The inclusion of dietary fatty acid (FA), especially PUFA, may play an important role in oxidative metabolism. Indeed, the administration of PUFA has been shown to increase the risk of plasma lipoperoxidation in ruminant animals such as steers( Reference Scislowski, Bauchart and Gruffat 1 ) and sheep( Reference Gladine, Rock and Morand 2 ). Although oxidative metabolism is essential for the survival of cells, a side effect of this dependence is the production of free radicals and other reactive oxygen species that cause oxidative changes. Sies et al. ( Reference Sies, Stahl and Sevanian 3 ) suggested that antioxidants are required to provide protection against the oxidative effect of diets rich in fat. The inclusion of antioxidants in the diet alleviates the negative effects of oxidised fat by scavenging peroxides and reducing FA peroxidation( Reference Frankel and Frankel 4 ) and enhances the lactation performance and antioxidant status of cows( Reference Vázquez-Añón, Nocek and Bowman 5 ). It is well known that high-producing dairy cows are prone to oxidative stress and that this situation can be exacerbated under certain environmental, physiological and dietary conditions( Reference Bernabucci, Ronchi and Lacetera 6 ). Strong positive correlations between several antioxidant enzymes and vascular adhesion molecules indicate a protective response of antioxidants to the enhanced proinflammatory state observed in transition dairy cows( Reference Aitken, Karcher and Rezamand 7 ). Some antioxidants such as sesamin lignans also increase the expression of genes involved in β-oxidation in rats( Reference Kiso 8 ) and modulate the transcript abundance of lipogenic genes such as lipoprotein lipase (LPL) and stearoyl-CoA desaturase (SCD) in adipose tissue and of the acetyl-coenzyme A carboxylase-α (ACACA) in the liver( Reference Ide, Lim and Odbayar 9 ). Antioxidants could thus contribute to the enhancement of mechanisms protecting against oxidative stress with various immunity, reproduction and health benefits. Therefore, the inclusion of natural antioxidants in cows' diet appears interesting for exploiting the full potential of PUFA while decreasing oxidative stress.
Research has demonstrated several health benefits of n-3 FA in humans including the prevention of CVD( Reference Baum and Hamm 10 ) and reduction of the incidence of breast and prostate cancers( Reference Stephenson, Al-Taan and Arshad 11 ). Thus, supplementation with PUFA from the n-3 family has been used as a strategy to increase these FA in animal products to improve their nutritive value( Reference Wood and Enser 12 , Reference Kouba and Mourot 13 ). In dairy cows, flaxseed, which is rich in the n-3 linolenic acid, has been shown to decrease the proportions of SCFA and medium-chain FA and to increase those of PUFA in milk fat( Reference Petit 14 ). However, lipid oxidation of milk is highly influenced by the content of long-chain FA, which are particularly susceptible to oxidation and can give rise to the development of off-flavour( Reference Timmons, Weiss and Palmquist 15 ).
Plant lignans are natural strong antioxidants, and flaxseed (Linum usitatissimum) is known as the richest dietary source of lignans, including matairesinol and glycosides of secoisolariciresinol as the major compounds( Reference Mazur, Fotsis and Wähälä 16 ). In dairy cows, rumen microbiota converts plant lignans into mammalian lignans such as enterolactone, which are later absorbed and transferred into urine, blood and milk( Reference Gagnon, Côrtes and Da Silva 17 ). The greatest concentration of enterolactone in milk is obtained when flax meal and not flax seed is added to the diet of dairy cows( Reference Petit, Gagnon and Priyadarshini 18 ), as lignans are concentrated in the outer fibre-containing layers( Reference Adlercreutz and Mazur 19 ). The mammalian lignan enterolactone has been shown to have greater antioxidant activity than vitamin E( Reference Prasad 20 ), which suggests that flax may contribute to the enhancement of the oxidative status of cows provided PUFA supplementation. Indeed, recent results have indicated that supplementation of n-3 PUFA along with antioxidants such as vitamin E and plant polyphenols reduces lipoperoxidation in lactating cows, thereby contributing to their protection against the deleterious consequences of lipoperoxidation( Reference Gobert, Martin and Ferlay 21 ). Besides the well-known antioxidant properties of plant lignans, recent papers have also reported their effects on the expression of lipogenic genes. For example, higher hepatic mRNA abundance of sterol regulatory element binding transcription factor 1 (SREBF1), fatty acid synthase (FASN) and ACACA has been observed in rats fed green tea, which is rich in the lignans matairesinol and secoisolariciresinol( Reference Chen, Bezzina and Hinch 22 ). We have recently shown that flax hulls, which are rich in lignans, increase the mammary transcript abundance of some antioxidant genes (e.g. catalase, glutathione peroxidase 1 and superoxide dismutase 1), which can contribute to the protection against oxidative stress damage occurring in the mammary gland of dairy cows( Reference Côrtes, Palin and Gagnon 23 ). However, the effect of flax hulls on the expression of lipogenic genes has never been investigated. Taking all these results into account, we hypothesised that the inclusion of a source of antioxidants such as flax lignans to the diet of dairy cows supplemented with PUFA may modulate the mammary expression of genes involved in the metabolism of lipids, thus affecting milk FA profile. Therefore, the present study aimed to determine whether dietary flax hulls with or without flax oil bypassing the rumen can affect the expression of lipogenic genes in mammary tissue. The effects of flax hulls and flax oil on milk production and composition, milk FA profile and ruminal fermentation were also determined.
