There are two main and several minor types of diabetes mellitus. Of diabetic patients, 5% suffer from type 1 diabetes with absolute insulin deficiency, while about 90 % of all diabetics are affected by type 2 diabetes, which is associated with insulin resistance and obesity. The prevalence of obesity and diabetes mellitus is increasing worldwide. A few decades ago, mainly the industrialised countries were affected, but presently, diabetes mellitus is a problem in developing countries also(Reference Zimmet, Alberti and Shaw1). However, the population of developing countries often has no access to adequate medical care and drugs due to economic or infrastructure reasons. Therefore, nutrition and dietary measures play a crucial role in the treatment of insulin resistance in these countries. Vegetables and fruits with antidiabetic effects may contribute to the improvement of metabolic control.
In traditional Asian medicine, the bitter gourd (BG, Momordica charantia) is known for its blood glucose-lowering effects in hyperglycaemic patients. Powder and extracts of the fruit, as well as teas from the stems and leaves of the plant, are used(Reference Cummings, Hundal and Wackerhage2–Reference Krawinkel and Keding5). In vivo studies, especially those done on rodents with type 1 diabetes, but also a few studies done on insulin-resistant rodents demonstrated the blood glucose-lowering effects of BG and its extracts. However, due to the many different experimental models and extracts, it is not sufficiently clear what substances or substance groups are mainly responsible for the blood glucose-lowering effect of BG and what biochemical mechanisms underlie this effect. Saponins, triterpenes, conjugated fatty acids and other substances which, depending on the experimental design, inhibit the intestinal absorption of monosaccharides(Reference Oishi, Sakamoto and Udagawa6), enhance insulin secretion(Reference Singh, Adeghate and Cummings3) or increase insulin sensitivity of insulin-dependent tissues(Reference Nerurkar, Lee and Motosue7, Reference Miura, Itoh and Iwamoto8) are discussed.
One possible explanation for the increased insulin sensitivity is a decreased activity of protein tyrosine phosphatase 1B (PTP 1B). PTP 1B acts as a physiological antagonist of the insulin receptor and its signal by dephosphorylating both the insulin receptor and the insulin receptor substrates(Reference Müller, Bosse and Most9, Reference Clampit, Meuth and Smith10). Increased PTP 1B levels or an increased activity of this enzyme were found in insulin-resistant and obese patients(Reference Ahmad, Azevedo and Cortright11). Mohammad et al. (Reference Mohammad, Wang and McNeill12) showed that untreated Zucker fatty rats exhibit a 200 % increased PTP 1B activity in the skeletal muscle in comparison with their healthy companions. Increased PTP 1B levels were also found in insulin-resistant obese patients(Reference Ahmad, Azevedo and Cortright11). In contrast, non-diabetic mice without PTP 1B gene (PTP 1B − / − mice) showed increased insulin sensitivity and, even under a high-fat diet, no weight gain(Reference Elchebly, Payette and Michaliszyn13), a reduced fat cell mass and an increased BMR(Reference Klaman, Boss and Peroni14). Several studies suggest that the reduction in PTP 1B expression and activity is sufficient to enhance the insulin signalling pathway and to improve insulin sensitivity(Reference Gum, Gaede and Koterski15). Therefore, the inhibition of PTP 1B or the reduction in PTP 1B levels is a potential target for the prevention and treatment of insulin resistance and type 2 diabetes. Substances that reduce PTP 1B expression or activity are more and more being considered as important.
The aim of the present study was to investigate the molecular aspect of the antidiabetic effects of different BG fractions in type 2 diabetic db/db mice and in particular their tissue-specific effect on PTP 1B regulation. Additionally, we measured the concentration of thiobarbituric acid-reactive substances (TBA-RS) as a marker for oxidative stress and damage in the liver, adipose tissue and skeletal muscle.
Materials and methods
Preparation of bitter gourd extracts
The saponin fraction, the lipid fraction and the hydrophilic residue were extracted using the methods published by Oishi et al. (Reference Oishi, Sakamoto and Udagawa6) and Chao & Huang(Reference Chao and Huang16) combinedly.
