During the past few years, numerous studies and reviews have underlined the beneficial effects of n-3 long-chain PUFA (n-3 LC-PUFA) found in fish and fish oils in the prevention and management of both cardiovascular and chronic inflammatory diseases, such as rheumatoid arthritis (RA)(Reference James and Cleland1–Reference Breslow5).
n-3 LC-PUFA, e.g. EPA (20 : 5n-3) and DHA (22 : 6n-3), as well as n-6 PUFA, e.g. arachidonic acid (AA, 20 : 4n-6), are present in cell phospholipids (PL). These fatty acids (FA) are involved in many physiological processes through their influence on membrane fluidity, eicosanoid synthesis, receptor affinity, cell signalling and gene expression(Reference Holub2, Reference Adam, Beringer and Kless3, Reference Calder6–Reference Adam8). The eicosanoids derived from AA are biologically active in very small quantities; however, in larger concentrations, they may contribute to thrombus formation, the development of atheromas, as well as allergic and inflammatory diseases(Reference Simopoulos9). On supplementing the diet with n-3 LC-PUFA, the concentrations of n-3 FA in individual tissues increased, whereas that of AA decreased(Reference Calder7, Reference Volker, Fitzgerald and Garg10). Additionally, consumption of n-3 LC-PUFA may lead to a reduction in the production of pro-inflammatory cytokines and cartilage-degrading enzymes(Reference Cleland, James and Proudman11). Hence, the intake of FA can directly modulate the synthesis and action of regulatory eicosanoids and cytokines.
RA characterised by an autoimmune inflammation, involving both small and large joints, is mediated by an exaggerated production of eicosanoids and cytokines. The chronic inflammation process in the affected joints leads to the formation of degenerative and erosive lesions in cartilage and bones. Improvements in the clinical and immunological parameters of RA via n-3 LC-PUFA consumption have been shown in several intervention studies(Reference Adam, Beringer and Kless3, Reference Kremer, Lawrence and Jubiz12–Reference Volker, Fitzgerald and Major14).
In the present study, dairy products were enriched with n-3 LC-PUFA. Conventional dairy products are poor in LC-PUFA, although they are a source of anti-inflammatory effectuating conjugated linoleic acids (18 : 2n-6)(Reference Jaudszus, Foerster and Kroegel15, Reference Jaudszus, Krokowski and Möckel16) and SCFA. Dairy products are, however, rich in oleic acid (18 : 1n-9), although they contain only marginal concentrations of AA. Moreover, dairy products are the most important source of bioavailable calcium.
The aim of the present long-term study with RA patients was to compound the positive effects of dairy products (yoghurt, cheese and butter) with moderate doses of n-3 LC-PUFA on the risk factors of CVD (serum lipids and inflammation parameters), immune biomarkers, collagen crosslinks, markers of oxidative stress, and on the disease activity of RA.
Subjects and methods
Subjects
Forty-five patients (forty-three females and two males) with RA, diagnosed according to the 1987 revised criteria of the American Rheumatism Association, were included in the study after giving their informed consent.
Patients receiving either non-steroidal anti-inflammatory drugs or corticosteroids ( ≤ 15 mg/d) or both were eligible if the dosage had been stable for at least 4 weeks before day 1 of the study and remained below this limit throughout the study. Those on disease-modifying antirheumatic drugs had to be on a constant dosage for at least 8 weeks before and throughout the study. Subjects diagnosed with gastrointestinal or metabolic diseases, and alcohol abuse, those taking dietary supplements (e.g. fish oil capsules), and with known food allergy or food intolerance were excluded.
Patients were withdrawn from the study at any time after enrolment for the following reasons: patient's request; acquisition of a serious infection; an inadequate control of the arthritic symptoms ( ≥ 50 % increase in the number of swollen or tender joints); the reinstitution of therapy with disease-modifying antirheumatic drugs; if the patient compliance with the study protocol was doubtful. The study protocol was approved by the Ethical Committee of the Friedrich Schiller University of Jena.
Study design and diet
The randomised, double-blind, placebo-controlled cross-over study consisted of two 12-week investigation periods and an 8-week washout phase between the two periods (Fig. 1). Patients received approximately 40 g fat in the form of 200 g yoghurt with 3·8 % fat, 30 g cheese with about 50 % fat in the DM and 20–30 g butter, daily. The milk fat was partially exchanged by special oils such as fish, rapeseed and Dracocephalum ibericum oil containing high concentrations of EPA, DHA and α-linolenic acid (18 : 3n-3). The daily dose of n-3 FA, consisting of 1·1 g α-linolenic acid, 0·7 g EPA, 0·1 g DPA and 0·4 g DHA, amounted to 2·4 g. Commercial dairy products with comparable fat contents were used as a placebo. The intake of AA was about 50 mg/d through the intervention products and about 70 mg/d through the control products. All products used in the study were offered in neutral packaging.