Materials and methods
Animals and experimental treatments
The present study is part of a larger project where results on mammary gene expression and activity of antioxidant enzymes, along with the concentration of the mammalian lignan enterolactone in milk and plasma, have been reported previously( Reference Côrtes, Palin and Gagnon 23 ). A total of eight multiparous Holstein cows fitted with ruminal cannulas (10 cm; Bar Diamond, Inc.) with milk production averaging 163 (se 11) d were assigned to a replicated 4 × 4 Latin square design with four 21 d periods balanced for residual effect. Treatments were planned according to a 2 × 2 factorial arrangement: control diet with no flax hulls (CONT); diet with 9·88 % flax hulls in the DM (HULL); control diet with 500 g flax oil/d infused in the abomasum (COFO); diet with 9·88 % flax hulls in the DM and 500 g flax oil/d infused in the abomasum (HUFO). The two total mixed diets have been described in detail previously( Reference Côrtes, Palin and Gagnon 23 ) and have been formulated to meet the requirements of cows that were 750 kg in body weight and producing 30 kg milk/d with 3·5 % fat( 24 ). Diets with and without flax hulls had equal amounts of protein, acid-detergent fibre (ADF) and neutral-detergent fibre (NDF), but the addition of flax hulls to the diets resulted in a higher concentration of fat compared with the control diet (5·46 v. 2·73 % of the DM). The fatty acid composition of flax oil (Brenntag Canada, Inc.) and chemical composition of flax hulls (Natunola Health, Inc.) have been provided in detail by Côrtes et al. ( Reference Côrtes, Palin and Gagnon 23 ). Flax hulls contained 29·8 % of total lipids and 0·99 % of secoisolariciresinol diglucoside on a DM basis. The FA profile of flax hulls, expressed as a percentage of total FA, included 8·2 % of 16 : 0, 1·7 % of 18·0, 16·6 % of cis-9 18 : 1, 15·4 % of cis-6 18 : 2 and 58·1 % of cis-3 18 : 3. At the start of the experiment, the body weight of the cows averaged 742 (se 11) kg. The cows were kept in individual stalls and were given free access to water. National guidelines for the care and use of animals were followed as recommended by the Canadian Council on Animal Care( Reference Offert, Cross and McWilliam 25 ), and all experimental procedures were approved by the local Animal Care Committee.
Sampling
Each experimental period consisted of 21 d with 7 d of adaptation to the diets and 14 d of infusion. The cows were milked twice a day at 08.00 and 19.00 hours and were fed ad libitum (10 % refusals on an as-fed basis) twice a day (08.30 and 14.30 hours). Feed intake and milk yield were measured daily throughout the experimental period. Abomasal infusions were carried out by inserting an infusion line through the rumen cannula and the sulcus omasi into the abomasum as described previously( Reference Côrtes, Palin and Gagnon 23 ). Samples of diets and of flax hulls were collected daily from day 15 to day 20 and pooled within a period for each cow. Samples were frozen at − 20°C for subsequent drying at 55°C and analysed according to the procedures used by Côrtes et al. ( Reference Côrtes, da Silva-Kazama and Kazama 26 ). Milk samples were obtained from each cow from day 15 to day 21 and pooled on a yield basis. Some samples were kept frozen without a preservative for further analysis of milk FA profile, whereas some samples were stored at 4°C with a preservative (bronopol-B2) until analyses of protein, fat, urea N, lactose and total solids and somatic cell count.
Faecal output and digestibility were predicted by inserting a capsule of Cr2O3 into the rumen once daily at 09.00 hours from day 11 to day 20 (10 g Cr2O3/d). Oil infused in the abomasum was prepared daily for each cow and weighed into tarred bottles. Therefore, the exact amount of oil used for infusion was considered as intake for the determination of diethyl ether extract digestibility. Faecal grab samples were collected twice daily from day 15 to day 20 at 08.30 and 16.30 hours. Faecal samples were then processed as described by Côrtes et al. ( Reference Côrtes, Kazama and da Silva-Kazama 27 ). On day 20, ruminal contents were collected at 0, 1, 2, 4 and 6 h after the morning meal, and the pH was immediately monitored as described previously( Reference Côrtes, da Silva-Kazama and Kazama 26 ). The ruminal contents were then strained through four layers of cheesecloth, and the filtrate was acidified to pH 2 with 50 % H2SO4 and kept at − 20°C for the determination of volatile fatty acid (VFA) and NH3-N concentrations. On day 21, biopsies (approximately 800 mg) were taken from the upper portion of the mammary gland on the last day of each period as described previously( Reference Farr, Stelwagen and Cate 28 ).
