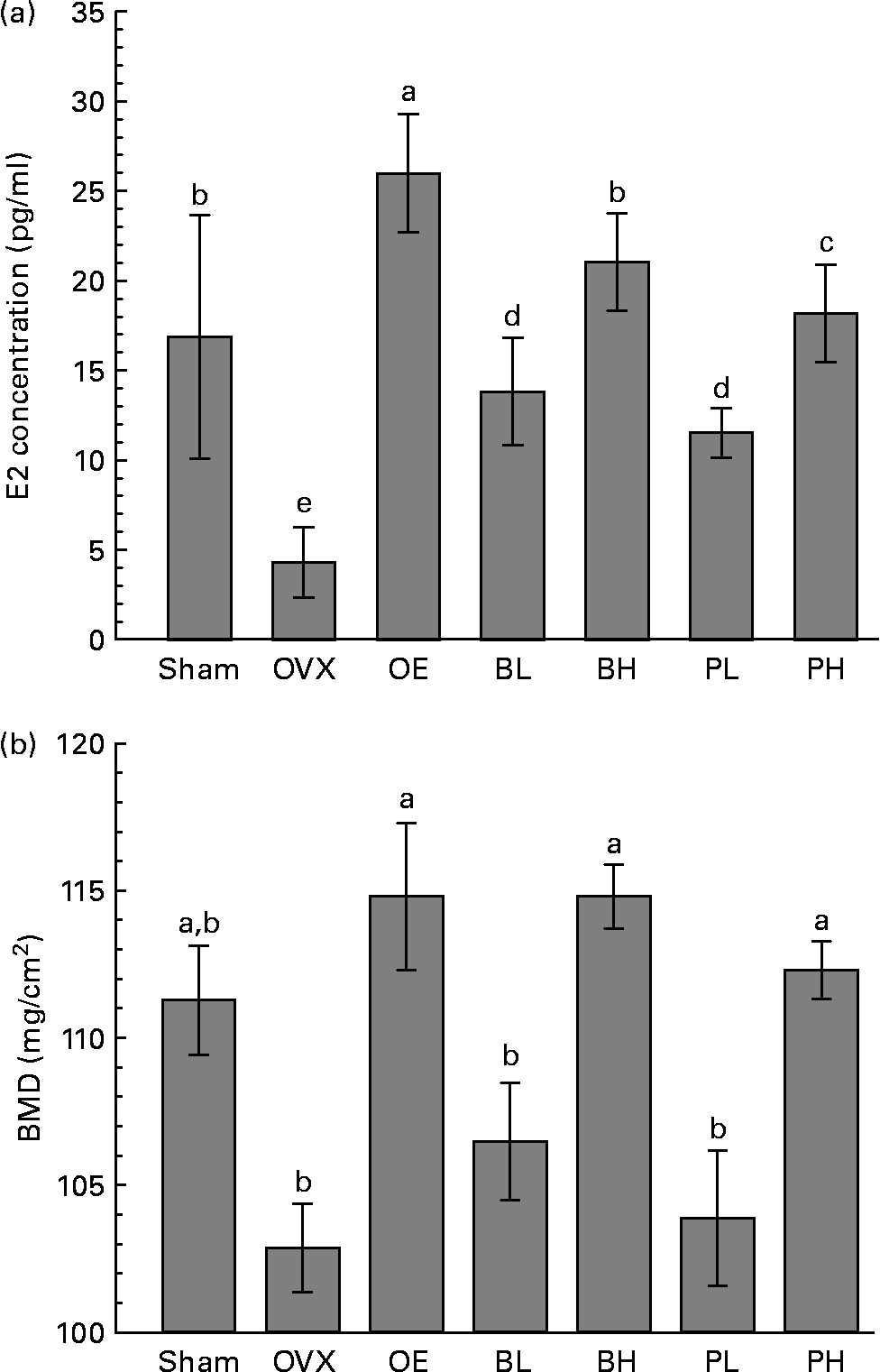
Fig. 1 Effect of ethanol extract of Psoralea corylifolia L. (PCE) and bakuchiol treatments on 17β-oestradiol (E2) serum concentration (A) and bone mineral density (BMD) of the proximal femur (B) in ovariectomised rats. OVX, ovariectomised vehicle-treated control group; OE, E2-administered group (+20 μg E2/kg per d); BL, bakuchiol-administered group with low dose (+15 mg/kg per d); BH, bakuchiol-administered group with high dose (+30 mg/kg per d); PL, PCE-supplemented group with low dose (+0·25 % PCE); PH, PCE-supplemented group with high dose (+0·5 % PCE). Values are means, with standard errors represented by vertical bars. a–e Mean values with unlike letters were significantly different (P < 0·05; Duncan's multiple-range test). For E2 and BMD, P < 0·001 (ANOVA).