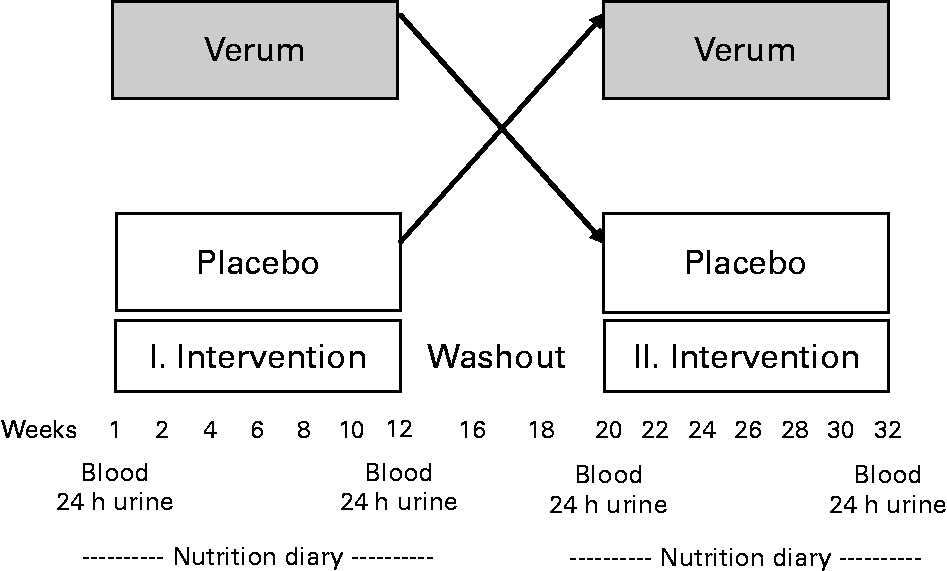
Fig. 1 Design of the clinical study.
Patients had to fill a nutrition diary in both study periods, where they documented the daily consumption (kind and amount) of meat, meat products, fish, fish products, oils and fats.
The following criteria were assessed both at the beginning and at the end of each period: body weight; blood pressure; duration of morning stiffness as estimated by the patients; the disease activity score (DAS28).
Blood and urine collection
Between 07.30 and 08.00 hours, fasting blood samples were drawn by venepuncture into evacuated tubes (Sarstedt®). The venous blood was collected into one citrate tube, two evacuated serum tubes (5 and 10 ml), one evacuated tube (10 ml) containing K3-EDTA (1 mg/ml) and one evacuated tube (10 ml) containing Li-heparin.
The blood samples were processed as follows. After plasma and platelets were separated by centrifugation (10 min, 2000 rpm), plasma and serum samples were placed in individual tubes and stored at − 80°C until analysis. The C-reactive protein, alkaline phosphatase, γ-glutamyltransferase concentrations and cyclo-oxygenase (COX) expression were analysed in plasma. For serum, total cholesterol, HDL, LDL, TAG and lipoprotein a levels were evaluated.
Urine samples were collected during the day before blood samples were taken (Fig. 1). The morning urine from the first day was not kept, but samples of urine from the rest of the day until the morning urine on the next day (24 h urine collection) were collected in a special 24 h urine collection tank (10 ml; Sarstedt®, Nümbrecht, Germany).
The samples were placed in urine monovettes (Sarstedt®) and stored at − 80°C until analysis. The 24 h urine was tested for collagen crosslinks, 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-oxodG), 8-iso-PGF2α, 15-keto-dihydro-PGF2α and creatinine (Cr).
Lipid extraction and fatty acid analysis
Plasma lipids were extracted by employing a methanol–chloroform mixture according to the method of Bligh & Dyer(Reference Blight and Dyer17). PL and cholesteryl esters (CE) were isolated from plasma lipid extracts by TLC with the use of hexane–diethyl ether–acetic acid (85:15:0·2, by vol.). Furthermore, an acid-catalysed methylation was performed using anhydrous HCl–methanol (5 % w/v; Supelco, Bellefonte, PA, USA). The resulting fatty acid methyl esters were isolated by means of TLC. The FA analysis was conducted via GC (GC-17 V3; Shimadzu, Tokyo, Japan) equipped with an autosampler and a flame ionisation detector. FA ranging from four to twenty-five carbon atoms in length, as well as the total conjugated linoleic acids, were determined using a fused silica capillary column (CP Select for fatty acid methyl esters (Chrompak, Walton-on-Thames, UK), 200 m × 0·25 mm × 0·25 μm; Shimadzu, Canby, OR, USA). The FA concentrations were expressed as a percentage of the total area of all FA peaks (percentage of total fatty acid methyl esters).