Chemical analyses
DM content of the diets and faeces was determined in a forced-air oven according to procedure 934.01( 29 ). Total mixed dried diets and freeze-dried faeces were ground to pass through a 1 mm screen in a Wiley mill before analyses of total N, diethyl ether extract, ADF and NDF. The analyses of total N, diethyl ether extract, ADF and NDF were carried out as described previously by Côrtes et al. ( Reference Côrtes, da Silva-Kazama and Kazama 26 ). Faecal samples were analysed for chromium according to the procedure of Fenton & Fenton( Reference Fenton and Fenton 30 ). The concentrations of NH3-N and VFA in ruminal fluid were determined, respectively, using the indophenolblue method( Reference Novozamsky, Van Eck and Van Schouwenburg 31 ) and a HPLC Waters Alliance 2695 system (Waters) fitted with a flame-ionisation detector. The concentration of milk fat was determined by the method of Rose-Gottlieb( 29 ). The concentrations of protein, lactose and urea N in the milk samples were determined by IR spectrophotometry (System 4000 Milkoscan; Foss Electric) following procedure 972.16 of the Association of Official Analytical Chemists( 29 ). Milk fat was extracted and FA were methylated according to the method of Chouinard et al. ( Reference Chouinard, Lévesque and Girard 32 ), while in situ transesterification of the diets was carried out according to the method of Park & Goins( Reference Park and Goins 33 ). Individual FA were identified according to the procedures described by Côrtes et al. ( Reference Côrtes, da Silva-Kazama and Kazama 26 ) and trans-10 18 : 1 were coeluted with trans-11 18 : 1.
Real-time quantitative RT-PCR amplifications of the studied genes
Total RNA was extracted from biopsies using TRIzol Reagent (Invitrogen Life Technologies). The synthesis of complementary DNA and quantification of mRNA abundance in mammary gland biopsies were carried out as described previously by Labrecque et al. ( Reference Labrecque, Beaudry and Mayhue 34 ). Primer pairs were designed using the Primer express software 3.0 (PE Applied BioSystems). The studied genes were SREBPF1, FASN, LPL, PPARγ1, PPARγ2, SCD and ACACA. Table 1 summarises the primer sequences, GenBank accession numbers, amplified product sizes, primer concentrations used and amplification efficiencies (%) for all the studied genes. Moreover, three reference genes were used to identify the gene that was the least affected by the treatments. These reference genes were glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylproplyl isomerase A (PPIA) and actin β (ACTB). The ACTB gene was identified as the best reference gene for the normalisation of the data sets of the present study according to the NormFinder statistical algorithm( Reference Andersen, Jensen and Orntoft 35 ). Data were analysed using the comparative C t method, and amplification efficiencies were determined as described previously by Côrtes et al. ( Reference Côrtes, Palin and Gagnon 23 ).
Table 1 Primer sequences used for real-time quantitative PCR amplifications of the studied genes in mammary tissue

nt, Nucleotides; SREBF1, sterol regulatory element binding transcription factor 1; F, forward; R, reverse; FASN, fatty acid synthase; LPL, lipoprotein lipase; SCD, stearoyl-CoA desaturase (delta-9-desaturase); ACACA, acetyl-CoA carboxylase-α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PPIA, peptidylproplyl isomerase A; ACTB, actin β.
Statistical analysis
All the results were analysed using the MIXED procedure of SAS (SAS 2000; SAS Institute, Inc.) within a 2 × 2 factorial arrangement of treatments. Data on faecal output and feed intake were averaged over the 6 d of the digestibility week (e.g. day 15 to day 20) and subjected to ANOVA. Data on milk production, milk composition, mRNA abundance and feed intake were analysed using a replicated 4 × 4 Latin square design with the following general model:
where Y ijkl is the dependent variable, μ is the overall mean, S i is the fixed effect of square (i= 1–2), C j(i) is the random effect of cow within a square (j= 1–4), P k is the fixed effect of period (k= 1–4), H l is the fixed effect of hull treatment, O m is the fixed effect of oil treatment, HO lm is the interaction and e ijklm is the residual error. Data on ruminal fermentation and digestibility were analysed using a single 4 × 4 Latin square design. The model for ruminal fermentation characteristics (pH, VFA and NH3-N) was augmented with time and time × treatment interaction for repeated measurements, and values are reported with their adjusted mean values with their standard errors. The two-sided level of significance was set at P≤ 0·05, although probability values up to P< 0·1 are reported if the data suggest a trend. Results are reported as least squares means with their standard errors. Real-time quantitative RT-PCR data were analysed using normalised mRNA quantities (e.g. normalised with ACTB) and are presented as relative quantification of mRNA abundance using the comparative C t method and comparing treatments with CONT. The compound symmetry was used as the covariance structure.
