Introduction
Overview of depression
Major depressive disorder (MDD) is one of the most common psychiatric disorders, which affects >264 million people worldwide (GBD, 2017 Disease & Injury Incidence…, 2018). Depression is associated with a high mortality rate, with a hazard ratio of 1.61 (Pratt, Druss, Manderscheid, & Walker, Reference Pratt, Druss, Manderscheid and Walker2016) and a particularly high suicide rate. These factors, in part, have contributed to the large societal, medical and economic burden of this disease. The first line of treatment for MDD includes psychotherapy and pharmacotherapy; however, at least one-third of patients are resistant to these treatments (Ionescu, Rosenbaum, & Alpert, Reference Ionescu, Rosenbaum and Alpert2015). Therefore, it is important to elucidate the pathophysiology of MDD to develop effective strategies for treatment-resistant depression.
Disrupted excitatory and inhibitory balance in MDD
Several lines of evidence suggest that there is an imbalance between cortical excitability and inhibition in patients with MDD, whereby there is excessive cortical excitability and reduced cortical inhibition (Gabbay et al., Reference Gabbay, Bradley, Mao, Ostrover, Kang and Shungu2017; Sanacora, Treccani, & Popoli, Reference Sanacora, Treccani and Popoli2012). For example, previous studies have reported decreased gamma-aminobutyric acid (GABA) concentrations and increased glutamate concentrations in the brain of patients with MDD, using proton magnetic resonance spectroscopy (1H-MRS) (Bhagwagar et al., Reference Bhagwagar, Wylezinska, Jezzard, Evans, Boorman, Matthews and Cowen2008; Moriguchi et al., Reference Moriguchi, Takamiya, Noda, Horita, Wada, Tsugawa and Nakajima2019; Sanacora et al., Reference Sanacora, Gueorguieva, Epperson, Wu, Appel, Rothman and Mason2004). In addition, ketamine, which is a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, represents an effective treatment approach for treatment-resistant depression (McGirr et al., Reference McGirr, Berlim, Bond, Fleck, Yatham and Lam2015). Taken together, these findings suggest that patients with depression may have disrupted GABA and glutamate NMDA receptor-mediated activity. Furthermore, functional magnetic resonance imaging studies have noted a reduction of functional connectivity in several brain regions including the prefrontal cortex (PFC) (Zeng et al., Reference Zeng, Shen, Liu, Wang, Li, Fang and Hu2012), as well as a reduction of PFC volumes in patients with MDD (Botteron, Raichle, Drevets, Heath, & Todd, Reference Botteron, Raichle, Drevets, Heath and Todd2002). These findings support the notion that neuroplasticity in the PFC may be lower in patients with MDD compared to healthy controls (HCs) (Noda et al., Reference Noda, Zomorrodi, Vila-Rodriguez, Downar, Farzan, Cash and Blumberger2018; Pittenger & Duman, Reference Pittenger and Duman2008).
Transcranial magnetic stimulation (TMS) neurophysiological paradigms
Cortical excitability, inhibition, and neuroplasticity can be measured by TMS paradigms. Output measures of TMS can be assessed in two ways: coupling of TMS with peripheral electromyography (EMG) or with concurrent electroencephalography (EEG) (Farzan et al., Reference Farzan, Barr, Hoppenbrouwers, Fitzgerald, Chen, Pascual-Leone and Daskalakis2013). Single- and paired-pulse TMS paradigms have been shown to assess intracortical facilitation (ICF), and intracortical inhibition, which includes short-interval cortical inhibition (SICI) and long-interval cortical inhibition (LICI) (Chen, Reference Chen2000). SICI consists of a subthreshold condition pulse and suprathreshold test pulse with an interstimulus interval of 1–5 ms and is thought to reflect GABAA receptor-mediated activity (Hanajima et al., Reference Hanajima, Ugawa, Terao, Sakai, Furubayashi, Machii and Kanazawa1998; Ilić et al., Reference Ilić, Meintzschel, Cleff, Ruge, Kessler and Ziemann2002; Ziemann, Rothwell, & Ridding, Reference Ziemann, Rothwell and Ridding1996). LICI is composed of a suprathreshold condition pulse and test pulse with an interstimulus interval of 100–200 ms (Nakamura, Kitagawa, Kawaguchi, & Tsuji, Reference Nakamura, Kitagawa, Kawaguchi and Tsuji1997). LICI is thought to reflect GABAB receptor-mediated activity. GABAB activity can also be measured using a TMS paradigm known as cortical silent period (CSP), whereby a strong test pulse is delivered during a voluntary muscle contraction (McDonnell, Orekhov, & Ziemann, Reference McDonnell, Orekhov and Ziemann2006; Siebner, Dressnandt, Auer, & Conrad, Reference Siebner, Dressnandt, Auer and Conrad1998; Wilson, Lockwood, Thickbroom, & Mastaglia, Reference Wilson, Lockwood, Thickbroom and Mastaglia1993). In contrast to these measures, ICF is thought to be a measure of cortical excitability, specifically glutamate NMDA receptor-mediated activity (Hunt & Castillo, Reference Hunt and Castillo2012). ICF consists of a subthreshold condition pulse and suprathreshold test pulse with an interstimulus interval of 10–15 ms (Liepert, Schwenkreis, Tegenthoff, & Malin, Reference Liepert, Schwenkreis, Tegenthoff and Malin1997; Ziemann et al., Reference Ziemann, Rothwell and Ridding1996). An additional TMS paradigm called short-latency afferent inhibition (SAI) is an index of the central cholinergic activity (Tokimura et al., Reference Tokimura, Di Lazzaro, Tokimura, Oliviero, Profice, Insola and Rothwell2000). SAI is measured by delivering TMS over the M1 immediately after contralateral peripheral median nerve stimulation, which attenuates the motor-evoked potential (MEP) (Tokimura et al., Reference Tokimura, Di Lazzaro, Tokimura, Oliviero, Profice, Insola and Rothwell2000). Furthermore, neuroplasticity can be indexed using a TMS paradigm called paired associative stimulation (PAS). This paradigm combines repeated electrical stimulation to the peripheral median nerve of the wrist with TMS to the contralateral primary motor cortex, for over 30 min (Stefan, Kunesch, Cohen, Benecke, & Classen, Reference Stefan, Kunesch, Cohen, Benecke and Classen2000). Depending on the time interval between the PNS and TMS pulses, PAS can induce either long-term potentiation (LTP)-like (e.g. ~25 ms interval) and long-term depression (LTD)-like (e.g. ~10 ms interval) neuronal activity (Buonomano & Merzenich, Reference Buonomano and Merzenich1998).
