Neuroticism refers to a relatively stable personality trait that is characterized by a tendency to respond with negative emotions to threat, frustration or loss (Costa & McCrae, Reference Costa and McCrae1987). High-neuroticism has been associated with economic costs (Cuijpers et al., Reference Cuijpers, Smit, Penninx, de Graaf, ten Have and Beekman2010) and prospectively linked with both mental and physical disorders (Lahey, Reference Lahey2009). More specifically, neuroticism has been linked to common mental disorders such as anxiety and depression (Ormel et al., Reference Ormel, Jeronimus, Kotov, Riese, Bos, Hankin and Oldehinkel2013b) and physical disorders such as cardiovascular disease (Suls & Bunde, Reference Suls and Bunde2005), atopic eczema (Buske-Kirschbaum et al., Reference Buske-Kirschbaum, Geiben and Hellhammer2001), and ultimately, mortality (Terracciano et al., Reference Terracciano, Löckenhoff, Zonderman, Ferrucci and Costa2008; Wilson et al., Reference Wilson, Krueger, Gu, Bienias, Mendes de Leon and Evans2005).
In spite of its established association with health and disease, only limited knowledge of the etiology of neuroticism is available (Ormel et al., Reference Ormel, Bastiaansen, Riese, Bos, Servaas, Ellenbogen and Aleman2013a). In order to clarify its biological basis, neuroticism has been previously linked with deregulation in two major stress axis: the autonomic nervous system, and the hypothalamic-pituitary-adrenal (HPA) axis and other underlying biological pathways. However, in their review, Ormel and colleagues (2013) did not review papers on the relationship between neuroticism and deregulation of immunological mechanisms and/or inflammatory markers.
This is remarkable, as inflammatory markers such as neuroticism have been linked to a variety of mental and physical disorders (as mentioned above). More specifically, higher levels of neuroticism have been (prospectively) associated with increased serum levels of C-reactive protein (CRP) and interleukin (IL)-6 (McManus, Reference McManus, Beaulieu, Mick, Tanriverdi, Larson, Keaney, Benjamin and Freedman2013, Turiano et al., Reference Turiano, Mroczek, Moynihan and Chapman2013), as well as higher leukocyte counts (Daruna, Reference Daruna1996; Sutin et al., Reference Sutin, Terracciano, Deiana, Naitza, Ferrucci, Uda and Costa2010). However, another study found no evidence for a direct association between neuroticism and the inflammatory markers CRP, IL-6 and fibrinogen (Millar et al., Reference Millar, Lloyd, McLean, Batty, Burns, Cavanagh and Tannahill2013). Thus, the literature on the relationship between neuroticism and inflammatory markers is inconsistent.
To the best of our knowledge, no study has examined the shared genetic background of neuroticism and baseline levels of inflammatory markers, although neuroticism scores and the majority of commonly used immunological markers are both found to be substantially heritable (Heath et al., (Reference Heath, Jardine, Eaves and Martin1989), Su et al., Reference Su, Snieder, Miller, Ritchie, Bremner, Goldberg and Vaccarino2008, Reference Su, Miller, Snieder, Bremner, Ritchie, Maisano and Vaccarino2009). In order to assess these potentially shared (genetic) influences, in the present study we will investigate the phenotypic relationship between neuroticism and three commonly used inflammatory markers: CRP, fibrinogen, and Immunoglobulin-G (IgG). Using a classical twin study design, we hypothesize that the markers are heritable, and may (partly) share their genetic influences with neuroticism.