Results
DM intake and digestibility
There was no interaction between flax hull supplementation and flax oil infusion for the intake of DM and digestibilities of DM, crude protein, ADF, NDF and diethyl ether extract (data not shown). The intake of DM and digestibilities of DM, crude protein, ADF and NDF were similar among the treatment groups. However, the intake of DM, expressed as a percentage of body weight, was lower (P= 0·05) in cows infused with flax oil. The digestibility of diethyl ether extract was higher (P= 0·03) in cows fed diets supplemented with flax hulls, whereas flax oil infusion had no effect.
Ruminal fermentation characteristics
There was no interaction between treatment and sampling time for ruminal fermentation characteristics. Therefore, only mean values for the 6 h ruminal sampling time are given in Table 2. Ruminal pH and NH3-N concentration were similar among the treatment groups. Flax hull supplementation had no effect on total VFA concentration and proportion of acetate, but it increased the proportion of propionate and decreased those of butyrate and valerate and the acetate:propionate ratio in the rumen. The proportion of isobutyrate tended (P= 0·06) to decrease with flax hull supplementation. Abomasal infusion of flax oil decreased the concentration of VFA and proportions of propionate and increased those of acetate, butyrate and isobutyrate in the rumen. Flax oil infusion increased the acetate:propionate ratio and decreased the propionate:(acetate+butyrate) ratio in the rumen, while flax hull supplementation increased the propionate:(acetate+butyrate) ratio. There was an interaction between flax hull supplementation and flax oil infusion for the proportions of valerate and isovalerate; the lowest and highest proportions of valerate and isovalerate were observed, respectively, in cows fed HULL and COFO.
Table 2 Ruminal fermentation characteristics of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g/d flax oil in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g/d flax oil in the abomasum (HUFO) (Mean values with their overall standard errors)
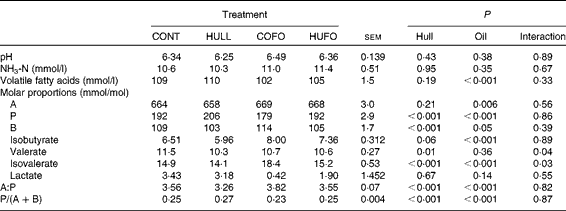
A, acetate; P, propionate; B, butyrate.
Milk production and milk composition
There was no interaction between flax hull supplementation and flax oil infusion for milk production and milk composition, except for the percentage of milk protein, which was lower in cows fed HULL than in those fed the other diets (Table 3). Flax hull supplementation had no effect on milk production, percentages of fat, lactose, urea N, and total solids in milk and yield of milk components. However, flax hull supplementation decreased the yield of milk protein. Abomasal infusion of flax oil decreased milk production and had no effect on the percentages of fat, lactose and total solids in milk, which resulted in lower yield of milk components. Milk urea N content (mg/l) was increased and somatic cell counts tended (P= 0·06) to increase with the infusion of flax oil in the abomasum.
Table 3 Milk production and milk composition of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g/d flax oil in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g/d flax oil in the abomasum (HUFO) (Mean values with their overall standard errors)
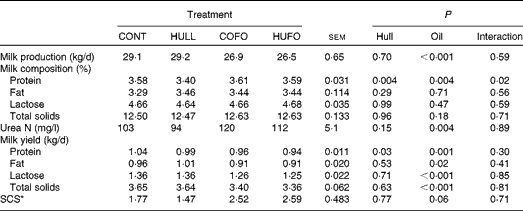
* Somatic cell score = log10(somatic cell count/ml).
Milk fatty acid profile
Flax hull supplementation decreased the proportions of SCFA (6 : 0, 7 : 0, 8 : 0, 9 : 0, 10 : 0, 11 : 0, 12 : 0, cis-11 12 : 1 and 13 : 0) in milk fat, with the exception of the proportions of 4 : 0 and 5 : 0, which were unaffected (Table 4). Abomasal infusion of flax oil decreased the proportions of 5 : 0 in milk fat and had no effect on those of other SCFA. Feeding HUFO and CONT resulted in the lowest and highest proportions of medium-chain FA (cis-9 14 : 1, 15 : 0 and 16 : 0) in milk fat, respectively, as a result of the interaction between flax hull supplementation and flax oil infusion. Cows fed CONT had the highest proportion of cis-9 16 : 1 in milk fat, but there was no difference in the proportion of cis-9 16 : 1 in milk fat between cows fed HULL and those fed COFO and between cows fed COFO and those fed HUFO. Flax hull supplementation increased the proportion of trans-9 16 : 1 in milk fat; however, abomasal infusion of flax oil had no effect.
Table 4 Fatty acid profile of milk fat (percentage of total fatty acids) of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g/d flax oil in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g/d flax oil in the abomasum (HUFO) (Mean values with their overall standard errors)

MCFA, medium-chain fatty acids; LCFA, long-chain fatty acids.
* 4 : 0+5 : 0+6 : 0+7 : 0+8 : 0+9 : 0+10 : 0+11 : 0+12 : 0+cis-11 12 : 1+13 : 0.
† 14 : 0+cis-9 14 : 1+15 : 0+16 : 0+cis-9 16 : 1+trans-9 16 : 1+17 : 0.
