Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairment of social communication and repetitive/restrictive behaviours (Lord et al., Reference Lord, Elsabbagh, Baird and Veenstra-Vanderweele2018). Notably, 40–80% of patients with ASD suffer from sleep alterations, which is a significantly higher rate compared with their typically developing (TD) counterparts (Cohen et al., Reference Cohen, Conduit, Lockley, Rajaratnam and Cornish2014). Sleep alterations in ASD could be partly explained by its underlying neurobiology. Accumulated evidence has supported lower melatonin levels in individuals with ASD, and a previous review found that disturbance of sleep/circadian rhythm was associated with the progression of ASD (Wu et al., Reference Wu, Huang, Zou, Wang, Naveed, Bao, Wang, Fukunaga and Han2020). Moreover, the overlaps of sleep maturation and synaptic physiology are believed to play a patho-etiological role in sleep alterations in various neurodevelopmental disorders, including ASD (Crocker and Sehgal, Reference Crocker and Sehgal2010; Gaetz et al., Reference Gaetz, Bloy, Wang, Port, Blaskey, Levy and Roberts2014; Veatch et al., Reference Veatch, Maxwell-Horn and Malow2015; Veenstra-VanderWeele et al., Reference Veenstra-VanderWeele, Muller, Iwamoto, Sauer, Owens, Shah, Cohen, Mannangatti, Jessen, Thompson, Ye, Kerr, Carneiro, Crawley, Sanders-Bush, McMahon, Ramamoorthy, Daws, Sutcliffe and Blakely2012).
Sleep alterations in patients with ASD are associated with core symptoms and problematic behaviours. A recent study reported that short sleep duration showed a strong relationship with patients’ tendency to fail in developing peer relationships (Veatch et al., Reference Veatch, Sutcliffe, Warren, Keenan, Potter and Malow2017), which supports the statement that sleep alterations are associated with impairment in social function in ASD (Richdale and Schreck, Reference Richdale and Schreck2009; Schreck et al., Reference Schreck, Mulick and Smith2004). Internalizing and externalizing behavioural problems have also been found to be strongly correlated with sleep alterations in ASD (Adams et al., Reference Adams, Matson and Jang2014; Goldman et al., Reference Goldman, McGrew, Johnson, Richdale, Clemons and Malow2011; Henderson et al., Reference Henderson, Barry, Bader and Jordan2011; Mayes and Calhoun, Reference Mayes and Calhoun2009; Schreck et al., Reference Schreck, Mulick and Smith2004; Sikora et al., Reference Sikora, Johnson, Clemons and Katz2012). Therefore, sleep alterations in these individuals may substantially affect the quality of life of patients and their caregivers (Levin and Scher, Reference Levin and Scher2016).
To appropriately manage these problems, it is necessary to elucidate the characteristics of sleep alterations in children and adolescents with ASD. Numerous primary studies including meta-analysis have investigated sleep alterations in ASD using objective measures such as actigraphy, polysomnography and other subjective measures (Chen et al., Reference Chen, Liu, Wu, Xuan, Zhao and Sun2021; Díaz-Román et al., Reference Díaz-Román, Zhang, Delorme, Beggiato and Cortese2018; Elrod and Hood, Reference Elrod and Hood2015). However, previous analyses included studies that enrolled patients with ASD taking psychotropic drugs. Considering that psychotropic drugs have various effects on sleep, their results may not represent the genuine characteristics of sleep alterations in children and adolescents with ASD. In this regard, the present study aims to explore sleep alterations in medication-naïve children and adolescents with ASD compared with TD controls. We meta-analysed each of the various sleep parameters of actigraphy, polysomnography and other subjective measures to (1) accurately elucidate sleep alterations in this population and (2) generate results that could be compared with previous meta-analyses that included studies enrolling patients with ASD prescribed with psychotropic medications.