The BMD of the proximal femur was 112·3 (se 0·9) mg/cm2 in the PH group and 114·8 (se 1·1) g in the BH group, and these values were significantly higher than in the OVX group (102·8 (se 1·5) mg/cm2; P < 0·001). BMD in the PL group (103·8 (se 2·3) mg/cm2) and in the BL group (106·4 (se 2·0) mg/cm2) did not differ significantly from that of the OVX group. The BMD was similar in the BH and OE groups, and 11 % higher than in the OVX group. The BMD in the PH group was 9 % higher than in the OVX group (Fig. 1 (B)).
Discussion
Based on the results of observational and epidemiological studies, phyto-oestrogens have been shown to help alleviate symptoms of menopause and to reduce the risk of CVD and oestrogen-dependent cancers. Despite evidence supporting the health benefits of phyto-oestrogens(Reference Anderson, Anthony, Cline, Washburn and Garner19), genistein and soya protein stimulate tumour growth in a dose-dependent manner in ovariectomised athymic mice implanted with MCF-7 cells(Reference Messina and Loprinzi20). Many new potent phyto-oestrogens from medicinal plants such as red clover, dong quai, soya and fo-ti have been investigated for development into new drugs that will hopefully provide alternatives to HRT(Reference Liu, Buredette and Xu21–Reference Mishima, Suzuki, Isohama, Kuratsu, Araki, Inoue and Miyata24). P. corylifolia L. is also a source of phyto-oestrogens because PCE contains oestrogen-like compounds such as coumarin and members of the flavonoid family.
The yeast transactivation assay and E-screen assay are routinely used for phyto-oestrogen screening, and the ER binding assay is performed to compare binding affinity for the ER(Reference Coldham, Dave, Sivapathasundaram, McDonnell, Connor and Sauer25). In the present study, only one of ninety fractions by gel chromatography was identified by the in vitro assays as the most oestrogenic fraction. The active component from that fraction was identified as bakuchiol by HPLC and LC/MS. Wang et al. proposed that corylin and bavachin can stimulate bone formation and may have potential activity against osteoporosis in osteoblast-like UMR106 cells(Reference Wang, Li and Jiang15). However, the present results show that only the bakuchiol-containing fraction had oestrogenic activity in the in vitro assays. In the ER binding assay, the affinity of bakuchiol for ERα was three times higher than for ERβ. ERα has been shown to cause breast cancer cell proliferation, and ERβ has been demonstrated to be a tumour suppressor(Reference Paruthiyil, Parmar, Kerekatte, Cunha, Firestone and Leitman26, Reference Strom, Hartman, Foster, Kietz, Wimalasena and Gustafsson27). However, Hertrampf et al. (Reference Hertrampf, Gruca, Seibel, Laudenbach, Fritzemeier and Diel28) suggested that only ERα agonists confer bone-protective effects. The present results showing a higher selectivity of bakuchiol for ERα may result in a protective effect for bone loss.
We evaluated the effects of PCE and bakuchiol treatment on body-weight gain, uterine weight and bone loss in ovariectomised rats. Body-weight gain is associated with metabolic abnormalities in postmenopausal women, and oestrogen treatment decreases weight gain and visceral fat development in animals and humans(Reference Paruthiyil, Parmar, Kerekatte, Cunha, Firestone and Leitman26, Reference Strom, Hartman, Foster, Kietz, Wimalasena and Gustafsson27). The PCE-supplemented groups gained significantly less weight than the OVX group and a similar amount of weight as the sham and OE groups, whereas weight gain was similar in the bakuchiol-treated groups and the OVX group. Szkudelska, Nogowski reported that genistein decreased body- and fat tissue-weight gain, and that these changes are accompanied by lower feed intake(Reference Szkudelska and Nogowski29). However, in our experiments, the feed intake did not differ significantly between the bakuchiol- and PCE-treated groups and the OVX and OE groups. The feed efficiency ratio was similar in all groups except the OVX and BH groups.