Blood and urinary parameters
The erythrocyte sedimentation rate was measured using the Westergren technique. C-reactive protein was quantified by means of the turbidimetric immunoassay on the Synchron LX®20 system (Beckman Coulter, Fullerton, CA, USA). Serum total cholesterol, HDL, LDL, TAG, Cr, the activity of γ-glutamyltransferase and alkaline phosphatase were ascertained by enzymatic methods employing commercially available kits (Beckmann, Krefeld, Germany) and using the autoanalyser Synchron LX systems (Beckman Coulter), according to the methods of the International Federation of Clinical Chemistry and Laboratory Medicine.
The WBC counts were determined using the haematology analyser (KX-21; Sysmex). The immunophenotyping of leucocytes was performed by flow cytometry. The lymphocytes, monocytes, granulocytes and lymphocyte subpopulations (CD3+, CD19+, CD4+, CD8+, NK+, CD3+NK+, CD57+, CD8+CD57+, CD25+, CD4+CD25+ and CD3+HLA-DR+) were analysed on a flow cytometer FACScan using the SimulSET software, as described by Klein et al. (Reference Klein, Friedrich and Vogelsang18).
COX-1 and COX-2 expressions in whole-blood monocytes were determined by flow cytometry (FACScan™ instruments) utilising a test kit for monoclonal antibodies that detected human antigens (BD Biosciences, Heidelberg, Germany), modified according to Ruitenberg & Waters(Reference Ruitenberg and Waters19). The results were expressed as a percentage of positively stained cells (%).
The 24 h urine samples were prepared for pyridinoline (Pyr) and deoxypyridinoline (Dpyr) analyses via HPLC, as described by Müller et al. (Reference Mueller, Hein and Franke20) and Hein et al. (Reference Hein, Franke and Mueller21).
A highly specific and sensitive RIA was used to determine the urinary concentrations of free 8-iso-PGF2α and 15-keto-dihydro-PGF2α(Reference Basu22, Reference Basu23).
The analysis of 8-oxodG was conducted by means of HPLC and electrochemical detection(Reference Kuhnt, Wagner and Kraft24). After Cr correction, the urinary parameters were expressed as nmol/mol Cr.
Statistical analysis
The statistical assessment of the data was performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A value of P ≤ 0·05 indicates significant intra- and intergroup changes. The results are presented as means and standard deviations. The Kolmogorov–Smirnov test was used to verify the distribution of the data.
Possible differences in the intervention or the control period as well as between the end values of the intervention and control periods were tested with repeated measurements (ANOVA). The intervention sequence of the cross-over design was considered as a covariate. The differences between the start and end values after 2 × 12 study weeks were analysed by means of the paired t test. If the data were not normally distributed, the Wilcoxon test was used. Relationships between variables were calculated using Pearson's correlation or Kendall's tau, in case of not normally distributed data.
Results
Six patients, representing a dropout rate of 13 %, did not complete the study because they showed side effects due to disease-modifying antirheumatic drugs. Thirty-nine test subjects aged 57·9 ± 10·8 years completed the 8-month intervention study. Of these, eleven needed an intra-articular injection of corticosteroids less than 4 weeks before blood sampling was carried out. Hence, these patients were excluded from the statistical evaluation. Furthermore, seven patients were omitted because the EPA increase in erythrocyte membranes was less than 10 % after the 12 weeks of the intervention period and adherence to the protocol was doubtful. We assume that the compliance of these patients was not given and they are ‘non-consumers’.
Fatty acid distribution in plasma lipids, cholesteryl esters and phospholipids
In plasma lipids, α-linolenic acid, EPA, DPA, DHA, n-3 FA and n-3 LC-PUFA increased significantly. In addition, there was a significant decrease of the AA:EPA, AA:n-3 LC-PUFA as well as n-6 FA:n-3 FA ratios during the verum treatment (Table 1). In the control group, the concentrations of these FA were not affected. There was a low increase in the AA levels in this group. The final values of all n-3 LC-PUFA, the ratios, n-3, n-6 FA and SFA differed significantly between the verum and control periods (P ≤ 0·05), respectively.