Previous studies of TMS neurophysiological paradigms in MDD
As mentioned earlier, research suggests that patients with MDD may have excessive cortical excitability and reduced cortical inhibition, in addition to lower levels of neuroplasticity in the PFC. Several neurophysiological studies using TMS in patients with MDD have attempted to establish support for these hypotheses (Kaskie & Ferrarelli, Reference Kaskie and Ferrarelli2018); however, the results of these studies are inconsistent. Therefore, continued research is necessary in order to elucidate if these hypotheses are valid.
Aim of this review study
Here, we conducted a systematic review and meta-analysis to compare TMS-EMG indices between patients with MDD and HC. Our hypotheses were as follows: patients with MDD would have lower GABAA/B receptor-mediated activity, higher glutamate NMDA receptor-mediated activity, and lower levels of neuroplasticity compared to HC. In addition, we explored the effects of clinic-demographic factors such as age, sex, and depression severity on the TMS findings in patients with MDD.
Methods
Search strategy
Research articles written in English were screened by three reviewers using EMBASE, Medline, and PsycINFO from the earliest record to 29 April 2019. The search terms included ‘non-invasive brain stimulation’ or ‘TMS’ or ‘transcranial magnetic stimulation’, ‘brain activity’ or ‘brain waves’ or ‘EEG’ or ‘electroencephalogram’ or ‘electroencephalography’ or ‘EMG’ or ‘MEP’ or ‘motor evoked potential’ or ‘neurophysiolo’ or ‘neuroplasticity’ or ‘plasticity’ or ‘plastic’ or ‘short interval intracortical inhibition’ or ‘SICI’ or ‘intracortical facilitation’ or ‘ICF’ or ‘long interval intracortical inhibition’ or ‘LICI’ or ‘paired associative stimulation’ or ‘PAS’ or ‘short latency afferent inhibition’ or ‘SAI’ or ‘contralateral silent period’ or ‘CSP’, and ‘depression’.
Inclusion criteria
Studies were included in the analysis if they met the following criteria: (1) depression was diagnosed by operational diagnostic criteria; (2) TMS-EMG was conducted using any of the following paradigms, SICI, LICI, ICF, SAI, PAS, or CSP; and (3) results were included for both patients with depression and HCs. Various types of depression, such as atypical depression and melancholic depression, were also included. Vascular depression (VD) was excluded from the main meta-analyses, however, we included VD to sub-analyze its effect on the results for certain TMS paradigms. Of note, any discrepancies on the data extraction process were reviewed and resolved by the senior researcher (Y.N.).
Analysis
The meta-analyses and meta-regression analyses were conducted using the comprehensive meta-analysis (CMA) Software (Biostat Inc.). Outcome variables were denoted as standardized mean differences (SMD). A 95% confidence interval (CI) was calculated following summary statistics. Study heterogeneity was evaluated using the I2 statistic with I 2 ≥ 50% indicating significant heterogeneity. When a two-sided p value was <0.05, it was statistically considered to be significant. Further, we conducted meta-regression analyses to examine the effects of additional factors including patients' age, gender rate, and severity of depression. For the severity of the depression factor, the Hamilton Rating Scale for Depression (HRSD) with 17 items was selected for the moderator variable. Studies that included the Montgomery–Åsberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), or BDI-II scores, were converted to HRSD scores, as there are strong correlations between HRSD score and correlation coefficients of r = 0.88, r = 0.73, and r = 0.74, respectively (Furukawa et al., Reference Furukawa, Reijnders, Kishimoto, Sakata, DeRubeis, Dimidjian and Cuijpers2019; Heo, Murphy, & Meyers, Reference Heo, Murphy and Meyers2007). The formula used for the conversion of the MADRS to the HRSD was: HDRS17 = −1.58 + 0.86 × MADRS (Heo et al., Reference Heo, Murphy and Meyers2007). For the conversion of scores on the BDI or BDI-II to the HRSD, published data from a previous study were used (Furukawa et al., Reference Furukawa, Reijnders, Kishimoto, Sakata, DeRubeis, Dimidjian and Cuijpers2019, Table 2).
For the included studies with missing data values, we supplemented them using one of the following options: (1) contacting the authors for additional data or (2) enlarging the graphic charts, if present, and measuring the data values with R Studio Software or a ruler. Thus, we also conducted the analyses only on the studies that had complete data and confirmed if the pattern of findings held the same.