The uterotrophic effects of oestrogen treatment are undesirable in postmenopausal women because they may increase the risk of endometrial cancer(Reference Weiderpass, Adami, Baron, Magnusson, Bergstrom, Lindgren, Correia and Persson9). The goal of many studies is to identify a new phyto-oestrogen as an alternative to HRT for preventing osteoporosis without causing uterine hormone stimulation. However, there is an ongoing controversy about phyto-oestrogens and their uterotrophic effects. Anderson et al. (Reference Anderson, Ambrose and Garner30) provided evidence that genistein treatment (0·5, 1·6 and 5·0 mg/d) did not exhibit any uterotrophic activity, but showed dosage-dependent biphasic effects on bone tissue in the ovariectomised rat model. However, Fanti et al. (Reference Fanti, Monier-Faugere, Geng, Schmidt, Morris, Cohen and Malluche31) reported that higher genistein doses (50 μg/g body weight) caused an increase in uterine mass. Other studies with genistein and daidzein have also demonstrated weak oestrogenic effects, as reflected by changes in uterine weight(Reference Szkudelska and Nogowski29, Reference Richter and Uhlenhuth32, Reference Tchernof, Calles-Escandon, Sites and Poehlman33). The effect of dietary equol on uterotrophic activity is 3·5 times less than the effect of E2 treatment, and equol shows weak uterotrophic activity in mice(Reference Rachon, Vortherms, Seidlova-Wuttke and Wuttke7). In the present study, uterine weight was much lower in the OVX group with oestrogen deficiency than in the sham and E2-treated groups. Bakuchiol administration and PCE supplementation had no effect on uterine weight increase. These results suggest that bakuchiol and PCE treatment have no uterotrophic activity, despite having significant oestrogenic activity. Taken together, these data suggest that bakuchiol and PCE treatments may alleviate menopausal symptoms without increasing the risk of cancer in postmenopausal women.
Decreased plasma E2 and increased bone loss are the most prevalent symptoms in postmenopausal women. It has been well documented that isoflavone or other phyto-oestrogen supplements modulate plasma E2 and exert a protective effect on bone loss(Reference Mehmood, Smith, Tucker, Chuzel and Carmichael34). However, genistein has been shown to have adverse effects on tumours and on the uterus in women with postmenopausal breast cancer, despite beneficial effects on bone(Reference Picherit, Dalle, Neliat, Lebecgue, Davicco, Barlet and Coxam35). Genistein also shows both positive and negative effects on experimental endothelial dysfunction(Reference Santell, Chang, Nair and Helferich36). In addition, pomegranate extract inhibits ovariectomy-stimulated bone turnover(Reference Alekel, Germain, Peterson, Hanson, Stewart and Toda37), and high dietary levels of soya isoflavones do not stimulate breast or uterine proliferation in postmenopausal monkeys and may contribute to an oestrogen profile associated with reduced breast cancer risk(Reference Power, Ward, Chen, Saarinen and Thompson38). Our present data suggest that bakuchiol and PCE supplementation significantly increase the plasma E2 level, suggesting that bakuchiol acts as a potent phyto-oestrogen.
We found that both bakuchiol and PCE treatments in ovariectomised rats resulted in lower ALP and IP in serum, higher Ca in serum, and increased BMD of the proximal femur. ALP is a key enzyme for bone calcification and provides an index of cell differentiation for bone formation(Reference Squadrito, Altavilla and Squadrito39). Increased ALP levels indicate a positive effect on osteoblastic differentiation, particularly bone mineralisation(Reference Mori-Okamoto, Otawara-Hamamoto, Yamato and Yoshimura40). The ALP concentration was significantly higher in the OVX group than in other groups, suggesting that ovariectomy increases bone turnover rate(Reference Wood, Register, Anthony, Kock and Cline41). E2 administration significantly suppressed the increase of serum ALP levels. Rats treated with bakuchiol or PCE had lower ALP levels than those in the OVX and sham groups. Bakuchiol administration was most effective and had only a slightly different effect than E2 treatment. Usually, osteoclasis is induced when the Ca:IP ratio decreases(Reference Xiong, Wang, Xu and Li42). In the present study, bakuchiol and PCE treatments may have attenuated bone loss by decreasing the IP levels and by slightly increasing Ca concentration in serum. An increase in BMD was also observed in the proximal femur of ovariectomised rats. Surprisingly, the BH and PH groups exhibited significantly higher BMD than the sham group, and similar BMD to the E2-treated group. The serum E2 concentration was also consistent with the BMD results. Zhang et al. reported that PCE inhibits bone resorption in vitro (Reference Zhang, Wang, Zhang, Chen and Liang14) and that an acetone extract of P. corylifolia L. significantly increases serum IP and promotes bone calcification in rats(Reference Miura, Nishida and Iinuma10). The present results suggest that bakuchiol and PCE supplementation can reduce postmenopausal bone loss without the need for oestrogen.