Fresh fruits, described as green BG by Chao & Huang(Reference Chao and Huang16), were grown in Frankfurt/Main, Germany. The fruits were cut, freeze-dried (Gamma 1-20; Christ, Osterode, Germany) and ground. Then, 200 g of homogeneous powder were stirred in 3 litres of ethyl acetate in the dark for 2 h and filtered. Using the rotary evaporator (Laborota digitally 4002; Heidolph, Schwabach, Germany), the ethyl acetate was evaporated at 35°C to obtain the lipid fraction with a concentration of 3·2 % of the whole fruit on a DM basis. The non-ethyl acetate-soluble filter residue was again stirred in 3 litres of methanol in the dark for 2 h and filtered. The filtrate was reduced to a dry residue in a rotary evaporator at 42°C, and the dry residue was dissolved in 250 ml of distilled water and 250 ml of n-butanol. The water phase and the n-butanol phase were separated, and were then evaporated at 40°C to obtain the hydrophilic residue (12·2 % of DM) and the saponin fraction (4·2 % of DM). Concentrations of the fractions were similar to the data reported in the literature(Reference Oishi, Sakamoto and Udagawa6, Reference Chuang, Hsu and Chao17).
Animal model and experiment
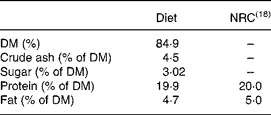
A total of forty-five 4-week-old male db/db mice (BKS. Cg-+Leprdb/+Leprdb/01aHsd; Harlan Winkelmann GmbH, Borchen, Germany) were assigned to five groups of nine each. For the 5-week trial, the mice were kept individually in plastic cages at 22°C and under a 12 h day–12 h night cycle. The mice had free access to a self-made non-purified standard diet (Table 1) with all nutrients at the level of the National Research Council(18) recommendations (Table 2) and to drinking-water.
Table 1 Composition of the self-made standard diet

* Minerals and amino acids were added by taking the native concentrations of the minerals and amino acids in wheat, maize, barley, soyabean meal, wheat bran and oats into consideration to achieve the recommended amounts(18).
† All vitamins were added according to the National Research Council(18) recommendations without correction.
Table 2 Macronutrients of the diet and comparison with the National Research Council (NRC)(18) recommendations
In addition, the mice were given the whole fruit powder, the lipid fraction, the saponin fraction or the hydrophilic residue of BG at a daily oral dosage of 150 mg/kg body weight for 5 weeks. The whole fruit powder and the extracts were dissolved in sterile tap water (45 mg/ml), and were given via stomach feeding. The lipid fraction was dispersed to ensure that hydrophobic substances were homogeneously distributed in the water. The control group was given sterile tap water.
After 5 weeks and fasting for 6 h, the mice were anaesthetised with CO2, blood was collected from the heart using EDTA-Monovettes (KABAVETTE®; KABE Laboratory GmbH, Nümbrecht-Elsenroth, Germany), and then the mice were decapitated. Liver, skeletal muscle and adipose tissue were immediately frozen in liquid N2 and stored for further analysis at − 80°C. As a long-term parameter of blood glucose levels, glycated Hb levels were measured using a test kit (HA1C Kit) and an analyser (Dimension® Xpand™) obtained from Siemens Healthcare Diagnostics (Eschborn, Germany). Mouse erythrocytes have a lifespan of about 22 d(Reference Wilmanns von, Sauer and Gelinsky19).
Institutional and national guidelines for the care and use of animals were followed, and the protocol of the animal study was approved by the Animal Protection Unit of the Regional Council of Giessen, Germany (reference: V54-19c20/15cGI 19/1).
Determination of protein tyrosine phosphatase 1B activity (Zhu & Goldstein method)
PTB 1B activity was determined according to the method of Zhu & Goldstein(Reference Zhu and Goldstein20). Liver, skeletal muscle and adipose tissue were homogenised using buffer A (50 mm-HEPES, 50 mm-NaCl, 0·5 mm-EDTA, 0·1 mm-phenylmethanesulphonylfluoride, pH 7·2), and were centrifuged (Labofuge 400R, Heraeus Instruments, Hanau, Germany) for 30 min at 13 000 rpm and 2°C. A quantity of 10 μl of the cytosolic supernatant was incubated with 240 μl of buffer A at room temperature for 5 min. Subsequently, 250 μl of buffer A containing 10 mm-p-nitrophenylphosphate were added. Due to the activity of PTP 1B, p-nitrophenylphosphate was dephosphorylated to p-nitrophenol. This reaction was stopped after 20 min using 500 μl of a 2 m-NaOH solution. In an alkaline environment, p-nitrophenol changes to p-nitrophenolate anion, which is coloured intensely yellow and can be quantified by a photometer (Cary 50 Bio; Varian, Melbourne, Vic, Australia) at 405 nm.
The measurement was repeated with a buffer containing 2 mm-dithiothreitol, a reducing agent, to measure the reversible inhibition of PTP 1B.
In order to refer PTP 1B activity to the protein content, the protein content of the prepared samples was determined by the Bradford(Reference Bradford21) method.