Risk of bias of the included studies
We used the risk of a bias assessment tool for non-randomized studies (Kim et al., Reference Kim, Park, Lee, Seo, Sheen, Hahn and Son2013) to assess the risk of bias for the following factors: the selection of participants, confounding variables, measurement of exposure, blinding of outcome assessments, incomplete outcome data, and selective outcome reporting. The assessment is shown in online Supplementary Fig. S1.
Publication bias
The publication bias was assessed by Egger's test using the CMA Software.
Results
Out of 882 initial records, 16 studies were included in this meta-analysis. The Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement flow diagram is presented in online Supplementary Fig. S2. The characteristics of the included studies are detailed in Table 1. There were nine studies which measured SICI (Bajbouj et al., Reference Bajbouj, Lisanby, Lang, Danker-Hopfe, Heuser and Neu2006; Concerto et al., Reference Concerto, Lanza, Cantone, Pennisi, Giordano, Spampinato and Bella2013; Croarkin et al., Reference Croarkin, Nakonezny, Husain, Melton, Buyukdura, Kennard and Daskalakis2013; Lefaucheur et al., Reference Lefaucheur, Lucas, Andraud, Hogrel, Bellivier, Del Cul and Paillère-Martinot2008; Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010; Lewis et al., Reference Lewis, Nakonezny, Blacker, Vande Voort, Port, Worrell and Croarkin2018; Maeda, Keenan, & Pascual-Leone, Reference Maeda, Keenan and Pascual-Leone2000a, Reference Maeda, Keenan, Tormos, Topka and Pascual-Leone2020b; Münchau et al., Reference Münchau, Langosch, Gerschlager, Rothwell, Orth and Trimble2005; Veronezi et al., Reference Veronezi, Moffa, Carvalho, Galhardoni, Simis, Benseñor and Brunoni2016), two studies for LICI (Croarkin et al., Reference Croarkin, Nakonezny, Lewis, Zaccariello, Huxsahl, Husain and Daskalakis2014; Lewis et al., Reference Lewis, Nakonezny, Blacker, Vande Voort, Port, Worrell and Croarkin2018), nine studies for CSP (Bajbouj et al., Reference Bajbouj, Lisanby, Lang, Danker-Hopfe, Heuser and Neu2006; Concerto et al., Reference Concerto, Lanza, Cantone, Pennisi, Giordano, Spampinato and Bella2013; Croarkin et al., Reference Croarkin, Nakonezny, Husain, Melton, Buyukdura, Kennard and Daskalakis2013; Lefaucheur et al., Reference Lefaucheur, Lucas, Andraud, Hogrel, Bellivier, Del Cul and Paillère-Martinot2008; Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010; Lewis et al., Reference Lewis, Nakonezny, Blacker, Vande Voort, Port, Worrell and Croarkin2018; Münchau et al., Reference Münchau, Langosch, Gerschlager, Rothwell, Orth and Trimble2005; Steele, Glabus, Shajahan, & Ebmeier, Reference Steele, Glabus, Shajahan and Ebmeier2000; Veronezi et al., Reference Veronezi, Moffa, Carvalho, Galhardoni, Simis, Benseñor and Brunoni2016), nine studies for ICF (Bajbouj et al., Reference Bajbouj, Lisanby, Lang, Danker-Hopfe, Heuser and Neu2006; Concerto et al., Reference Concerto, Lanza, Cantone, Pennisi, Giordano, Spampinato and Bella2013; Croarkin et al., Reference Croarkin, Nakonezny, Husain, Melton, Buyukdura, Kennard and Daskalakis2013; Lefaucheur et al., Reference Lefaucheur, Lucas, Andraud, Hogrel, Bellivier, Del Cul and Paillère-Martinot2008; Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010; Lewis et al., Reference Lewis, Nakonezny, Blacker, Vande Voort, Port, Worrell and Croarkin2018; Maeda et al., Reference Maeda, Keenan and Pascual-Leone2000a, Reference Maeda, Keenan, Tormos, Topka and Pascual-Leone2020b; Münchau et al., Reference Münchau, Langosch, Gerschlager, Rothwell, Orth and Trimble2005; Veronezi et al., Reference Veronezi, Moffa, Carvalho, Galhardoni, Simis, Benseñor and Brunoni2016), and three studies for PAS (Bhandari et al., Reference Bhandari, Lissemore, Rajji, Mulsant, Cash, Noda and Blumberger2018; Kuhn et al., Reference Kuhn, Mainberger, Feige, Maier, Wirminghaus, Limbach and Nissen2016; Player et al., Reference Player, Taylor, Weickert, Alonzo, Sachdev, Martin and Loo2013). There were no studies that examined the SAI paradigm that met the inclusion criteria. Due to the insufficient number of studies, we did not conduct the meta-analysis on the LICI and SAI paradigms.