Material and Methods
Participants
This study is part of the Twin Interdisciplinary Neuroticism Study (TWINS) in which the genetic and environmental origins of neuroticism are studied. For this purpose, in 2002 (T1) the Groningen Twin Register (GTR) was established. A full description of the sample selection and procedures has been published before (Riese et al., Reference Riese, Rijsdijk, Snieder and Ormel2013). In short, in 2002 (T1), 1,047 participants of the GTR participated in a survey. The survey included, among others, a neuroticism questionnaire. From the GTR, neuroticism data of 206 female twin pairs were used in the statistical analyses of the current study. As gender differences in both mean level as well as variance of neuroticism are well established, including both men and women in our experimental sessions would have implied that gender needed to be included as a covariate in our statistical analyses, or statistical analysis had to be stratified for gender. Both statistical procedures would have resulted in less power in our statistical analyses. We therefore a priori decided to only include female twin pairs in the experimental session (and repeated the measurement of our core variable neuroticism multiple times). A subsample of 125 female twin pairs between 18 and 30 years from the GTR participated in TWINS in 2003/2004 (T2). TWINS participants did not differ from the other eligible women of the GTR, in age or neuroticism as assessed at T1. At T2, the subgroup of 125 twin pairs participated in a laboratory experiment in which additional neuroticism measures, CRP, fibrinogen and IgG data, information about smoking habits and oral contraceptive use were collected and body weight and height were assessed. All participants reported to be in good physical and mental health at T2. Zygosity was assessed by questionnaire (Nichols & Bilbro, Reference Nichols and Bilbro1966), and DNA samples. The study was approved by the Ethics Committee of the University Medical Center Groningen, and all participants gave written consent prior to participation.
Neuroticism
At T1, neuroticism was measured with the neuroticism subscale of the NEO-Five Factor Inventory (NEO-FFI) inventory (Costa & McCrae, Reference Costa and McCrae1992, Hoekstra et al., Reference Hoekstra, Ormel and De Fruyt1996). At T2, neuroticism was measured again in three different ways: (a) self report using the short form of the Eysenck Personality Questionnaire (Sanderman et al., Reference Sanderman, Eysenck and Arrindell1991), (b) self report using the NEO-FFI inventory (Costa & McCrae, Reference Costa and McCrae1992, Hoekstra et al., Reference Hoekstra, Ormel and De Fruyt1996), and (c) co-twin report using the NEO-FFI inventory (Costa & McCrae, Reference Costa and McCrae1992, Hoekstra et al., Reference Hoekstra, Ormel and De Fruyt1996) in order to adjust for self-report bias in neuroticism (descriptive data for these scales have been published previously in Riese et al., Reference Riese, Rosmalen, Ormel, van Roon, Oldehinkel and Rijsdijk2007). To simplify the analyses while maximizing the usefulness of all available information, for each individual a composite score was generated by the LAVASE program (Campbell et al., Reference Campbell, Rijsdijk and Sham2007) using the correlational structure of the four neuroticism scales to account for both rater bias and zygosity misclassification of twin pairs. Comparable models have shown a substantial decrease in variance attributed to individual-specific environment (including measurement error) and a proportional increase in heritability (Kendler et al., Reference Kendler, Gardner and Prescott2002). The neuroticism composite score (Ncomp) was available for 206 female twin pairs (115 monozygotic [MZ] and 91 dizygotic [DZ] pairs).
Biochemical Marker Measurement
Venous blood samples for inflammatory marker analysis were collected after an overnight fast at T2. Plasma fibrinogen was assessed using commercially available Trombin Reagent kits (Dade Behring, Marburg, Germany). The coefficient of variation (CV) range for this assay was 3.8–7.1% (within run) and 0.0–2.4% (between run) over 8 samples, reproduced 5 times. IgG was assessed using N antisera to Human Immunoglubulins (Dade Behring, Marburg, Germany). CV range for this assay was 1.8–3.0% (within run) and 1.4–2.1% (between run) over 8 samples, reproduced 5 times. CRP was assessed using the CardioPhase® hsCRP kit (Siemens Healthcare Diagnostics inc., The Hague, Netherlands). CV range was 2.1–4.6% (within run) and 1.1–4.0% (between run) over 8 samples, reproduced 5 times. The main principle of these kits is the aggregation of the inflammatory marker with specific antigens in the kit, thereby forming antigen-antibody complexes. By measuring the scattering of a beam of light through the sample, a serum concentration (proportional to the light scatter) can be estimated. All assays were performed according to the manufacturer's specifications.