‡ 18 : 0+trans-9 18 : 1+trans-10+11 18 : 1+trans-13+14 18 : 1+cis-6+8 18 : 1+cis-9 18 : 1+cis-11 18 : 1+cis-9, cis-12 18 : 2+cis-9, trans-11 18 : 2+trans-9, trans-12 18 : 2+trans-10, cis-12 18 : 2+cis-6, cis-9, cis-12 18 : 3+cis-9, cis-12, cis-15 18 : 3+19 : 0+cis-7 19 : 1+20 : 0+cis-5 20 : 1+cis-8 20 : 1+cis-11 20 : 1+cis-11, cis-14 20 : 2+cis-11, cis-14 cis-17 20 : 3+cis-8, cis-11, cis-14 20 : 3+cis-5, cis-8, cis-11, cis-14 20 : 4+cis-5, cis-8, cis-11, cis-14, cis-17 20 : 5+22 : 0+cis-7, cis-10, cis-13, cis-16 22 : 4+cis-7, cis-10, cis-13, cis-16, cis-19 22 : 5+24 : 0.
§ cis-11 12 : 1+cis-9 14 : 1+cis-9 16 : 1+trans-9 16 : 1+trans-9 18 : 1+trans-10+11 18 : 1+trans-13+14 18 : 1+cis-6+8 18 : 1+cis-9 18 : 1+cis- 11 18 : 1+cis-7 19 : 1+cis-5 20 : 1+cis-8 20 : 1+cis-11 20 : 1.
∥ cis-9, cis-12 18 : 2+cis-9, trans-11 18 : 2+trans-9, trans-12 18 : 2+cis-6, cis-9, cis-12 18 : 3+cis-9, cis-12, cis-15 18 : 3+cis-11,14 20 : 2+cis-11, cis-14 cis-17 20 : 3+cis-8, cis-11, cis-14 20 : 3+cis-5, cis-8, cis-11, cis-14 20 : 4+cis-5, cis-8, cis-11, cis-14, cis-17 20 : 5+cis-7, cis-10, cis-13, cis-16 22 : 4+cis-7, cis-10, cis-13, cis-16, cis-19 22 : 5.
¶ 4 : 0+5 : 0+6 : 0+7 : 0+8 : 0+9 : 0+10 : 0+11 : 0+12 : 0+13 : 0+14 : 0+15 : 0+16 : 0+17 : 0+18 : 0+19 : 0+20 : 0+22 : 0+24 : 0.
All eighteen-carbon FA in milk fat were affected by the diets. There was an interaction between flax hull supplementation and flax oil infusion for the proportions of 18 : 0 and cis-9 18 : 1 in milk fat as well as a trend for the proportions of trans-13+14 18 : 1+cis-6+8 18 : 1, cis-9, cis-12 18 : 2 and trans-9, trans-12 18 : 2 (P= 0·09, 0·05 and 0·05 respectively). Flax hull supplementation increased the proportions of 18 : 0, trans-9 18 : 1, trans-11 18 : 1, trans-13+14 18 : 1+cis-6+8 18 : 1 and cis-9, trans-11 18 : 2 in milk fat, while abomasal infusion of flax oil had the opposite effect. There was an interaction between flax hull supplementation and flax oil infusion for the proportions of cis-9, cis-12, cis-15 18 : 3 and cis-11, cis-14, cis-17 20 : 3 in milk fat, which resulted in the highest proportions when cows were fed COFO and HUFO and the lowest when cows were fed CONT. On the other hand, cows fed CONT had the highest proportion of cis-6, cis-9, cis-12 18 : 3 in milk fat, and there was no difference between cows fed COFO, HULL and HUFO.
Expression of lipogenic enzymes in the mammary gland
The addition of flax hulls to the diet (HULL) increased (P< 0·05) the mRNA abundance of lipogenic genes in mammary tissue, with the exception of PPARγ2, which was not affected by the treatment (Table 5). Abomasal infusion of flax oil (COFO) up-regulated the mRNA abundance of FASN, LPL and ACACA in mammary tissue and decreased the mRNA levels of PPARγ2 and SCD when compared with CONT (P< 0·05). The addition of both flax hulls and flax oil to the diet (HUFO) increased the mRNA abundance of ACACA and PPARγ1 in mammary tissue and decreased the expression of LPL and SCD genes (P< 0·05).
Table 5 Relative quantification of the mRNA of lipogenic genes in the mammary gland of Holstein cows fed diets containing no flax hulls (CONT), 9·88 % flax hulls (HULL), no flax hulls and infused with 500 g/d flax oil in the abomasum (COFO) or 9·88 % flax hulls and infused with 500 g/d flax oil in the abomasum (HUFO)
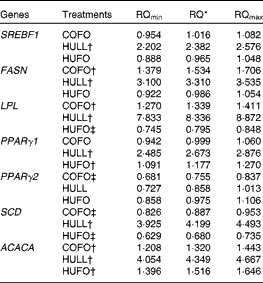
RQ, relative quantification; SREBF1, sterol regulatory element binding transcription factor 1; FASN, fatty acid synthase; LPL, lipoprotein lipase; SCD, stearoyl-CoA desaturase (delta-9-desaturase); ACACA, acetyl-CoA carboxylase-α.