Table 1 Fatty acid (FA) distribution in plasma lipids, cholesteryl esters (CE) and phospholipids (PL) at baseline and after a 12-week intake of n-3 long-chain PUFA (n-3 LC-PUFA)- supplemented or control dairy products†
(Mean values and standard deviations)
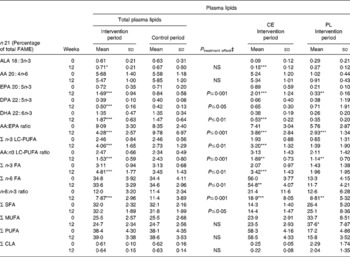
FAME, fatty acid methyl esters; ALA, α-linolenic acid; AA, arachidonic acid; CLA, conjugated linoleic acids.
Significantly different compared with the start value: *P ≤ 0·05, ** P ≤ 0·01, *** P ≤ 0·001.
† Fatty acids were separated gas chromatographically as corresponding FAME.
‡ End values are significantly different from the control period with baseline values as covariates.
In CE, the concentrations of α-linolenic acid, EPA, DHA, n-3 FA and n-3 LC-PUFA were increased significantly by the intervention treatment. The ratios, together with n-6 FA, were significantly decreased by the consumption of n-3 LC-PUFA-supplemented dairy products (P ≤ 0·05; Table 1).
In PL, the concentration of EPA and MUFA increased significantly in the intervention period. The ratios decreased by the consumption of n-3 LC-PUFA-supplemented diary products (P ≤ 0·05; Table 1). The increase of DPA, DHA, n-3 FA and n-3 LC-PUFA was not significant (Table 1).
Nutrition diary
The analysis of the nutrition diary provided information about the basal diet without consideration of the study products. There were no differences in the intake of oleic acid, AA, EPA, DHA, SFA, MUFA, PUFA, n-3 LC-PUFA, n-3 and n-6 PUFA, and trans-FA between the intervention and control periods (Table 2). The patients consumed 0·2–0·3 g n-3 LC-PUFA/d and 0·8–0·9 g AA/d by the intake of meat and fish during the study.
Table 2 Consumption of chosen fatty acids (FA) by meat, meat products, fish, fish products, oils and fats (g FA/d)*
(Mean values and standard deviations)
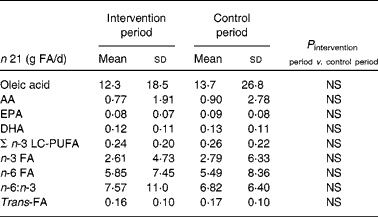
AA, arachidonic acid; n-3 LC-PUFA, n-3 long-chain PUFA.
* Without the study products.
Anthropometric data and clinical parameters
There were no differences observed in the body weight, BMI, blood pressure, pulse rate, alkaline phosphatase and γ-glutamyltransferase in the intervention or the control period and between the end points of both the periods.
After 2 × 12 study weeks, there was a tendency for an increase in the body weight, BMI and the pulse rate. During this time, the diastolic blood pressure decreased significantly from 91 (sd17) to 84 (sd13) mm Hg (P ≤ 0·01) and the alkaline phosphatase decreased significantly from 1·25 (sd 0·49) μmol/l to 1·16 (sd0·46) μmol/l (P ≤ 0·05).
Blood lipids
Total cholesterol was not affected by the intervention with dairy products (Table 3), although HDL increased significantly on consumption of dairy products rich in n-3 LC-PUFA. The HDL increase in the control period was not significant. LDL and the LDL:HDL ratio remained unaffected by both the intervention and control treatments. In addition, supplementation with verum products was associated with significantly lower concentrations of lipoprotein a. In the intervention period, TAG values decreased from 1·1 (sd 0·4) to 1·0 (sd0·4) mmol/l and, in the control period, the TAG concentrations increased. The end points of these study parameters showed no changes between the intervention and control treatments (Table 3).
Table 3 Blood lipids, acute-phase reactants and disease activity at baseline and after a 12-week intake of n-3 long-chain PUFA (n-3 LC-PUFA)-supplemented or control dairy products
(Mean values and standard deviations)
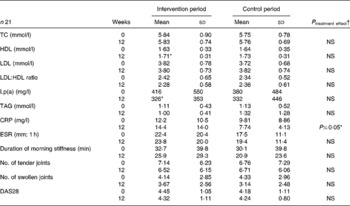
TC, total cholesterol; Lp(a), lipoprotein a; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; DAS28, disease activity score 28.