For 3 participants no valid blood samples were available, leaving 247 samples for statistical analyses. Of these, 11 CRP-results were below the assays detection limit (0.16 mg/L) and therefore excluded. Additional robustness analyses after imputing random values between 0 and 0.16 for these missing values gave slightly lower point estimates for variance components and correlations as reported in the results section, but did not result in different conclusions as presented in the current study. For 22 subjects CRP values were above 10 mg/L. These values are assumed to reflect clinical inflammation and were therefore excluded from the final analysis (Rahman et al., Reference Rahman, Bennet, Pedersen, de Faire, Svensson and Magnusson2009; Su et al., Reference Su, Snieder, Miller, Ritchie, Bremner, Goldberg and Vaccarino2008). Fibrinogen and IgG measurements were also excluded for these subjects, leaving data of 214 participants for the final statistical analyses.
Preparation of the Data
Data distributions were checked prior analyses in SPSS (SPSS for Windows, Version 16.0. Chicago, USA).
CRP and IgG data were log-transformed to obtain a better approximation of a normal distribution. Linear regression analysis was used to create residual scores adjusted for potential confounding influences on inflammatory markers. Residual scores were used in the twin modeling analyses. General Estimating Equations analyses were used to test for significant differences in Ncomp-score, inflammatory markers, age, and BMI, smoking and oral contraceptive (OC) use between MZ and DZ twin pairs.
Statistical Analyses
Twin modeling. The classical twin model allows estimation of the effects of (latent) genetic and environmental factors on the variance of an observed trait. The power to estimate these variance components is derived by the differential predictions of the covariance (or correlation) of the trait among MZ and DZ twin pairs. MZ pairs correlate 1 for the additive genetic component (A), whereas DZ pairs correlate 0.5, as they share, on average, only 50% of their genes. However, both MZ and DZ pairs correlate 1 for the shared environmental component (C) and both are uncorrelated for the unshared environmental component (E), which also includes measurement error. Assuming that MZ and DZ twins experience the same degree of similarity in their environments, a higher MZ than DZ twin correlation is interpreted as caused by the greater proportion of genes shared by MZ twins allowing estimation of A. An estimate for C is given by the difference in MZ correlation and the estimated effect of A. The phenotypic differences between MZ twins can only be due to E (Neale & Cardon, Reference Neale and Cardon1992). When measuring multiple traits in each twin, the logic of the twin model can be extended. Significant phenotypic correlations between traits within twins suggest a common etiology. Significant cross-trait cross-twin correlations suggest that the common etiology is familial. The ratio of the MZ and DZ cross-trait cross-twin correlations indicates to what extent the common etiology is genetic or environmental in origin: a 2:1 ratio suggests the effects of A, whereas a 1:1 ratio suggests the effects of C. Non-significant cross-trait cross-twin correlations suggest that the common etiology is due to E (Neale & Cardon, Reference Neale and Cardon1992). Thus, when more than one trait is measured in each twin, the model can be extended to a multivariate case, in which the cross-trait cross-twin correlations of the MZ and DZ pairs provide the additional information to partition the phenotypic correlation between variables within individuals into A, C and E components. In this case, estimates are derived from a set of bivariate ACE Cholesky decompositions (Neale & Cardon, Reference Neale and Cardon1992) performed in the Mx program (Neale et al., Reference Neale, Boker, Xie and Maes2003).
Analyses were run three times. First, the raw inflammatory marker data was used for twin modeling. Second, prior modeling, the marker data were adjusted for age. This is a common procedure in twin analyses because age can spuriously introduce a C effect if there is a significant correlation between the phenotype and age, because twins of a twin pair are always of the same age. Third, prior modeling, the marker data were additionally adjusted for body mass index (BMI), smoking habits (yes/no) and OC use. In all models, the Ncomp score was adjusted for age. Due to the small differences in point estimates and largely overlapping confidence intervals between the different models, only the results of the analyses on age-corrected data are presented.
Results
Baseline characteristics of MZ and DZ-twins are given in Table 1. Prevalence of smoking and OC use was higher among MZ twins compared to DZ twins. MZ and DZ twins did not differ on age, BMI, their neuroticism scores, and levels of the inflammatory markers CRP, fibrinogen and IgG.