Methods
Protocol, registration and study design
We performed a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Supplementary Appendix pp 4–7) (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021). The protocol was registered with PROSPERO (CRD42021243881) with the amendment for addressing reviewers’ comment (Supplementary Appendix p 13). The entire process of screening, data extraction and methodological appraisal of all the included articles was performed by at least two independent authors (HK, JHK and JK).
Search strategy and selection criteria
We systematically searched PubMed/Medline, Embase and Web of Science from database inception to March 22, 2021, without any language restrictions. Full search strategies for each database are presented in Supplementary Appendix p 8. To identify eligible articles, two independent authors (JHK and JK) screened titles, abstracts and full texts sequentially (Fig. 1). We also manually searched references of the relevant studies to find further eligible articles. Any disagreements were resolved by discussion with the other authors (HK, JIS and KAC).

Figure 1. Literature search process.
We included studies that met the following inclusion criteria: observational studies that compared the objective (i.e., actigraphy or polysomnography) and/or subjective sleep measures (e.g., Children’s Sleep Habits Questionnaire [CSHQ], Sleep Disturbance Scale for Children [SDSC], and Pediatric Sleep Questionnaire [PSQ]) between ASD patients and TD individuals, studies that clearly stated that patients were medication-naïve and studies that included child or adolescent patients only (age < 18 years). The ASD diagnosis was operationalized according to any version of the International Classification of Diseases, Diagnostic and Statistical Manual of Mental Disorders, Autism Diagnostic Interview or Autism Diagnostic Observation Schedule.
We excluded studies that met the following criteria: studies that did not examine sleep measures in ASD patients, studies that included patients who took medication, studies that did not compare the outcome with TD individuals, studies that did not provide enough data for analysis, studies with intervention, non-human and genetic studies and conference abstracts.
Data extraction
Two independent authors (JHK and JK) performed data extraction, and any discrepancies were resolved by discussion with the third author (HK). The following data were extracted from each eligible observational study: name of the first author, publication year, the country where the study was conducted, details of the study (number of participants, mean age and corresponding standard deviation [SD], percentage of boys, diagnostic criteria for ASD and comorbidity for intellectual disability), mean and SD for each outcome measure (polysomnography, actigraphy and other subjective measures) and nights recorded for assessment.
Methodological quality assessment
The quality of eligible articles was assessed independently by two authors (JHK and JK) based on Newcastle-Ottawa Scale (NOS) because all included studies were of a non-randomized design (Wells et al., Reference Wells, Shea, O’Connell, Peterson, Welch, Losos and Tugwell2000). The three categories for quality assessment of case–control studies were the selection of the study population, intergroup comparability of case and control groups and the measures of exposure factors. The maximum score of NOS is 9 points. The quality of the study was classified as follows: ‘good’, which required 3 or 4 stars in the selection, 1 or 2 in comparability and 2 or 3 in outcomes; ‘fair’, which required 2 in the selection, 1 or 2 in comparability and 2 or 3 in outcomes; the study was graded ‘poor’ otherwise.
Statistical analysis
We performed a meta-analysis for each sleep measurement (e.g., sleep latency or time in bed). Specifically, the differences in sleep measures between medication-naïve children and adolescents with ASD and TD controls were converted to standardized effect sizes (Hedges’ g) and corresponding 95% confidence intervals (95% CIs) and were combined using random-effects models weighted by the inverse of the study variance and between-study heterogeneity. Additionally, we conducted an influence leave-one-out analysis to examine the effect of each study on the overall estimate by omitting one study at a time.