* RQ of mRNA abundance using the comparative cycle threshold method and the control (CONT) treatment as reference. Values represent fold values relative to CONT with their corresponding RQmin and RQmax. Significant differences are observed when the range (RQmin–RQmax) does not include the value 1.
† Up-regulated genes (P< 0·05).
‡ Down-regulated genes (P< 0·05).
Discussion
PUFA have been shown to increase the peroxidation of tissues in sheep( Reference Gladine, Rock and Morand 2 ) and steers( Reference Scislowski, Bauchart and Gruffat 1 ). In the present study, flax oil was infused in the abomasum of cows to increase the susceptibility of their mammary tissue to lipoperoxidation. As another study( Reference Sies, Stahl and Sevanian 3 ) has suggested that antioxidants may provide protection against the oxidative effect of diets rich in fat, flax hulls were fed to dairy cows to determine the potential of this rich source of antioxidants to limit peroxidation in mammary tissue. Moreover, the fact that supplementation of flax hulls in the diet of dairy cows significantly increases the plasma concentration of enterolactone( Reference Côrtes, Palin and Gagnon 23 ), which is a strong antioxidant( Reference Prasad 20 ), clearly justifies looking at flax products as a source of antioxidants. The infusion of flax oil was associated with a 6-fold increase in the proportion of linolenic acid (cis-9, cis-12, cis-15 18 : 3) in milk fat, which is in agreement with previous observations made by Côrtes et al. ( Reference Côrtes, Kazama and da Silva-Kazama 27 ) in dairy cows that were infused with 250 or 500 g flax oil/d in the abomasum. This is also in agreement with the finding of an increase in the proportion of linolenic acid in the plasma of sheep infused with flax oil in the duodenum, which was related to a higher susceptibility of plasma lipids to lipoperoxidation( Reference Gladine, Rock and Morand 2 ).
In the present study, the lower intake of DM, expressed as a percentage of body weight, in cows infused with flax oil (COFO and HUFO) when compared with those not infused with flax oil (CONT and HULL) may be the result of a higher amount of fat reaching the small intestine as reported previously( Reference Côrtes, Kazama and da Silva-Kazama 27 ). Lower milk yield with the infusion of flax oil in the abomasum is in agreement with the results reported by Khas-Erdene et al. ( Reference Khas-Erdene, Wang and Bu 36 ), who infused a free FA mixture of linolenic acid, although the proportion of fat was not affected in the present study. The effects of flax hull supplementation and abomasal infusion of flax oil on milk FA profile were similar to those reported by Côrtes et al. ( Reference Côrtes, Kazama and da Silva-Kazama 27 ).
In the present study, higher total VFA concentration in cows not infused with flax oil was probably a result of higher numerical DM intake. The molar proportion of propionate increased with flax hull supplementation as reported previously by da Silva-Kazama et al. ( Reference da Silva-Kazama, Côrtes and Kazama 37 ). As flax hulls contain 54·3 % linolenic acid as a percentage of total FA, this FA may be responsible for these changes. It is unclear whether a change in the bacterial community or other mechanisms (e.g. change in substrate fermentation resulting from a lower intake of DM, expressed as a percentage of body weight) were responsible for the increased concentrations of butyrate and isovalerate in cows infused with flax oil. The lower molar proportions of acetate, butyrate and isobutyrate and higher proportion of propionate in cows not infused with flax oil in the abomasum were probably a result of numerical differences in DM intake.
In the present study, a higher mRNA abundance of SREBF1, FASN, LPL, PPARγ1, SCD and ACACA, but not of PPARγ2, was observed in cows fed HULL than in those fed CONT. As FASN and ACACA are known to be involved in de novo FA synthesis( Reference Harvatine, Boisclair and Bauman 38 ), up-regulation of these genes was unexpected. Indeed, according to the proportions of SCFA in milk fat, the lowest de novo synthesis of FA was observed in cows fed HULL. A similar decrease in the proportions of SCFA and medium-chain FA in milk fat with flax hull supplementation has been reported previously( Reference Côrtes, Kazama and da Silva-Kazama 27 ), thus reflecting reduced de novo FA synthesis. The FASN, LPL, SCD and ACACA genes are known downstream targets of SREBF1 and PPARγ transcription factors, both of which are involved in FA synthesis( Reference Harvatine, Boisclair and Bauman 38 , Reference Kadegowda, Bionaz and Piperova 39 ). In accordance with these studies, we found the mRNA levels of SERBF1 and PPARγ1 to be up-regulated in parallel with the mRNA abundance of FASN, LPL, SCD and ACACA. However, the reason for the lack of a treatment effect on the mRNA abundance of PPARγ2 remains to be determined. Nevertheless, rosiglitazone, a specific agonist of PPARγ, has been shown to up-regulate the expression of SREBF1, FASN, SCD and ACACA genes when added to bovine mammary epithelial cells( Reference Kadegowda, Bionaz and Piperova 39 ). However, the activation of PPARγ with rosiglitazone does not allow discriminating between the different PPARγ isoforms involved.