TABLE 1 Baseline Characteristics
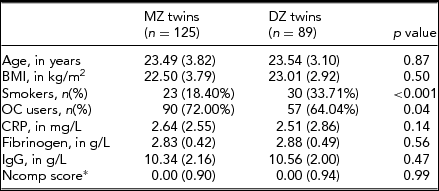
General characteristics of studied subjects by zygosity (mean (±SD), unless indicated otherwise).* Ncomp-score was available for n = 230 MZ-twins and n = 182 DZ-twins. MZ = monozygotic; DZ = dizygotic; BMI = body mass index; OC = oral contraceptives; CRP = C-reactive protein; IgG = Immunoglobulin-G; Ncomp = neuroticism composite score (see method section for details).
In Table 2, within-trait cross-twin correlations, phenotypic cross-trait (Ncomp vs. the inflammatory markers) correlations and cross-trait cross-twin correlations (for MZ and DZ twins separately) for neuroticism and the inflammatory markers are given. Point estimates of the phenotypic cross-trait or cross-twin cross-trait-correlations were low and not significant (all confidence intervals included the value zero). No phenotypic correlations were found between Ncomp and any of the inflammatory markers.
TABLE 2 Twin Correlations for the Neuroticism Composite Score and Inflammatory Markers
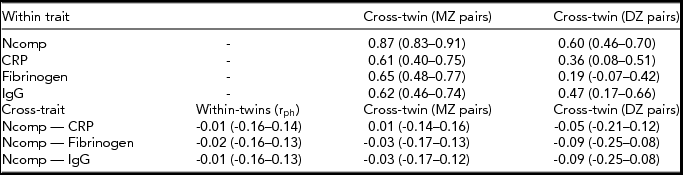
In the upper panel, within-trait cross-twin correlations (95% CI) for the neuroticism composite score and the three inflammatory markers are given. In the lower panel, the phenotypic cross-trait correlations (rph), 95% CI), and cross-trait cross-twin correlations for monozygotic and dizygotic twin pairs separately are given. Ncomp = neuroticism composite score (see method section for details); CRP = C-reactive protein; IgG = immunoglubulin-G; MZ = monozygotic; DZ = dizygotic.
In the upper panel of Table 3, standardized parameter estimates of the contribution of additive genetic, shared environmental and unique environmental components on the Ncomp score and inflammatory markers are given. Heritability's are fair for Ncomp (0.55), CRP (0.52) and fibrinogen (0.67) and moderate for IgG (0.43). A significant shared environmental influence (c2) of 33% on the neuroticism composite score was found. No significant contribution of c2 was observed for the inflammatory markers. Additional testing for genetic dominance (d2) of fibrinogen yielded no significant dominant effects (Δχ2 = 0.529, p = .47). The genetic shared environmental and non-shared environmental correlations between Ncomp and the inflammatory markers were not significant (lower panel of Table 3; all confidence intervals included the value zero).
TABLE 3 Standardized Variance Components for the Neuroticism Component Score and Inflammation Measures and the Genetic, Shared Environment and Non-Shared Environmental Correlation Between Them
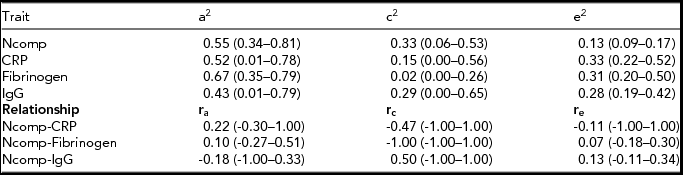
In the upper panel, standardized parameter estimates (95% CI) of the contribution of genetic (a2), shared environmental (c2) and non-shared environmental (e2) influences on the neuroticism composite score and the inflammation measures are given. In the lower panel, the genetic (ra), shared environment (rc) and non-shared environmental (re) correlations (95% CI) between the neuroticism composite score and the inflammation measures are given. Ncomp = neuroticism composite score (see method section for details); CRP = C-reactive protein; IgG = immunoglubulin-G; MZ = monozygotic; DZ = dizygotic.
Post-hoc fitting of interaction models in which interaction of neuroticism on the variance components of the inflammatory markers was calculated (Riese et al., Reference Riese, Rijsdijk, Rosmalen, Snieder and Ormel2009) yielded no significant moderation of these components by Ncomp.