Table 1. Characteristics of included studies
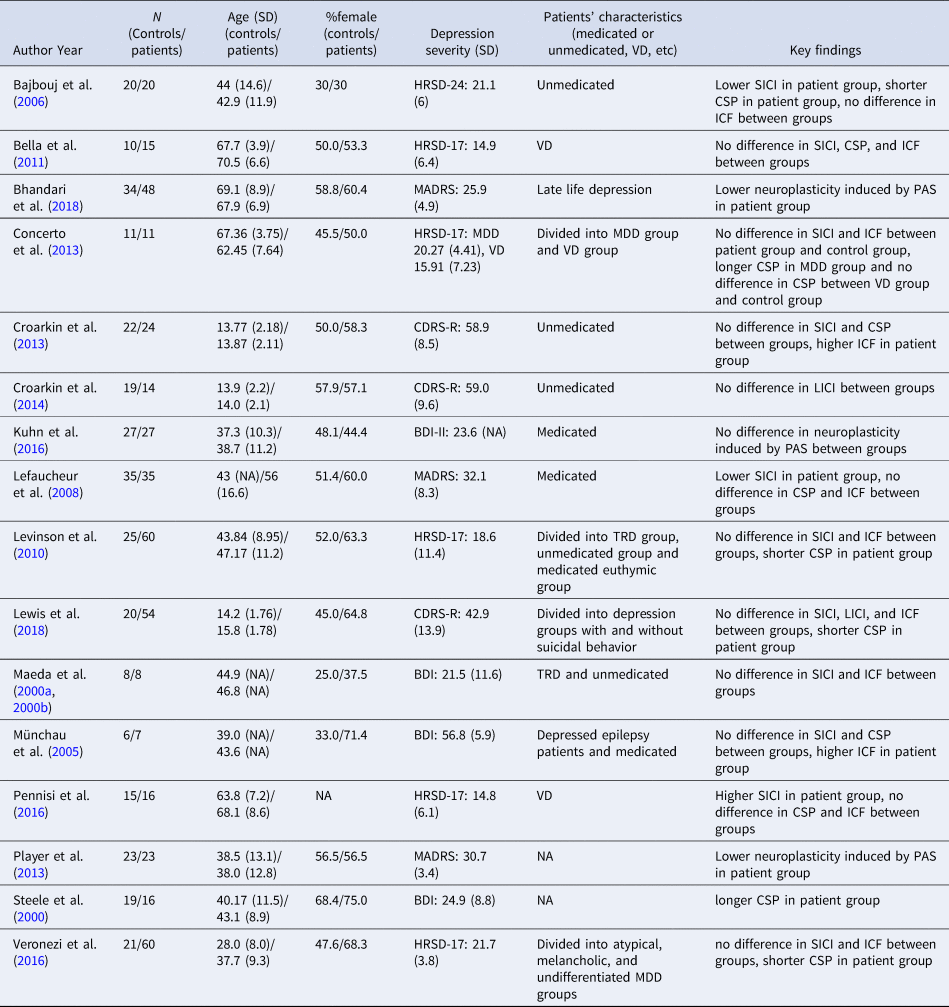
MDD, major depressive disorder; VD, vascular depression; TRD, treatment-resistant major depressive disorder; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; CDRS-R, Children's Depression Rating Scale-Revised; BDI, Beck Depression Inventory; BDI-II, Beck Depression Inventory second edition; SICI, short-interval cortical inhibition; LICI, long-interval cortical inhibition; CSP, cortical silent period; ICF, intracortical facilitation; PAS, paired associative stimulation.
Meta-analysis
The results of the meta-analysis for SICI, CSP, ICF and PAS are shown in Fig. 1.
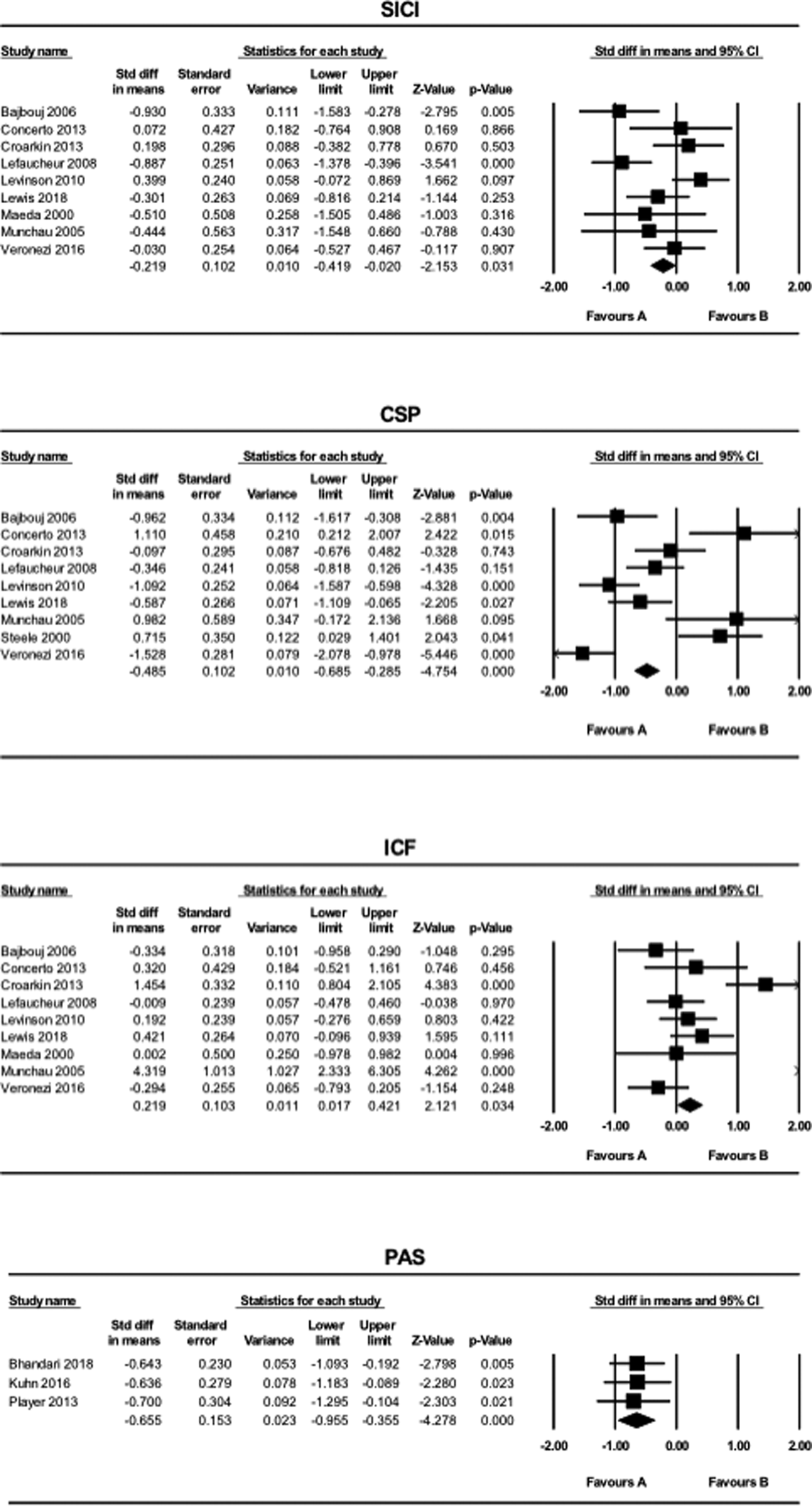
Fig. 1. The results of meta-analyses for the SICI, LICI, CSP, ICF, and PAS paradigms comparing patients with MDD and HCs. Favors A (left side): HC. Favors B (right side): MDD.