Determination of protein tyrosine phosphatase 1B expression via Western blot analysis
Skeletal muscle was homogenised using a radioimmunoprecipitation assay lysis buffer (50 mm-Tris–HCl, 150 mm-NaCl, 1 mm-phenylmethanesulphonylfluoride, 1 mm-EDTA, 10 g/l sodium deoxycholate, 1 g/l SDS, 10 ml/l Triton X-100, pH 7·4), and the protein content was measured by the Bradford(Reference Bradford21) method. A quantity of 40 μg of the protein was then separated on a 15 % (v/v) polyacrylamide gel, and the separated proteins were transferred onto a polyvinyldifluoride membrane (PALL BioTrace 0·45 μl™) via a semi-dry blotting technique. After blocking and washing, first the PTP 1B antibody and then the secondary antibody, linked to an alkaline phosphatase, were added. Blots were developed using a Mg-containing reaction buffer with nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate. Optical density of the protein bands was measured using GenTools from SynGene (Synoptics Limited, Cambridge, UK).
Determination of protein tyrosine phosphatase 1B gene expression via RT-PCR
Skeletal muscle RNA was isolated according to the method described by Chomczynski & Sacchi(Reference Chomczynski and Sacchi22). To analyse RNA purity and quantity, RNA was dissolved in nuclease-free water and measured photometrically (Genesys 10 UV; Thermo, Bonn, Germany) at 260 and 280 nm. RNA with a quotient of the absorbances between 1·6 and 1·8 at 260 and 280 nm was considered pure, and was used for further analyses. For further quality control, RNA was separated electrophoretically on a 1·5 % agarose gel.
For complementary DNA synthesis, a kit obtained from MBI-Fermentas (Revert AidTM First, Strand complementary DNA Synthesis Kit; Fermentas, St Leon-Rot, Germany) was used.
For gene-specific PCR, the following forward (fw) and reverse (rev) primers were used for β-actin and PTP 1B genes:
β-Actin fw (5′ → 3′): TGT TAC CAA CTG GGA CGA CA
β-Actin rev (5′ → 3′): TCT CAG CTG TGG TGG TGA AG
PTP 1B fw (5′ → 3′): GAT GGA GAA GGA GTT CGA GGA G
PTP 1B rev (5′ → 3′): CCA TCA GTA AGA GGC AGG TGT
Determination of thiobarbituric acid-reactive substances
The concentration of TBA-RS, in tissues and organs is a marker for oxidative stress, which is normally increased in diabetes. Products of lipid peroxidation and degradation, such as malondialdehyde, react with thiobarbituric acid, which can then be quantified photometrically (Cary 50 Bio; Varian) at 532 nm. We measured the concentration of TBA-RS in the cytosol of skeletal muscle, adipose tissue and liver according to the method described by Wong et al. (Reference Wong, Knight and Hopfer23) and modified by Khoschsorur et al. (Reference Khoschsorur, Winklhofer-Roob and Rabl24).
Statistical analysis
To test the differences between groups (P ≤ 0·05), we performed ANOVA, followed by post hoc testing (two-sided). Differences between the control and all the treated groups (P ≤ 0·05) were examined using t tests (two-sided) for unpaired samples. Increase in body weight (P ≤ 0·05) was tested using t tests (two-sided) for paired samples. Normal distribution and homoscedasticity were ascertained for all the tests. Correlations between parameters were analysed according to Pearson or Spearman for non-parametric correlations when normal distribution was not given. We used Statistical Package for Social Sciences 17.0 program for Windows (SPSS, Inc., Chicago, IL, USA).
Results
At the beginning of the study, the mean body weight of the mice (29·2 (sd 0·2) g) did not differ within the five groups. Body weight of all the mice increased significantly (P < 0·0001) during the 5-week trial. The control group showed the highest body weight gain from the first week onwards (Fig. 1). After 5 weeks, body weight was significantly lower when the mice were given the whole fruit powder (P = 0·005), the lipid fraction (P = 0·007), the saponin fraction (P = 0·007) or the hydrophilic residue (P = 0·05) in comparison with that of the control mice (Table 3).