Discussion
In the present study no phenotypic or genetic correlations between neuroticism and the inflammatory markers were found. We observed substantial heritabilities for all traits, which are in line with previous findings in the literature (e.g., neuroticism: 0.43–0.59 (e.g., Rettew et al., Reference Rettew, Vink, Willemsen, Doyle, Hudziak and Boomsma2006, Wray et al., Reference Wray, Birley, Sullivan, Visscher and Martin2007), CRP: 0.22–0.76 (e.g., Su et al., Reference Su, Miller, Snieder, Bremner, Ritchie, Maisano and Vaccarino2009; Wörns et al., Reference Wörns, Victor, Galle and Höhler2006), and fibrinogen: 0.34–0.52 (Bladbjerg et al., Reference Bladbjerg, de Maat, Christensen, Bathum, Jespersen and Hjelmborg2006; Su et al., Reference Su, Snieder, Miller, Ritchie, Bremner, Goldberg and Vaccarino2008). To our knowledge, we are the first to report on the heritability of IgG.
The lack of phenotypic correlations in the present study is in contrast with a recent study in a large Sardinian population of 4,923 individuals in which higher levels of IL-6 were associated with higher scores on neuroticism (Sutin et al., Reference Sutin, Terracciano, Deiana, Naitza, Ferrucci, Uda and Costa2010). When looking at the wide 95% CIs for the point estimates of the phenotypic correlations, we could assume that our twin sample might have been too small to pick up these effects as significant. The same argument might be true for the lack of a genetic association. This is despite assessing the key variable of our TWINS study, neuroticism, four times to increase the statistical power. However, it has been suggested that the neuroticism trait itself may be too broad and heterogeneous, and that focusing on more homogenous lower order facets of neuroticism (e.g., impulsiveness or self-consciousness; Ormel et al., Reference Ormel, Bastiaansen, Riese, Bos, Servaas, Ellenbogen and Aleman2013a) may have revealed existing relationships.
An alternative explanation is the potential lack of stability of the inflammatory markers as this would have had implications for the interpretation and expectation of research findings regarding its association with neuroticism. However, the MZ cross-twin correlations of the inflammatory markers can be considered as a lower bound of the measurement reliability within the same individual. As all markers showed reasonably large correlations (>0.60) it is unlikely that instability of the measurements would have had a major effect.
On the other hand, is it plausible that the present findings are realistic. Prior studies with null findings may not have been published, possibly due to publication bias. This is supported by a study in a population sample of 666 men and women that found no relationship between neuroticism and the inflammatory markers, CRP and fibrinogen (Millar et al., Reference Millar, Lloyd, McLean, Batty, Burns, Cavanagh and Tannahill2013). An alternative explanation is that a relationship between neuroticism and inflammatory markers is only present in individuals in the acute phase of a mental disorder. This view is in line with findings in a large sample of persons (18–65 years) with current and remitted anxiety disorders (a disorder closely related to high neuroticism) and healthy controls (Vogelzangs et al., Reference Vogelzangs, Beekman, de Jonge and Penninx2013). In this study, men with a current anxiety disorder had somewhat increased levels of CRP. Moreover, elevated inflammation in particular was found in those men and women with a late onset of an anxiety disorder (between ages 50–65).
In the present study, only data of healthy premenopausal women were assessed. The benefit of this homogenous sample is that the results cannot be confounded by gender or a wide age range, since these covariates have previously been shown to have significant effects (Sutin et al., Reference Sutin, Terracciano, Deiana, Naitza, Ferrucci, Uda and Costa2010). A limitation of this strategy, however, is that our conclusions are not generalizable to men, older subjects or subjects with somatic or mental diseases.
The present study shows that in healthy young women there is no evidence for a shared (genetic) predisposition or the presence of possible pleiotropic effects of neuroticism and the inflammatory markers CRP, fibrinogen and IgG, meaning that although high neuroticism and plasma levels of the studied inflammatory markers can lead to similar unfavorable health outcomes, the underlying pathways for these two risk markers should be considered as independent of each other.
Acknowledgments
This research is part of the Twin Interdisciplinary Neuroticism Study (TWINS) supported by the Netherlands Organisation for Health Research and Development (ZonMw 904-57-130).