SICI and CSP values were smaller in the MDD group compared to the HC group (SICI: SMD = −0.22, CI −0.42 to −0.020, p = 0.031; CSP: SMD = −0.49, CI −0.69 to −0.29, p < 0.001). In contrast, ICF values were greater in patients with MDD compared to HCs (SMD = 0.22, CI 0.017–0.42, p = 0.034). MEP values generated using the PAS paradigm were smaller in the MDD group compared to the HC group (SMD = −0.66, CI −0.96 to −0.36, p < 0.001). Further, for the PAS paradigm, all of the three studies included in this systematic review indicated LTP-like activity.
The analyses when the studies with missing data values were excluded
There were four studies measuring SICI (Concerto et al., Reference Concerto, Lanza, Cantone, Pennisi, Giordano, Spampinato and Bella2013; Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010; Maeda et al., Reference Maeda, Keenan and Pascual-Leone2000a, Reference Maeda, Keenan, Tormos, Topka and Pascual-Leone2020b; Münchau et al., Reference Münchau, Langosch, Gerschlager, Rothwell, Orth and Trimble2005), one study for CSP (Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010), three studies for ICF (Levinson et al., Reference Levinson, Fitzgerald, Favalli, Blumberger, Daigle and Daskalakis2010; Maeda et al., Reference Maeda, Keenan and Pascual-Leone2000a, Reference Maeda, Keenan, Tormos, Topka and Pascual-Leone2020b; Münchau et al., Reference Münchau, Langosch, Gerschlager, Rothwell, Orth and Trimble2005), and two studies for PAS (Bhandari et al., Reference Bhandari, Lissemore, Rajji, Mulsant, Cash, Noda and Blumberger2018; Player et al., Reference Player, Taylor, Weickert, Alonzo, Sachdev, Martin and Loo2013) which had missing data values. Thus, we measured the values from graphic charts in the articles using R Studio Software or a ruler. When these studies with missing data values were excluded from the analyses, the pattern of the findings still remained. However, the finding of ICF became non-significant in this analysis (SICI: SMD = −0.38, CI −0.62 to −0.14, p = 0.0020; CSP: SMD = −0.37, CI −0.59 to −0.15, p = 0.0010; ICF: SMD = 0.18, CI −0.050 to 0.41, p = 0.12). On the other hand, we could not conduct the analysis of the PAS paradigm since there was only one study left.
The effect of VD on the results
Three studies employed the SICI, CSP, and ICF paradigms in patients with VD, while no studies were found in this population using the PAS paradigm. When VD was included for the analysis of the SICI paradigm, the result for the meta-analysis became non-significant (SMD = −0.098, CI −0.28 to 0.085, p = 0.30). For the analysis of the CSP paradigm, the result remained significant (SMD = −0.34, CI −0.53 to −0.16, p < 0.001). Similarly, for the ICF paradigm, the result remained significant when VD was included (SMD = 0.27, CI 0.085 to 0.45, p = 0.004) (Fig. 2).