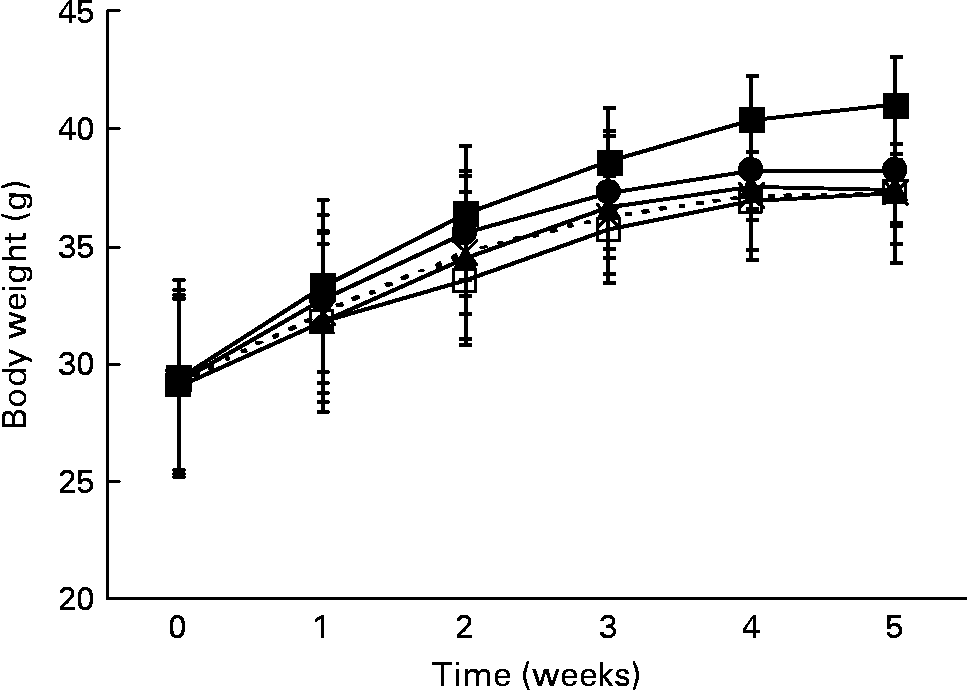
Fig. 1 Body weight gain of the male db/db mice during 5 weeks of bitter gourd treatment in comparison with that of the control mice (means and standard deviations). –■–, Control; –□–, whole fruit; –▲–, lipids; – × –, saponins; –●–, hydrophilic residue.
Table 3 Feed intake, body weight and glycated Hb of male db/db mice after 5 weeks of bitter gourd treatment in comparison with that of the control mice
(Mean values and standard deviations)
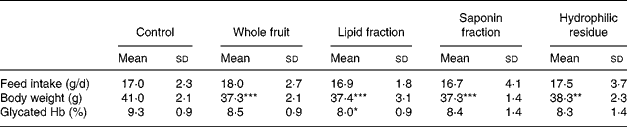
Mean values were significantly different from those of the control mice: *P ≤ 0·1, **P ≤ 0·05, ***P ≤ 0·01.
After 5 weeks, the control group also showed the highest levels of glycated Hb compared with all the four BG-treated groups (P = 0·02). However, the effect on glycated Hb level for each individual BG-treated group was not statistically significant. The lipid fraction had the strongest effect, and it tended (P = 0·075) to reduce glycated Hb levels from 9·3 % (in control mice) to 8·0 % (in lipid fraction-treated mice) (Table 3). No differences existed in feed intake (Table 3).
Native PTP 1B activity of liver and adipose tissue did not differ between the groups (data not shown). PTP 1B activity of skeletal muscle cytosol was significantly reduced in mice that were given the saponin fraction (P = 0·05), and it tended to be lower after treatment with the lipid fraction (P = 0·07) of BG compared with that of the control group. Mean PTP 1B activity was 25 or 23 % lower after the saponin or the lipid treatment, respectively (Fig. 2). The addition of the reducing agent dithiothreitol reversed the inhibition of PTP 1B in the saponin-treated mice (P = 0·02), indicating a reversible inhibition of the enzyme via oxidation (Fig. 3).

Fig. 2 Native protein tyrosine phosphatase 1B (PTP 1B) activity of skeletal muscle cytosol of the male db/db mice after 5 weeks of bitter gourd treatment in comparison with that of the control mice (means and standard deviations). Mean value was significantly different from that of the control mice: *P = 0·07, **P = 0·05.

Fig. 3 Protein tyrosine phosphatase 1B (PTP 1B) activity of skeletal muscle cytosol before and after addition of 2 mm-dithiothreitol (DTT) in the male db/db mice after 5 weeks of treatment with bitter gourd lipids or saponins in comparison with that of the control mice (means and standard deviations). ![]() , − 2 mm DTT; □,+2 mm DTT. *Mean value was significantly different from that before the addition of DTT (P = 0·02).