We performed Cochran’s Q test and calculated I 2 statistics to evaluate heterogeneity between studies (Higgins and Thompson, Reference Higgins and Thompson2002). The Q test assesses whether heterogeneity is statistically significant, and I 2 statistics measures the proportion of variance in the pooled effect size attributable to heterogeneity. Publication bias was evaluated using Egger’s test, p-curve analysis and visual inspection of the funnel plots (Egger et al., Reference Egger, Davey Smith, Schneider and Minder1997; Simonsohn et al., Reference Simonsohn, Nelson and Simmons2014a, Reference Simonsohn, Nelson and Simmons2014b). The p-curve analysis evaluates the presence of evidential value for a set of individual studies. The evidential value was denoted when either of the following condition was satisfied: P for the right-skewness test for the half curve was <0.05, or P for the right-skewness test for both half and full curve was <0.1. Moreover, we systematically searched observational studies that investigated sleep parameters in medication-naïve ASD patients without TD controls and performed exploratory independent T-tests between sleep parameters in two study designs (comparative studies [studies that enrolled both TD and ASD participants] versus non-comparative studies [studies that enrolled ASD participants only]) to gain further insights regarding publication bias. Details are presented in Supplementary Appendix pp 13–29. When potential publication bias was suspected by Egger’s test or p-curve analysis, we corrected the effect size using the trim-and-fill method (Duval and Tweedie, Reference Duval and Tweedie2000).
To evaluate potential moderating factors, we performed meta-regression and subgroup analysis. For continuous variables (publication year, mean age of ASD group and percentage of boys in ASD group), we conducted meta-regression analysis for each sleep measure except those with fewer than four studies. The moderating effects of categorical variables (inclusion of intellectual disability and questionnaires for sleep) were assessed by subgroup analysis. All statistical tests were performed using R version 4.1.0 software and its packages. All statistical tests were two-sided, and statistical significance was set at P < 0.05.
Results
Study selection and study characteristics
From the database search, we identified 4277 candidate articles after eliminating duplicates, of which 63 were included after the title and abstract screening. We also manually identified four candidate articles from citation screening. After the final screening, 16 studies were eligible for this systematic review and meta-analysis (Fig. 1). The list of included articles is provided in Supplementary Appendix p 8. The list of excluded articles in the full-text screening stage is presented in Supplementary Appendix pp 10–12.
The main characteristics of the eligible studies are presented in Table 1. A total of 981 patients with ASD (median 26.5 per study, interquartile range [IQR] 13–71.75, range 10–414) and 1220 TD individuals (median 27.5 per study, IQR 13–75.75, range 5–584) were included. Out of 16 eligible studies, four studies used actigraphy, seven polysomnography and nine subjective measures (Fig. 1).
Table 1. Characteristics of included studies

Abbreviations: ACT = actigraphy, ADI-R = autism diagnostic interview-revised, ADOS = autism diagnostic observation schedule, ASD = autism spectrum disorder, CARS = Childhood Autism Rating Scale, CSHQ = Children’s Sleep Habits Questionnaire, DSM = Diagnostic and Statistical Manual of Mental Disorders, ICD = International Classification of Diseases, N = the number, NA = not available, PSQ = polysomnography, SD = standard deviation, SDSC = Sleep Disturbance Scale for Children and TD = typically development.
Actigraphy
Among the 16 included articles, four measured sleep parameters with actigraphy. Meta-analyses were performed for sleep efficiency (n = 4), sleep latency (n = 2), total sleep time (n = 4) and wake after sleep onset (n = 2). The results showed that the patients with medication-naïve ASD exhibited significantly lower sleep efficiency (Hedges’ g, −0.53; 95% CI, −1.05 to −0.01) and total sleep time (Hedges’ g, −0.58; 95% CI, −1.15 to −0.02) than TD individuals (Table 2 and Fig. 2). All parameters measured using actigraphy showed large heterogeneity. Subgroup analyses revealed that the effect sizes of total sleep time and wake after sleep onset were moderated by intellectual disability (Table 2, Fig. 2 and Supplementary Appendix p 38).
Table 2. Meta-analyses for each sleep alteration of objective sleep measures

Abbreviation: ASD = autism spectrum disorder, CI = confidence interval, ID = intellectual disability, k = the number of studies, N = the number, NA = not available and REM = rapid eye movement.
a Egger’s test may lack the statistical power to detect bias when the number of studies is small (i.e., k < 10).
b Blanked cells indicated the case where the p-curve analysis was not carried out due to the number of statistically significant studies were scarce.
c Meta-regression or subgroup analysis was not available for (a) publication year, (b) the mean age of ASD group, (c) percentage of boys in ASD group or (d) the inclusion of ID. The reasons for each note are presented in the Supplementary Appendix.