* Significantly different compared with the start value (P ≤ 0·05).
† End values are significantly different from the control period with baseline values as covariates.
Immunological parameters and cyclo-oxygenase expression
The following immunological parameters were not influenced by the consumption of n-3 LC-PUFA-supplemented dairy products: the concentration of granulocytes per μl; the percentage of CD3+, CD4+, CD8+, CD4+/CD8+, NK, CD3+HLA-DR+, CD8+CD57+, CD25+, CD4+CD25+ cells; as well as the degree of unstimulated COX-2 expression. However, there was a significant increase of granulocytes (percentage of leucocytes), CD19+ cells and COX-1 expression in the intervention period (Table 4; CD are not shown). Moreover, lymphocytes, monocytes, the lipopolysaccharide-stimulated COX-2 expression and CD3+NK+ decreased significantly by the consumption of n-3 LC-PUFA-supplemented dairy products (P ≤ 0·05).
Table 4 Immune markers and cyclo-oxygenase (COX) expression at baseline and after a 12-week intake of n-3 long-chain PUFA-supplemented or control dairy products
(Mean values and standard deviations)
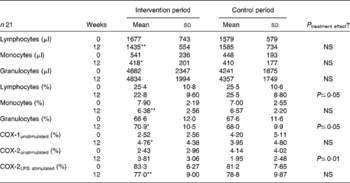
LPS, lipopolysaccharide.
Significantly different compared with the start value *P ≤ 0·05, ** P ≤ 0·01.
† End values are significantly different from the control period with baseline values as covariates.
In the control period, lymphocytes, monocytes, granulocytes, the percentage of CD3+, CD19+, CD4+, CD8+, CD4+/CD8+, NK+, CD3+NK+, CD3+HLA-DR+, CD25+, CD4+CD25+ cells and the COX-expression were not influenced. The numbers of CD57+ cells were significantly decreased (P ≤ 0·01). The final values for granulocytes, CD8+, CD3+HLA-DR+ and COX-2 expression (unstimulated) were significantly higher in the intervention period, whereas the final values of lymphocytes were higher in the control period (P ≤ 0·05).
Urinary parameters
No differences in the Pyr/Cr, Dpyr/Cr concentrations and the Pyr:Dpyr ratio were observed in either the intervention or the control period, and between the end points of the intervention and control phases (Table 5). During the entire study period (2 × 12 weeks), Pyr/Cr decreased significantly from 50·5 (sd 25·8) to 40·2 (sd 13·9) nmol Pyr/mmol Cr and Dpyr/Cr decreased significantly from 11·8 (sd 5·2) to 9·1 (sd 3·7) nmol Dpyr/mmol Cr (P ≤ 0·05).
Table 5 Urinary biomarkers at baseline and after a 12-week intake of n-3 long-chain PUFA-supplemented or control dairy products
(Mean values and standard deviations)
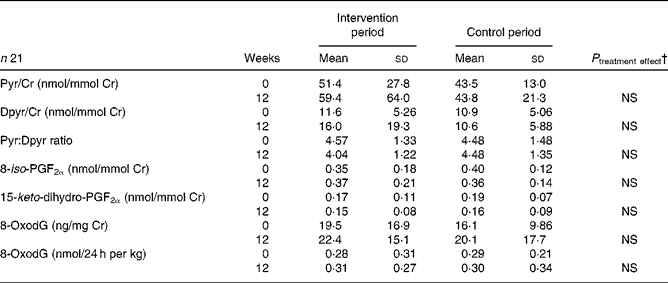
Pyr/Cr, pyridinoline/creatinine; Dpyr/Cr, deoxypyridinoline/creatinine; 8-oxodG, 7,8-dihydro-8-oxo-2′-deoxyguanosine.
† End values are significantly different from the control period with baseline values as covariates.
The urinary concentrations of 8-iso PGF2α (nmol/mmol Cr), 15-keto-dihydro-PGF2α (nmol/mmol Cr) and 8-oxodG (ng/mg Cr; nmol/24 h per kg) remained constant throughout the study. No significant differences of the afore-mentioned parameters were noted between the start and final concentrations of both periods, between the end points of the treatment groups and for the entire term of the study time (2 × 12 weeks; Table 5).
Acute-phase reactants and disease activity parameters
C-reactive protein, erythrocyte sedimentation rate (1 h), the number of tender and swollen joints and the DAS28 did not change significantly during the intervention or control period. The duration of morning stiffness decreased in both periods, but not significantly (Table 3). Here, the end-point values of C-reactive protein in the intervention period were significantly higher than in the control period (P ≤ 0·05).