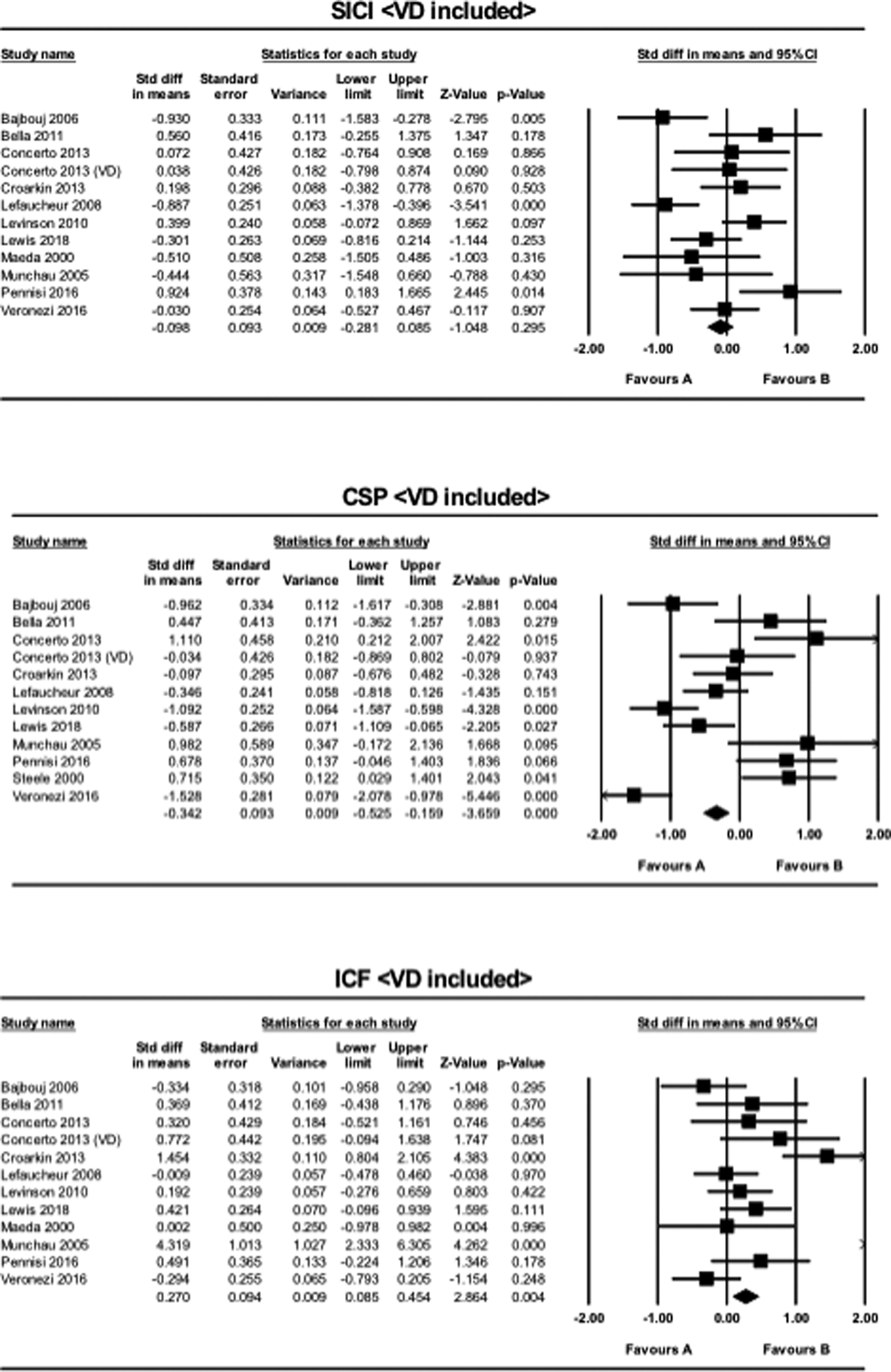
Fig. 2. The results of meta-analyses for the SICI, CSP, and ICF paradigms, when VD was included in MDD. Favors A (left side): HC. Favors B (right side): MDD.
Meta-regression analysis
Meta-regression analyses were conducted for the SICI, CSP, and ICF paradigms, while they could not be conducted for the PAS paradigm due to the insufficient number of studies.
First, patients' age was not associated with the SMD of the SICI, CSP, or ICF paradigms between patients with MDD and HCs (SICI: slope = −0.0084, CI −0.032 to 0.015, p = 0.24; CSP: slope = 0.013, CI −0.027 to 0.052, p = 0.26; ICF: slope = −0.018, CI −0.049 to 0.012, p = 0.12). The scatter plots are displayed in online Supplementary Fig. S3.
Second, the proportion of female patients was not associated with the SMD of the SICI, CSP, or ICF paradigms between patients with MDD and HCs (SICI: slope = 0.019, CI −0.0076 to 0.045, p = 0.083; CSP: slope = 0.018, CI −0.028 to 0.063, p = 0.23; ICF: slope = 0.027, CI −0.012 to 0.066, p = 0.085). The scatter plots are displayed in online Supplementary Fig. S4.
Finally, the severity of depression as assessed by the HRSD-17 was not associated with the SMD of the SICI and CSP paradigms between patients with MDD and HCs (SICI: slope = −0.037, CI −0.10 to 0.030, p = 0.14; CSP: slope = 0.052, CI −0.074 to 0.18, p = 0.21), while it was associated with higher SMDs in the ICF paradigm (slope = 0.15, CI 0.048–0.26, p = 0.0023). The scatter plots are displayed in online Supplementary Fig. S5.
Publication bias
Egger's test showed no publication bias in the analysis of the SICI, CSP, ICF, and PAS paradigms. The funnel plots are displayed in online Supplementary Fig. S6.
Risk of bias
The risk of bias of the included studies is summarized in online Supplementary Fig. S1. For ‘random sequence generation’, the risk of bias was ‘unclear’ for four studies which did not mention the method of recruitment of participants. The risk of bias for ‘incomplete outcome data’ was ‘unclear’ for one study which did not specify how data was excluded. For ‘selective reporting’, the risk of bias was ‘unclear’ for all studies since we did not have access to the experimental protocols.
In addition, no specific sponsorship bias was identified for the included studies in this review, as none of the studies were funded by the private sector. Although some studies received funding from companies, those companies was not likely to affect the results because their business was not related to TMS neurophysiology. Other than these studies, some of the included studies mentioned that they were funded by some foundations which were not likely to make sponsorship bias. The other studies stated that they had no conflict of interest, or did not mention about conflict of interest.