Figure 2. Summary of meta-analysis on objective sleep parameters in medication-naïve autism spectrum disorder.
Polysomnography
Among the 16 included articles, seven measured sleep parameters with polysomnography. Meta-analyses were available for 14 sleep parameters, which were numbers of awakenings per hour (n = 3), rapid eye movement (REM) density (n = 2), REM latency (n = 6), REM sleep (n = 6), S1 (n = 6), S2 (n = 6), slow wave sleep (n = 6), sleep efficiency (n = 6), sleep latency (n = 6), sleep period time (n = 3), stage shift per hour (n = 3), time in bed (n = 4), total sleep time (n = 6) and wake after sleep onset (n = 5). The results exhibited that patients with medication-naïve ASD showed significantly lower REM sleep (Hedges’ g, −0.33; 95% CI, −0.64 to −0.01), sleep efficiency (Hedges’ g, −0.59; 95% CI, −0.93 to −0.26), sleep period time (Hedges’ g, −1.17; 95% CI, −1.68 to −0.65), time in bed (Hedges’ g, −0.78; 95% CI, −1.17 to −0.40), total sleep time (Hedges’ g, −0.69; 95% CI, −1.25 to −0.14) and longer sleep latency (Hedges’ g, 0.56; 95% CI, 0.24 to 0.88) than TD individuals (Table 2 and Fig. 2). Slow wave sleep and total sleep time showed large heterogeneity. Meta-regression and subgroup analyses showed that the effect size of REM latency, slow wave sleep, total sleep time and wake after sleep onset were moderated by intellectual disability, publication year, publication year and percentage of boys in ASD group, respectively (Table 2, Fig. 2 and Supplementary Appendix pp 33–34, 39).
Actigraphy + polysomnography
For more concrete evidence, we pooled effect sizes of sleep parameters that were measured using objective sleep measures, and meta-analyses were performed for sleep efficiency (n = 10), sleep latency (n = 8), time in bed (n = 5) and total sleep time (n = 10). No study reported outcomes with both actigraphy and polysomnography. The results showed that the patients with medication-naïve ASD had significantly lower sleep efficiency (Hedges’ g, −0.58; 95% CI, −0.87 to −0.28), time in bed (Hedges’ g, −0.64; 95% CI, −1.02 to −0.26), total sleep time (Hedges’ g, −0.64; 95% CI, −1.01 to −0.27) and longer sleep latency (Hedges’ g, 0.59; 95% CI, 0.26 to 0.92) than TD individuals (Table 2 and Fig. 2). Sleep efficiency and total sleep time showed large heterogeneity. Meta-regression and subgroup analyses suggested that the effect sizes of sleep latency, time in bed and total sleep time were moderated by publication year, publication year and intellectual disability, respectively (Table 2, Fig. 2 and Supplementary Appendix pp 35, 40).
Subjective measures
Among the 16 included articles, nine measured sleep problems with subjective measures, which included CSHQ, SDSC, PSQ, sleep diary and sleep log assessment. Since measured sleep problems and their designation varied among sleep measures, we united them according to similarity (Details are in the Supplementary Appendix p 30). Meta-analyses were available for 12 sleep parameters, which were daytime sleepiness (n = 7), parasomnias (n = 5), sleep-disordered breathing (n = 6), sleep latency (n = 5), bedtime resistance (n = 2), disorders in initiating and maintaining sleep (n = 3), night waking (n = 2), sleep anxiety (n = 2), sleep duration (n = 3), sleep hyperhidrosis (n = 3), sleep–wake transition disorders (n = 3) and total sleep problem (n = 7). The results showed that the medication-naïve ASD patients showed significantly increased daytime sleepiness (Hedges’ g, 0.48, 0.26 to 0.71), sleep latency (Hedges’ g, 1.15; 95% CI, 0.72 to 1.58), disorders in initiating and maintaining sleep (Hedges’ g, 0.86; 95% CI, 0.39 to 1.33), sleep hyperhidrosis (Hedges’ g, 0.48; 95% CI, 0.29 to 0.66) and total sleep problem (Hedges’ g, 0.87; 95% CI, 0.58 to 1.16) compared to TD individuals (Table 3 and Fig. 3). All sleep parameters showed large heterogeneity except for sleep hyperhidrosis.