The baseline values of DAS28 rested in one patient < 3·2 ( → inactive) in the intervention period. In fifteen patients, the baseline values were between 3·2 and 5·1 ( → active) and in five patients >5·1 ( → highly active). Moreover, there was considerable improvement of the DAS28 in three patients (14·3 %), a moderate improvement in three patients (14·3 %), a moderate deterioration in four patients (19·0 %), a strong deterioration in one patient (4·8 %) and no changes in ten patients (47·7 %) in the intervention period.
In the control period, on the other hand, the DAS28 baseline values rested in five patients < 3·2, in twelve patients, they were between 3·2 and 5·1 and, in four patients, >5·1.
In addition, a moderate improvement was seen in the control phase in one patient (4·8 %), a moderate deterioration in four patients (19·0 %), a strong deterioration in one patient (4·8 %) and no changes in fifteen patients (47·7 %).
Correlation analysis of acute-phase reactants, disease activity score and urinary parameters
The baseline values of C-reactive protein correlated significantly with erythrocyte sedimentation rate (r 0·381**) and DAS28 (r; P ≤ 0.01 0·389**). Erythrocyte sedimentation rate start values also correlated significantly with 8-oxodG (nmol/24 h per kg; r 0·334; P ≤ 0.05) and Pyr/Cr (r 0·320; P ≤ 0.05). In addition, Pyr/Cr correlated significantly with Dpyr/Cr (r 0·722; P ≤ 0.01).
Furthermore, 8-iso-PGF2α correlated significantly with 8-oxodG (nmol/24 h per kg; r 0·376; P ≤ 0.05) and 15-keto-dihydro-PGF2α (r 0·770; P ≤ 0.01). The baseline values of COX-1 correlated significantly with COX-2-unstimulated (r 0·544; P ≤ 0.01), 8-iso-PGF2α (r 0·367; P ≤ 0.01) and 15-keto-dihydro-PGF2α (r 0·312; P ≤ 0.01). Additionally, there were correlations between COX-2 unstimulated and Dpyr/Cr (r 0·239; P ≤ 0.05), 8-iso-PGF2α (r 0·397; P ≤ 0.01) and 15-keto-dihydro-PGF2α (r 0·262; P ≤ 0.05).
Discussion
Changes in fatty acid distribution
The FA analysis of plasma lipids, CE and PL from study subjects consuming dairy products supplemented with moderate doses of n-3 LC-PUFA over a long-term intervention showed a significant increase in EPA and DHA, leading to a decreased AA:EPA and AA:n-3 LC-PUFA ratio (Table 1). The change in the ratio of the eicosanoid precursory FA is favourable and indicates a reduction of PG and leukotriene formation from AA.
The results indicate a good compliance with the dairy products and a high bioavailability of the supplemented FA. Interestingly, EPA and DHA accumulated more slowly in PL than in total plasma lipids and CE. Similar results regarding the accumulation of EPA and DHA were found by Adam et al. (Reference Adam, Beringer and Kless3). Furthermore, the concentrations of n-3 FA and n-6 FA were lower in PL in comparison with that in total plasma lipids and CE. Particularly, the concentrations of EPA and AA were very low in PL (Table 1).
Improvement in blood lipid values
The baseline mean concentrations of HDL and TAG were within the normal range, though the total cholesterol, LDL and lipoprotein a levels were elevated compared with the reference values. Although, the consumption of verum products improved blood lipids, in the control group, the effects were weaker (Table 3). The baseline values of TAG were low in all patients. Perhaps if TAG values would have been elevated, the observed TAG-lowering effect of n-3 LC-PUFA supplementation might be more pronounced. These results confirm the TAG-lowering effect of n-3 LC-PUFA described in previous studies and reviews(Reference Holub and Holub4, Reference Schacky25–Reference Milte, Coates and Buckley29).
Moreover, out results suggest an atherosclerosis-preventive and, thus, cardioprotective effect of long-term consumption of dairy products via the modulation of blood lipids and other cardiovascular risk factors. RA patients have a higher risk of CVD because of a deteriorated vascular function and a prolonged elevation of C-reactive protein values(Reference Wolfe, Mitchell and Sibley30–Reference Wong, Toh and Wilson34). Hence, inhibiting the primary development and a subsequent progression of CVD by means of controlling the risk factors is particularly important for RA patients.