Flax hulls are a rich source of plant lignans, including the glycosides of secoisolariciresinol and matairesinol, which have strong antioxidant properties( Reference Mazur, Fotsis and Wähälä 16 ). In the rumen, plant lignans are mainly converted into the mammalian lignan enterolactone, a polyphenol metabolite( Reference Gagnon, Côrtes and Da Silva 17 ). The presence of these polyphenol compounds in dairy cows fed HULL may account for the increases in the levels of some lipogenic gene transcripts. For example, Chen et al. ( Reference Chen, Bezzina and Hinch 22 ) had previously observed a higher hepatic mRNA abundance of SREBF1, FASN and ACACA in rats fed green and black tea and higher mRNA levels of PPARγ in the adipose tissue of rats fed black tea or epigallocatechin-3-gallate, which are all polyphenol-rich compounds leading to enterolactone production. Similar increases in the mRNA abundance of LPL and SCD in the adipose tissue of rats have been observed with the addition of sesame lignans to the diet, whereas lignans have been found to reduce the mRNA levels of ACACA in the liver( Reference Ide, Lim and Odbayar 9 ).
The increase in the mRNA abundance of SREBF1, FASN, LPL, PPARγ1, SCD and ACACA observed when cows were fed only flax hulls (HULL) was affected by the infusion of flax oil. Indeed, compared to cows fed the CONT diet, the increase in mRNA abundance of genes observed when only hulls were supplemented was changed for a decrease (LPL and SCD), moderate increase (PPARγ1 and ACACA) and lack of effect (SREBF1 and FASN) on mRNA abundance when hulls were combined with infusion of oil with the HUFO diet. Therefore, infusion of flax oil in the abomasum of cows that were fed flax hulls contributed to the down-regulation of the expression of lipogenic genes. This is in agreement with several studies that have reported a down-regulation of the expression of lipogenic genes with the addition of PUFA. For example, the transcriptional activity of SREBF1 promoter has been reported to be down-regulated in rat hepatocytes incubated with linolenic acid, γ-linolenic acid and EPA( Reference Deng, Cagen and Wilcox 40 ). Moreover, the addition of PUFA to HEK-293 cells has also been shown to result in lower mRNA levels of SREBF1 ( Reference Hannah, Ou and Luong 41 ), and rats fed n-6 or n-3 PUFA have been found to exhibit a reduced mRNA abundance of both SREBF1 and FASN in hepatic tissue( Reference Xu, Nakamura and Cho 42 ).
When flax oil was infused in the abomasum of cows fed CONT (e.g. COFO treatment), the mRNA abundance of SREBF1 and PPARγ1 transcription factors was not affected by flax oil infusion, whereas the transcript abundance of PPARγ2 was down-regulated. Conversely, when compared with cows fed CONT, cows fed COFO exhibited an increased expression of FASN and ACACA genes, which are involved in de novo FA synthesis. This was unexpected, as these two genes are known downstream targets of SERBF1 and PPARγ transcription factors, and flax oil infusion had no effect on the proportions of SCFA, which are synthesised de novo in mammary tissue, in milk fat. A possible explanation may be that the effects of dietary FA on SREBF1 and PPARγ are mediated through changes in activity rather than changes in transcript abundance. Interestingly, Bernard et al. ( Reference Bernard, Leroux and Faulconnier 43 ) observed a reduction in the de novo synthesis of SCFA in the milk fat of goats fed sunflower oil or linseed oil, and the reduction was found to be independent of the mRNA expression of ACACA and FASN. This suggests that the expression of ACACA and FASN gene is not always related to the secretion of SCFA in milk fat. The present results are in contrast with those reported by Ahnadi et al. ( Reference Ahnadi, Beswick and Delbecchi 44 ), who observed a decrease in the proportions of SCFA in milk fat and mRNA abundance of ACACA, FASN, SCD and LPL in the mammary tissue of dairy cows fed a diet supplemented with fish oil; however, the effect of fish oil on the mRNA abundance of LPL and SCD was dependent on the protection provided by oil against biohydrogenation by rumen microbes (e.g. unprotected v. glutaraldehyde-protected (protection level >90 %) fish oil). In another study( Reference Bernard, Rouel and Leroux 45 ), goats fed formaldehyde-treated linseed were found to exhibit a higher expression of LPL in mammary tissue compared with those fed a control diet, whereas the expression of SCD was reduced and that of FASN and ACACA was not affected.