Table 3. Meta-analyses for each sleep problem of subjective sleep measures
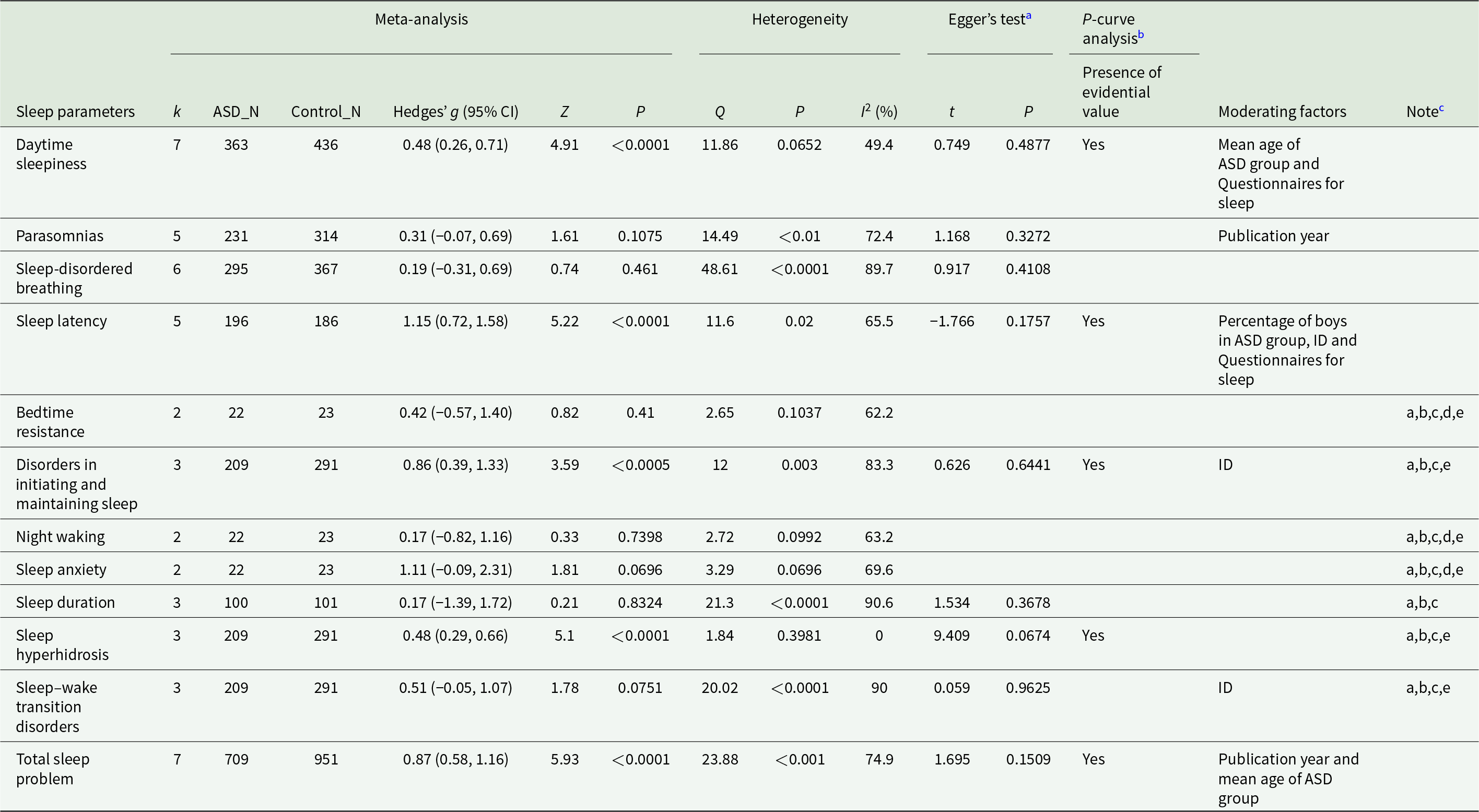
Abbreviation: ASD = autism spectrum disorder, CI = confidence interval, ID = intellectual disability, k = the number of studies, N = the number and NA = not available.