Discussion
This meta-analysis compared GABAA/B receptor-mediated activity, glutamate NMDA receptor-mediated activity, and neuroplasticity between patients with MDD and HC through comprehensive TMS-EMG neurophysiological indices. Our analyses revealed that compared to HCs, patients with MDD have lower SICI, CSP, and probably higher ICF, with small effect sizes, and lower PAS, with a medium effect size (Fig. 1). These results suggest that patients with MDD have lower GABAA/B receptor-mediated activity, a lower level of neuroplasticity, and might have higher glutamate NMDA receptor-mediated activity compared with HC. These findings provide support for the proposed cortical excitatory and inhibitory imbalance hypothesis and neuroplasticity hypothesis of MDD. Taken together, our results suggest that out of the five TMS paradigms, lower values of SICI, CSP, and PAS paradigms, and higher ICF could represent biomarkers for MDD, which can be used to distinguish MDD patients from HCs. In contrast to our ICF finding, a previous meta-analysis of glutamatergic neurometabolite levels in MDD as measured by 1H-MRS revealed decreased glutamate + glutamine levels in the medial frontal cortex in patients with MDD (Moriguchi et al., Reference Moriguchi, Takamiya, Noda, Horita, Wada, Tsugawa and Nakajima2019). This discrepancy may be due to differences in the region of interest and the modality of measurement. For example, TMS measures functional neural dynamics, while 1H-MRS measures static neurometabolite levels. Thus, the combination of these two may represent a more comprehensive measure of the difference in glutamate NMDA receptor-mediated activity between patients with MDD and HC.
One TMS-EEG study examined neuroplasticity differences in PFC activity between patients with MDD and HC using the PAS paradigm (Noda et al., Reference Noda, Zomorrodi, Vila-Rodriguez, Downar, Farzan, Cash and Blumberger2018). The study showed that prefrontal neuroplasticity was lower in patients with MDD compared to HCs (SMD = −0.78, CI −1.1 to −0.51, p = 0.004), supporting the result of the current meta-analysis findings for PAS in the motor cortex. These results indicate that reduced neuroplasticity is not limited to the motor cortex and might extend to broad cortical regions.
When studies of VD were included in the sub-analysis, the group differences in SICI no longer remained significant and differences in CSP became smaller. In contrast, the inclusion of the VD studies increased the group differences of the ICF paradigm (Fig. 2). The majority of patients with VD exhibit dementia-like pathology due to widespread microvascular insults, resulting in impaired inhibitory function. This is thought to lead to further disruption in cortical excitability and decrease in cortical inhibition (Alexopoulos et al., Reference Alexopoulos, Meyers, Young, Kakuma, Silbersweig and Charlson1997; Issac, Chandra, & Nagaraju, Reference Issac, Chandra and Nagaraju2013). Our meta-analyses indicated that patients with VD showed higher cortical excitability and higher cortical inhibition compared to the analysis where they were not included. Thus, our ICF findings were in line with previous research, whereas our analyses of SICI and CSP were not. This discrepancy is possibly due to the compensatory mechanism of interhemispheric inhibition. That is, when the excitability of one hemisphere increases (i.e. increased ICF), the inhibitory properties of the contralateral hemisphere also increases in response (i.e. increased SICI and CSP), and vice versa.
The results of meta-regression on patients' age suggest that cortical functions of the M1, including GABAA/B and glutamate NMDA receptor-mediated activity, may not be significantly influenced by age (online Supplementary Fig. S3). In general, however, neurophysiological activities have been shown to decrease with age (Talelli, Ewas, Waddingham, Rothwell, & Ward, Reference Talelli, Ewas, Waddingham, Rothwell and Ward2008). This discrepancy was possibly due to the small number of included studies. Further research with a larger number of studies is needed to confirm the effect of age on these neurophysiological indices.
The results of meta-regression on patients' sex suggest that cortical functions of the M1, including GABAA/B and glutamate NMDA receptor-mediated activity, do not differ between males and females (online Supplementary Fig. 4). A previous TMS study found a significant difference in TMS-induced MEPs of the lower limbs but not of the upper limbs between males and females (Cantone et al., Reference Cantone, Lanza, Vinciguerra, Puglisi, Ricceri, Fisicaro and Pennisi2019). This may be because the sex difference in the distance of the corticospinal tract from the M1 to the upper limbs is relatively smaller compared to the lower limbs. In the present systematic review, all of the studies included in this meta-analysis assessed TMS-EMG of the upper limbs. Thus, MEPs measured from the upper limbs may not detect subtle sex differences. In contrast, a previous study explored the effects of female hormones such as estrogen and progesterone on cortical excitability and found significantly higher motor threshold values at the first dorsal interosseous muscle using TMS applied to the M1 in women with amenorrhea compared to women in the early follicular stage (Chagas et al., Reference Chagas, Monteiro, Mazer, Baltar, Marques, Carneiro and Monte-Silva2018). This, therefore, highlights a potential difference in cortical functions of the M1 between males and females. Our analysis did not include information regarding the menstrual cycle of the study samples due to the lack of information provided.
The result of meta-regression on the severity of depression for the ICF paradigm suggests that the worse the HRSD-17 score, the higher the glutamate NMDA receptor-mediated activity (online Supplementary Fig. S5). Therefore, ICF may represent a state marker of MDD. In contrast, the results of meta-regression on depression severity for the SICI and CSP paradigms show no correlations between HRSD-17 scores and GABAA/B receptor-mediated activity (online Supplementary Fig. S5). Taken together, our results suggest that while SICI and CSP may be biomarkers of MDD, it is difficult to evaluate the state of depression from the inhibitory function of the corticospinal tract.