The results of many studies indicated that the intake of high quantities of n-3 LC-PUFA can make a contribution towards the prevention of CVD(Reference Holub and Holub4, Reference Demaison and Moreau35–Reference Hjerkinn, Seljeflot and Ellingsen39). In the present study, the consumption of dairy products supplemented with moderate doses of n-3 LC-PUFA improved cardiovascular risk factors to a similar degree achieved in other studies using high doses of n-3 LC-PUFA.
Influences on immunological parameters and cyclo-oxygenase expression
All immunological parameters were within the normal range, except the fact that the mean values of CD3+NK+, CD25+ and CD4+CD25+ were elevated compared with the reference values. The bulk of the analysed CD, e.g. T-cells and T-helper cells (CD3+ and CD4+), was not affected through the consumption of n-3 LC-PUFA-supplemented dairy products. However, the effects on lymphocytes, monocytes (Table 4) and CD3+NK+ (cytotoxic T-cells) were beneficial. It can be concluded that the intake of n-3 LC-PUFA-supplemented dairy products suppresses the specific and unspecific immune responses and, possibly, contributes to a reduction of the inflammatory process.
In the present study, n-3 LC-PUFA influenced COX expression in the whole-blood monocytes by increasing COX-1 and decreasing lipopolysaccharide-stimulated COX-2 (Table 4). Since COX-1 is not inducible by external stimuli under both physiological and pathological conditions(Reference Tapiero, Nguyen and Couvreur40), the significant increase of COX-1 cannot be the result of n-3 LC-PUFA supplementation. By contrast, COX-2 expression can be induced by various stimuli, e.g. cellular stress and inflammatory cytokines(Reference Smith, Garavito and DeWitt41, Reference Maloney, Kutchera and Albertine42). The COX-2 overexpression leads to an increase in PGE2 biosynthesis and angiogenesis, further mediating inflammation and mitogenesis(Reference Daniel, Liu and Morrow43, Reference Patrignani, Panara and Greco44). In the present study, the COX-2 expression induced by bacterial lipopolysaccharide was diminished by n-3 LC-PUFA (Table 4). Similarly, n-3 LC-PUFA could also reduce COX-2 expression induced by other stimuli. This is in accordance with the previous data that show a reduction of COX-2 expression by n-3 LC-PUFA(Reference Mantzioris, Cleland and Gibson45–Reference Horia and Watkins47). In summary, inflammatory mediators, such as COX-2 and PGE2, that are involved not only in the pathogenesis of degenerative joint diseases, but also in that of inflammatory joint diseases are reduced by n-3 LC-PUFA supplementation.
Thus, n-3 LC-PUFA act as selective COX-2 inhibitors. Furthermore, these innovative dairy products possibly support non-steroidal anti-inflammatory drugs or glucocorticoid therapy in RA patients. Side effects of non-steroidal anti-inflammatory drugs such as gastric lesions and renal toxicity are associated with the inhibition of COX-1(Reference Bombardier, Laine and Reicin48). In this regard, the increase in COX-1 expression despite the fact that seventeen of the study patients took a daily dose of non-steroidal anti-inflammatory drugs can be interpreted as a positive result.
Effects on hydroxypyridinium crosslinks
The baseline values of urinary collagen crosslinks were elevated in RA patients compared with the reference values (Table 5). These results were found to be in agreement with the data of previous studies(Reference Hein, Franke and Mueller21, Reference Spector, James and Hall50, Reference Kollerup, Hansen and Horslev-Petersen51). An increased crosslink excretion reflects an increased turnover and degradation of articular cartilage, as well as an increased bone resorption(Reference Robins, Duncan and Wilson52, Reference Takahashi, Kushida and Hoshino53). The correlations between the urinary crosslinks, inflammation markers and disease activity in the present study and from the previous data(Reference Kollerup, Hansen and Horslev-Petersen51, Reference Bölzer, Müller and Bräunig54Reference Kaufmann, Mueller and Voigt55) substantiate an association between rheumatic inflammation processes and collagen degradation.
Thus, the long-term consumption of dairy products prevents cartilage and bone resorption in RA patients, which is indicated by a decrease in the Pyr/Cr and Dpyr/Cr excretion designated by the normal end values recorded in the study.