Compared with cows fed CONT, cows fed COFO and HUFO had a lower mRNA abundance of SCD in mammary tissue. Similar reductions in the mRNA levels of SCD in the mammary tissue of goats fed formaldehyde-treated linseed or oleic sunflower oil have been reported( Reference Bernard, Rouel and Leroux 45 ). Cows fed rumen-protected fish oil have also been shown to have reduced mRNA abundance of SCD in mammary tissue( Reference Ahnadi, Beswick and Delbecchi 44 ). As SCD is involved in the synthesis of MUFA such as cis-9 16 : 1, cis-9 18 : 1 and the cis-9, trans-11 18 : 2 conjugated linoleic acid( Reference Corl, Baumgard and Dwyer 46 ), this may partly explain the lower proportions of MUFA in the milk fat of cows infused with flax oil in the abomasum. However, a decrease in the proportions of MUFA could also be due to a dilution effect, as the proportions of PUFA in milk fat were increased by more than 10 %. The ratios of cis-9 14 : 1/14 : 0, cis-9 16 : 1/16 : 0, cis-9 and trans-11 18 : 2/trans-11 18 : 1, which can be used as a proxy of SCD activity( Reference Bernard, Leroux and Chilliard 47 ), also decreased with the infusion of flax oil in the abomasum. Altogether, these results suggest that PUFA bypassing the rumen decrease the transcript abundance of SCD (as shown in the present study) and the activity of SCD( Reference Ahnadi, Beswick and Delbecchi 44 ) in the mammary tissue of dairy cows, which lower the proportions of MUFA in milk fat as observed in the present study.
The higher mRNA abundance of LPL in mammary tissue and proportions of long-chain FA in the milk fat of cows fed the COFO than in those fed CONT may reflect a higher need for LPL enzyme in blood TAG uptake with an increase in the levels of dietary PUFA. Similar increases in the mRNA abundance and activity of mammary LPL and proportions of long-chain FA in milk fat have been observed in goats fed formaldehyde-treated oleic sunflower oil( Reference Bernard, Rouel and Leroux 45 ). However, the addition of unprotected flax oil or sunflower oil has been found to have no effect on the activity and mRNA abundance of LPL in the mammary tissue of goats( Reference Bernard, Leroux and Faulconnier 43 ). These results suggest that the biohydrogenation of PUFA by the ruminal microbiota affects the transcript abundance and activity of the LPL gene.
Flax hulls contain 29·8 % lipids and high proportions of PUFA, which may also affect the expression of lipogenic genes( Reference Prado and Saldana 48 ). In the present study, FA from flax hulls were subjected to biohydrogenation by the rumen microbiota, while FA from flax oil bypassed the rumen (COFO and HUFO). With HUFO, PUFA originating from flax hulls were subjected to biohydrogenation, while those present in flax oil were not, which resulted in the highest proportions of total trans FA in milk fat in cows fed HULL followed by those fed HUFO. Moreover, the highest proportions of biohydrogenation intermediates, such as trans-11 18 : 1, trans-9 18 : 1, cis-9, trans-11 18 : 2 and trans-9, trans-12 18 : 2, were found in the milk fat of cows fed HULL. Therefore, differences in biohydrogenation intermediates observed in milk fat may account for some of the differences in the mRNA abundance of lipogenic genes when cows were fed COFO or HUFO v. CONT. Indeed, several studies have established that biohydrogenation intermediates can affect lipogenesis in mammary tissue and modulate the expression and activity of lipogenic genes( Reference Shingfield, Bernard and Leroux 49 , Reference Mach, Goselink and van Baal 50 ).
Acknowledgements
In conclusion, the results of the present study indicated that the mRNA levels of SERBF1 and PPARγ1 were up-regulated in parallel with the mRNA abundance of FASN, LPL, SCD and ACACA when cows were fed flax hulls, corroborating that the FASN, LPL, SCD and ACACA genes are the downstream targets of SREBF1 and PPARγ transcription factors. However, when compared with cows fed the control diet, cows fed the control diet with the infusion of oil exhibited an increased expression of FASN and ACACA genes, which was unexpected. Therefore, the measurement of activity rather than that of the abundance of transcripts may be more important to determine the effects of dietary FA on genes involved in lipogenesis. The present study shows that flax hulls with or without flax oil bypassing the rumen can affect the expression of lipogenic genes in the mammary tissue of dairy cows. However, more information is required to better predict which constituent of flax hulls and flax oil is responsible for the observed effects on lipogenic gene expression and milk FA profile.
C. C. was a recipient of a fellowship from the National Science and Engineering Research Council of Canada. The present study was funded by Agriculture and Agri-Food Canada, which was aware of, but did not influence the trial design and had no role in the data analysis and interpretation.
The authors express their gratitude to the staff of the Dairy and Swine Research and Development Centre for their contribution to the present study. They cordially thank Véronique Roy, Liette Veilleux, Danielle Beaudry and Sylvie Dallaire for their technical assistance and Steve Méthot for his help in the statistical analysis.
The authors' contributions are as follows: M.-F. P., C. C. and H. V. P. drafted the manuscript; H. V. P. and M.-F. P. conceived and directed the study; C. C. coordinated the study and was in charge of the infusions and of collecting data from animals; C. B. contributed to the conception and design of the experiment and to the interpretation and discussion of the results; P. L. carried out the mammary biopsies. All the authors were involved in the revision of the paper and approved the final version of the paper.
None of the authors has a personal or professional conflict of interest.