There are several novel therapeutic strategies for MDD that target the neurophysiological bases of MDD. For instance, ketamine, a non-competitive antagonist of the NMDA receptor (Anis, Berry, Burton, & Lodge, Reference Anis, Berry, Burton and Lodge1983), is now used in refractory depression at some specialized medical institutions. Since patients with MDD have higher ICF compared to HCs, ketamine may rapidly suppress hyperexcitation in glutamate NMDA receptor-mediated activity, resulting in improvement of depression symptoms.
Another promising pharmacological treatment for MDD is a novel GABAA receptor positive allosteric modulator known as SAGE-217 (3α-hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one) (Martinez Botella et al., Reference Martinez Botella, Salituro, Harrison, Beresis, Bai, Blanco and Robichaud2017). Our SICI findings showing that GABAA receptor-mediated activity may be lower in patients with MDD compared to HCs is suggestive of the effectiveness of SAGE-217 for the treatment of MDD.
Some neurosteroids have also been shown to affect the state of depression. For example, estrogen attenuates GABAA/B receptor-mediated activities (Lagrange, Wagner, Rønnekleiv, & Kelly, Reference Lagrange, Wagner, Rønnekleiv and Kelly1996; Mukherjee et al., Reference Mukherjee, Cardarelli, Cantaut-Belarif, Deeb, Srivastava, Tyagarajan and Moss2017). However, no relationship was found between the proportion of females in the included studies and GABAA/B receptor-mediated inhibitory functions in the present meta-regression analysis (see online Supplementary Fig. S4). Another neurosteroid example is allopregnanolone, which is a positive allosteric modulator of the GABAA receptor (Faroni & Magnaghi, Reference Faroni and Magnaghi2011). Allopregnanolone also stimulates GABA synthesis by increasing the level of glutamic acid decarboxylase of 67 kDa, resulting in the activation of both GABAA/B receptor-mediated activities (Magnaghi et al., Reference Magnaghi, Parducz, Frasca, Ballabio, Procacci, Racagni and Fumagalli2010). The results of our analyses on SICI and CSP suggest the effectiveness of allopregnanolone as a potential treatment for MDD in women. In support of this, allopregnanolone has recently been approved by the FDA to treat postpartum depression, a condition that is associated with disrupted GABAergic functioning due to a rapid postpartum drop in progesterone (Walton & Maguire, Reference Walton and Maguire2019).
Other than pharmacotherapy, repetitive TMS (rTMS) has emerged as a promising treatment for treatment-resistant depression. rTMS is thought to exert its therapeutic effect through the induction of neuroplasticity in both excitatory and inhibitory synapses (Lenz et al., Reference Lenz, Galanis, Müller-Dahlhaus, Opitz, Wierenga, Szabó and Vlachos2016). Our PAS analysis indicates a lower level of neuroplasticity in patients with MDD compared to HCs, thus suggesting that rTMS could be a useful treatment to target the underlying pathophysiological impairments associated with depression.
The present study has several limitations. First, we failed to perform the meta-analysis for the PAS due to the limited number of the included studies, warranting further research on PAS in MDD. Ongoing investigation as the field continues to grow would improve the accuracy and reliability of the results. Second, for some studies, we had to impute the results from figures using R Studio software or a ruler which also impacted the accuracy of the findings. When the studies with missing data were excluded from the analyses, the difference of the ICF results between HCs and patients with MDD became non-significant. Therefore, ICF findings in patients with MDD should be interpreted with caution at this time. Further research on the ICF paradigm is needed comparing larger sizes of patients with MDD and HCs. Third, the concomitant medication administered to patients with MDD was not standardized across the studies. Furthermore, as mentioned earlier, the effect of hormonal fluctuation due to the menstrual cycle in females on neurophysiological findings was not considered as a confounding factor. Additionally, there are other potential confounding factors that could affect the results, including, alcohol, drugs, smoking, and physical activity that were not measured in the studies (Huang et al., Reference Huang, Lu, Antal, Classen, Nitsche, Ziemann and Rothwell2017; Kähkönen, Wilenius, Nikulin, Ollikainen, & Ilmoniemi, Reference Kähkönen, Wilenius, Nikulin, Ollikainen and Ilmoniemi2003; Kalivas & O'Brien, Reference Kalivas and O'Brien2008). Finally, three studies using the scales of depression other than HRSD-17, MADRS, and BDI (Bajbouj et al., Reference Bajbouj, Lisanby, Lang, Danker-Hopfe, Heuser and Neu2006; Croarkin et al., Reference Croarkin, Nakonezny, Husain, Melton, Buyukdura, Kennard and Daskalakis2013; Lewis et al., Reference Lewis, Nakonezny, Blacker, Vande Voort, Port, Worrell and Croarkin2018) could not be included in the meta-regression since the scales other than these three did not correlate with HRSD-17 score, which we selected as the moderator variable.
In summary, our results provided support for the cortical excitatory and inhibitory imbalance hypothesis as well as the neuroplasticity hypothesis. The present systematic review and meta-analyses on TMS neurophysiology in MDD warrants further research with larger sample sizes to replicate our findings and the consideration of potential confounding factors that may affect neural activity as mentioned above. Finally, given the results of this study, TMS neurophysiology has the potential not only to distinguish MDD from HCs but also to be a useful neuroscientific tool to elucidate the pathophysiology of MDD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004729
Acknowledgement
The authors would like to acknowledge the assistance of Michelle Goodman for editing related to grammar and language.
Conflict of Interest
None.