Parameters of oxidative stress and inflammation
The urinary concentrations of 8-iso PGF2a and 15-keto-dihydro-PGF2a were not significantly altered by the intervention (Table 5). The major F2-isoprostanes 8-iso-PGF2α is a reliable biomarker for in vivo measurement of lipid peroxidation. It is derived from AA using non-enzymatic free-radical-induced peroxidation and oxidative stress(Reference Basu22, Reference Quaggiotto, Leitch and Falconer56). 15-Keto-dihydro-PGF2α is a major metabolite of PGF2α and serves as an inflammation marker that indicates COX-2-dependent lipid peroxidation(Reference Basu23). A strong correlation between oxidative injury (8-iso-PGF2α) and inflammation response (15-keto-dihydro-PGF2α) was also found by Basu et al. (Reference Basu, Whiteman and Mattey57). Oxidative injury is an important mechanism involved in the chronic inflammatory state in rheumatic patients(Reference Basu, Whiteman and Mattey57, Reference Halliwell and Chirico58). Possibly, an increased consumption of PUFA can be associated with a higher oxidative stress and a rise in lipid peroxides(Reference Halliwell and Chirico58). However, other investigations have shown a significant decrease of 8-iso-PGF2α after intervention with n-3 PUFA(Reference Higdon, Liu and Du59, Reference Nälsen, Vessby and Berglund60). The study results also indicate that the consumption of moderate doses of n-3 LC-PUFA does not lead to an increase in oxidative injury (Table 5). The 8-iso-PGF2α and 15-keto-dihydro-PGF2α concentrations determined in RA patients (Table 5) are comparable with concentrations in healthy subjects(Reference Kuhnt, Wagner and Kraft24).
8-OxodG, induced by reactive oxygen species, is a biomarker of promutagenic DNA lesions(Reference Bashir, Harris and Denman61). In the present study, the urinary 8-oxodG concentrations of RA patients were strongly elevated in comparison with healthy subjects (Table 5)(Reference Kuhnt, Wagner and Kraft24). Furthermore, elevated concentrations of 8-oxodG were found in lymphocytes and synovial fluid of RA patients(Reference Bashir, Harris and Denman61, Reference Hajizadeh, DeGroot and TeKoppele62). An increased inflammation-related production of reactive oxygen species may explain the elevated 8-oxodG values in RA patients. Our result indicates that the consumption of moderate doses of n-3 LC-PUFA causes no additional DNA damage (Table 5).
Influence on clinical symptoms
In the present study, an influence of n-3 LC-PUFA-supplemented dairy products on clinical symptoms or inflammation markers was not evident. A few previously published studies and reviews have shown potentially therapeutic benefits of n-3 LC-PUFA in RA patients(Reference Adam, Beringer and Kless3, Reference Kremer, Lawrence and Jubiz12, Reference Cleland, Proudman and Hall63). The consumption of n-3 LC-PUFA-supplemented dairy products diminished the disease activity in only a small number of our patients correlating with the data from other studies(Reference Geusens, Woulters and Nijs13, Reference Volker, Fitzgerald and Major14). The values of C-reactive protein remained largely unaltered in other investigations with n-3 LC-PUFA(Reference Adam, Beringer and Kless3, Reference Madsen, Christensen and Schmidt64). This low effect of n-3 LC-PUFA supplementation on disease activity and acute-phase reactants could be accounted for the unaltered AA concentrations observed in plasma lipids, CE and PL due to an unlimited AA intake in the present study and the continuous AA intake with the study products. The results indicated that the limitation of AA intake might be the precondition for the anti-inflammatory effects and the benefit for RA patients through the consumption of n-3 LC-PUFA.
Conclusions
The consumption of dairy products enriched with moderate doses of n-3 LC-PUFA improves blood lipids, suppresses the immune response and reduces the lipopolysaccharide-stimulated COX-2 expression. Additionally, a long-term consumption of dairy products prevents elevated cartilage and bone resorption in RA, indicated by decreased excretion of hydroxypyridinium crosslinks. Finally, the intake of moderate doses of n-3 LC-PUFA over a long period does not induce oxidative injury, lipid peroxidation or DNA damage.
Acknowledgements
The present study was supported by a grant from the German Federal Ministry of Education and Research (BMBF). C. D., R. S. and G. J. were involved in the study design. C. D. was responsible for the conduct of the study, data acquisition, statistical analysis and for producing the manuscript. C. D. and G. J. were responsible for data interpretation and critical revision of the manuscript. R. S. was in charge of obtaining the funding and G. H. and T. E. were responsible for medical care of the study patients. C. D., A. M. and S. B. performed the experimental work. G. J. supervised the present work. None of the authors had any personal or financial conflicts of interest. Special thanks go to the HERZGUT™ creamery for creating and allocating the study products.