a Egger’s test may lack the statistical power to detect bias when the number of studies is small (i.e., k < 10).
b Blanked cells indicated the case where the p-curve analysis was not carried out due to the number of statistically significant studies were scarce.
c Meta-regression or subgroup analysis was not available for (a) publication year, (b) the mean age of ASD group, (c) percentage of boys in ASD group, (d) the inclusion of ID or (e) questionnaires for sleep. The reasons for each note are presented in the Supplementary Appendix.

Figure 3. Summary of meta-analysis on subjective sleep parameters in medication-naïve autism spectrum disorder.
Meta-regression and subgroup analysis showed that intellectual disability moderated the effect sizes of sleep latency, disorders in initiating and maintaining sleep and sleep–wake transition disorders; publication year moderated the effect sizes of parasomnias and total sleep problems; the type of questionnaires for assessing sleep moderated the effect sizes of daytime sleepiness and sleep latency; the mean age of the ASD group moderated the effect size of daytime sleepiness and total sleep problems and the percentage of boys in ASD group moderated the effect size of sleep latency (Table 3 and Fig. 3). Detailed results are provided in Supplementary Appendix pp 36–37, 41–42.
Newcastle-Ottawa scale
We performed the quality assessment of the 16 included studies. The median score was 8, IQR 7.25–8.75 and the range was 6–9. All included studies met the criteria for ‘high’ quality except for two, which were graded as ‘poor’ mainly because they did not provide any factor for comparability between cases and controls. Details of the NOS score for each study with reason are presented in Supplementary Appendix p 31.
Publication bias assessment and effect size correction
Our Egger’s test and p-curve analysis revealed that sleep latency, sleep period time and total sleep time measured by polysomnography might be affected by publication bias (Table 2). We corrected the effect size of sleep latency and total sleep time measured by polysomnography, and statistical significances of both were retained, whereas the effect size was slightly reduced (Table 4). However, the effect size of sleep period time measured by polysomnography was not corrected via the trim-and-fill method possibly because this meta-analysis contained only three individual studies. Our exploratory investigation for publication bias by independent T-tests between sleep parameters in two study designs (comparative versus non-comparative studies) suggested that the results from the CSHQ might be suspected to have publication bias. Detailed results are displayed in Supplementary Appendix p 13.
Table 4. Correction of the effect sizes by trim-and-fill method

Abbreviations: CI = confidence interval and k = the number of studies.
a Three studies were added for effect size correction.
b One study was added for effect size correction.
Discussion
This meta-analysis aimed to explore sleep alterations in medication-naïve children and adolescents with ASD compared with TD individuals. We found that these patients’ group had a variety of sleep issues that were supported by objective and subjective measures. The medication-naïve ASD group had significantly longer sleep latency and lower sleep efficiency, time in bed and total sleep time than the TD group in objective measures combining actigraphy and polysomnography. Polysomnography alone revealed that the medication-naïve ASD group had a lower proportion of REM sleep and shorter sleep period time. According to subjective measures, the medication-naïve ASD group reported increased problems with daytime sleepiness, sleep latency, disorders in initiating and maintaining sleep and sleep hyperhidrosis. Among the more prominent sleep alterations in ASD, we found that daytime sleepiness may become more severe with ageing and that prolonged sleep latency is more evident in girls than in boys. Moreover, moderators of variations in total sleep time and sleep latency were related to concurrent intellectual disability.