In the present study, we screened oestrogenic compounds from PCE by in vitro assays and identified bakuchiol as the most active component. Bakuchiol showed a higher selectivity for ERα than for ERβ, which could be related to its bone-protective properties. In our animal study, bakuchiol or PCE treatments were effective in preventing bone loss without significant uterotrophic activity in ovariectomised rats. In conclusion, bakuchiol and PCE treatments should be effective in preventing bone loss and could be potent phyto-oestrogens and useful alternatives to HRT.
Acknowledgements
The present study was supported by a grant of the Korea Science and Engineering Foundation (KOSEF) funded by the Ministry of Science and Technology and by a grant from the Korea Food Research Institute, Republic of Korea. The authors thank Y. S. Kim, Korea Research Institute of Chemical Technology, Republic of Korea for providing pure standard compounds and Professor K. S. Kang, College of Veterinary Medicine, Seoul National University, Republic of Korea for providing Saccharomyces cerevisiae ER+ LYS 8127. S.-H. L., H. J. P. and S. K. developed the initial idea, S.-H. L., S.-R. K. and S. K. collected and analysed the data from in vitro assays and instrumental analyses, T.-Y. H. and J. A. performed the animal study, and S. K. drafted the manuscript. The authors declare no conflict of interest.
Appendix

Appendix 1 Total phenolic contents of each fraction separated by Sephadex LH-20 (1·5 × 120 cm, 1 ml/min; Sigma Co., St Louis, MO, USA) from Psoralea corylifolia L. extract using the Folin–Ciocalteu protocol(Reference Singleton and Rossi16). A total of 100 μl of ethanol extract of Psoralea corylifolia L. was mixed with 2 ml of 2 % Na2CO3 for 2 min at room temperature and then added to 100 μl of 50 % Folin–Ciocalteu reagent. After 30 min, the total polyphenol content was measured at 750 nm using a UV spectrophotometer (V-530; Jasco, Tokyo, Japan). Catechin was used as the standard.

Appendix 2 Oestrogenic activities of the main fractions evaluated by in vitro yeast recombinant assay (a) and E-screen assay (b). Data represent only the five main fractions shown in Appendix 1. The fractions evaluated by the in vitro yeast recombinant assay were: no. 40 (●); no. 44 (○); no. 50 (▾); no. 56 (▿); no. 66 (■); 17β-oestradiol (10− 9m) (□); control (♦). The fractions evaluated by the E-screen assay were: no. 40 (●); no. 44 (▾); no. 50 (Δ); no. 56 (■); no. 66 (□); 17β-oestradiol (10− 9m) (♦); control (◇).

Appendix 3 Identification of bakuchiol (retention time 43·25 min) as the oestrogenic component from fraction no. 40 by HPLC (a) and LC/MS (b). (a) Chromatographic separation using an XTerra RP18 column (4·6 × 250 mm, 5 μm; Waters Co., Milford, MA, USA) at 25°C in an HPLC system (PU-2089; Jasco, Tokyo, Japan). The mobile phase comprised 0·1 % water–acetic acid (A) and acetonitrile (B); the A:B ratio was as follows: 0 min, 60:40; 15 min, 50:50; 35 min, 40:60; 45 min, 30:70; 55 min, 20:80 and maintained for 5 min. The flow rate was 1·0 ml/min; the detector wavelength was 245 nm; the injection volume was 20 μl. (b) A Quattro LC triple-quadrupole Tandem MS (Hewlett Packard Co., Palo Alto, CA, USA), equipped with an electrospray ionisation source, was used for MS analyses. The ionisation mode was positive, and the interface and mass selective detector parameters were as follows: flow, 0·001–10 ml/min; diode array detector, 190–950 nm; gas flow, 91 litres/h; desolvation gas flow, 473 litres/h; capillary, 3·7 kV; cone, 30 V; extractor, 3 V; Rangefinder (RF) lens, 0·51 V; source block temperature, 80°C; desolvation temperature, 200°C.