Our analysis concerning only objective sleep measures found that the medication-naïve ASD group had more noticeable sleep alterations than TD, which is consistent with a recent meta-analysis that included all ASD patients independent of the drug usage (Chen et al., Reference Chen, Liu, Wu, Xuan, Zhao and Sun2021). However, a lower proportion of REM sleep in children and adolescents with ASD has not been consistently reported in previous research. Considering that REM sleep is most abundant in early life and tends to correlate with the developmental phases of synaptogenesis and cortical plasticity, researchers had attempted to investigate the link between REM sleep and the clinical features of ASD. According to studies that included children with ASD, a lower proportion of REM sleep was correlated with a higher Childhood Autism Rating Scale score (Maski et al., Reference Maski, Holbrook, Manoach, Hanson and Stickgold2013) and Childhood Behaviour Checklist internalizing score (Bruni et al., Reference Bruni, Ferri, Vittori, Novelli, Vignati, Porfirio, Aricò, Bernabei and Curatolo2007), which indicated that REM sleep deficiency in ASD is associated with severity of symptoms and behavioural problems. In addition, the cholinergic system was suggested to be a potential contributor to the decreased REM sleep in ASD, considering that acetylcholine is the main driver of REM sleep coordinating REM and non-REM sleep (McCarley, Reference McCarley2007). This may imply the possibility of intervention targeting the cholinergic system in ASD. Indeed, an open-label trial reported that donepezil (acetylcholinesterase inhibitor) could increase REM sleep and improve behavioural problems and inattention in children with ASD (Buckley et al., Reference Buckley, Sassower, Rodriguez, Jennison, Wingert, Buckley, Thurm, Sato and Swedo2011). However, further research is needed since the evidence is not yet fully established regarding this issue.
Regarding subjective measures, fewer sleep problems were observed compared to the most recent meta-analysis that pooled the results of 39 studies using subjective sleep parameters in the ASD (Díaz-Román et al., Reference Díaz-Román, Zhang, Delorme, Beggiato and Cortese2018). However, caution is needed when interpreting these results. Since we excluded studies that involved ASD patients taking medications, individuals with relatively mild symptoms or lesser comorbidities were selected for this study. Moreover, only nine studies were included in our analysis; thus, a comparatively smaller number of studies reduced the statistical power. Therefore, we should not overlook the potential for genuine sleep problems in ASD patients to be underestimated. For example, while ‘sleep duration’ in subjective parameters did not retain statistical significance, ‘total sleep time’ in objective parameters was supported by concrete evidence.
Moreover, we performed meta-regression and subgroup analysis to identify potential moderating factors. First, among the subjective parameters, the sex of the ASD group was a significant moderator in sleep latency, and our results suggested that sleep latency was more pronounced in girls than in boys. Second, patients’ age was found to be another moderating factor. Our findings suggest that those with ASD experienced more daytime sleepiness and reported more total sleep problems than the TD individuals, and these problems appeared to increase with age. It is noticeable considering that sleep problems usually decrease with age in the normal development (Gregory and O’Connor, Reference Gregory and O’Connor2002; Li et al., Reference Li, Vitiello and Gooneratne2018). Moreover, since daytime sleepiness in ASD may worsen symptoms such as irritability and thus negatively affect social relationships (Cohen et al., Reference Cohen, Conduit, Lockley, Rajaratnam and Cornish2014), it may be essential to monitor ASD patients’ complaints of sleep problems during their growth to provide appropriate intervention in time. Third, concurrent intellectual disability has also been discovered to be a moderating factor. Our subgroup analysis reported that the ASD group with intellectual disability had more problems with total sleep time on objective measures and sleep latency on subjective measures than the ASD group without intellectual disability. There are two hypotheses regarding the relationship between sleep problems and intellectual disability in children with ASD. One is that preceding sleep alteration negatively affects cognitive function, and the other is that behavioural problems accompanying intellectual disability may interfere with the current sleep (Elia et al., Reference Elia, Ferri, Musumeci, Del Gracco, Bottitta, Scuderi, Miano, Panerai, Bertrand and Grubar2000). However, further studies in this field are required because a small number of studies may lead to overestimation or underestimation, and case–control studies cannot establish a causal relationship.
Our results should be interpreted in light of potential publication bias. Indeed, our Egger’s test and p-curve analysis revealed that the results of three sleep parameters (sleep latency, sleep period time and total sleep time measured by polysomnography) were influenced by potential publication bias, and their effect sizes were attenuated after the correction by the trim-and-fill method. This indicated that other sleep parameters might also be susceptible to publication bias, and their effect sizes might be overestimated even if not detected by our Egger’s test and p-curve analysis, possibly due to their small number of studies (k < 10). Moreover, our further investigation that compared the CSHQ scores between two study designs (comparative studies versus non-comparative studies) suggested that consideration is needed for the possibility of underlying publication bias that originated from the study design (i.e., authors of comparative studies might tend to report more prominent results relative to ASD patients than those in non-comparative studies). However, these analyses were conducted post hoc to address reviewers’ comments, and results are based on few studies and small sample sizes and should be considered exploratory. Therefore, further studies with a non-comparative design and a large number of participants are warranted to establish robust evidence in this field, and until then, careful interpretation with consideration for potential publication bias is required.
Limitations
This study has some limitations. First, the number of studies and participants included in each meta-analysis was small, resulting in reduced statistical power to identify genuine sleep alterations in ASD patients. This issue became more problematic when subgroup analyses were performed. In this context, we conducted a meta-analysis by combining the results of actigraphy and polysomnography, which contained up to 10 studies with 560 participants, to obtain more reliable evidence. Second, various subjective measures for sleep were united for meta-analysis, which seemed to partly contribute to the large heterogeneity across the results of the subjective measures. However, since we used standardized effect sizes (Hedges’ g) and the content between subjective measures was very similar, it appeared to not exert a substantial impact on our results. Third, considering that ASD often requires the administration of psychotropic drugs with various accompanying symptoms, it is possible that the inclusion of medication-naïve patients led to the selection of patients with mild clinical symptoms, which might result in the underestimation of sleep problems in patients with ASD.
Conclusion
In conclusion, our study performed a meta-analysis that included only medication-naïve children and adolescents to investigate sleep alterations in patients with ASD. Our results suggest that children and adolescents with ASD experience diverse sleep alterations. We also investigated some moderating factors such as the patient’s age, sex and concurrent intellectual disability. Future research should analyse how these sleep alterations are linked to core symptom severity and comorbid behavioural problems to provide an integrated therapeutic intervention for patients with ASD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796023000574.
Availability of data and materials
The data used for this study cannot be presented online because included articles are protected by copyright. Additional data from our analysis can be shared by contacting the corresponding author (Keun-Ah Cheon; [email protected]).
Authors contributions
HK, JHK, JK, JIS and KAC contributed to the study concept and design. JHK and JK independently reviewed studies and extracted data. JHK did the statistical analyses. HK, JHK, JK, JIS and KAC analysed and interpreted the data. HK and JHK drafted the manuscript. All authors contributed to critical revision of the manuscript for intellectual content. All authors had full access to the study data and the corresponding authors had the final responsibility for the decision to submit for publication. HK, JHK, JK and JIS contributed equally to this article as co-first authors.
Financial support
This research was supported by a grant of the R&D project, funded by the National Center for Mental Health (grant number: MHER22A01).
Competing interests
We declare no competing interests.
Ethical standards
Ethical approval was not required because this study extracted data from published studies.