, − 2 mm DTT; □,+2 mm DTT. *Mean value was significantly different from that before the addition of DTT (P = 0·02).
In the lipid-treated mice, dithiothreitol did not increase PTP 1B activity of the skeletal muscle cytosol (Fig. 3). This might be due to an irreversible inhibition or due to a decreased expression of the enzyme. Consequently, RT-PCR and Western blot analysis were performed to compare PTP 1B gene expression and PTP 1B expression of the control and lipid fraction-treated mice. Contrary to expectations, there was no significant regulation at the gene (Fig. 4(a)) or protein (Fig. 4(b)) level. Western blot analysis showed even an increase of 40 % rather than a decrease in PTP 1B levels (Fig. 4(b)) in the lipid-treated mice. However, this finding was NS.

Fig. 4 Protein tyrosine phosphatase 1B (PTP 1B) gene expression (a) and PTP 1B expression (b) (means and standard deviations) in the skeletal muscle cytosol of the male db/db mice treated with bitter gourd lipids for 5 weeks in comparison with that of the control mice.
Concentrations of TBA-RS were more than 40 % reduced in the adipose tissue of the mice treated with BG saponins (P = 0·005) or lipids (P = 0·003) compared with those of the control mice (Fig. 5(a)). While BG saponins and lipids had no significant effect on the TBA-RS concentration in the skeletal muscle cytosol, the concentration of TBA-RS in the skeletal muscle cytosol was 65 % lower in the mice treated with the hydrophilic residue of BG in comparison with that in the control mice (P = 0·001) (Fig. 5(b)). There was no influence of BG administration on TBA-RS concentration in the liver (data not shown).

Fig. 5 Concentration of thiobarbituric acid-reactive substances (TBA-RS) (μmol/g protein) in adipose tissue (a) and skeletal muscle (b) of the male db/db mice after 5 weeks of bitter gourd treatment compared with that of the control mice; data shown as means and standard deviations. Mean value was significantly different from that of the control mice: *P = 0·005, **P = 0·003, ***P = 0·001.
Discussion
There are far more patients suffering from type 2 diabetes mellitus than from type 1 diabetes mellitus. Therefore, investigations on the antidiabetic effects of BG in insulin-resistant and type 2 diabetic rodents are relevant for the development of new applications. Data obtained from in vitro and in vivo studies show positive effects of BG on insulin sensitivity(Reference Yibchok-Anun, Adisakwattana and Yao25, Reference Huang, Hong and Wong26). However, some results are contradictory(Reference Singh, Adeghate and Cummings3, Reference Roffey, Atwal and Johns27, Reference Yibchok-Anun, Adisakwattana and Yao28), and the knowledge about active substances, the most effective dosage and the biochemical mechanism is still insufficient.
While some in vitro studies showed that 5′-AMP-activated protein kinase activation is involved in the increased glucose uptake of adipocytes or myocytes(Reference Tan, Ye and Turner29, Reference Cheng, Huang and Chang30), others could demonstrate that BG influences insulin signalling. In these studies, the addition of wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase, to the medium inhibited cellular glucose uptake with or without BG treatment(Reference Singh, Adeghate and Cummings3, Reference Cummings, Hundal and Wackerhage2, Reference Roffey, Atwal and Johns27). Thus, it can be assumed that BG influences insulin signalling before the phosphatidylinositol 3-kinase reaction, presumably at the PTP 1B regulation level.
Miura et al. (Reference Miura, Itoh and Iwamoto8) were among the first to prove the antidiabetic effect of BG in type 2 diabetic animals. A water-soluble extract of BG resulted in significantly (P ≤ 0·01) lower blood glucose and insulin levels, and improved glucose tolerance and insulin sensitivity in male KK-Ay mice. Regarding the mechanism of the insulin-sensitising effect of BG, they could show that the level of GLUT4 in the cell membrane was 144 % higher in the skeletal muscle of the mice treated with BG than in that of the control mice(Reference Miura, Itoh and Iwamoto8). Increased GLUT4 recruitment into the cell membrane is the result of increased insulin signalling, starting at the insulin receptor. In this context, Nerurkar et al. (Reference Nerurkar, Lee and Motosue7) could demonstrate that BG juice increased tyrosine phosphorylation of the insulin receptor and of the insulin receptor substrates 1 and 2 in the livers of female mice fed a high-fat diet (58·0 kJ % fat). Furthermore, an enhanced interaction of insulin receptor substrate 1 and phosphatidylinositol 3-kinase was observed. Similar results for the effect of BG after the consumption of a high-fat diet (59 % of total energy from fat) on the insulin signalling pathway were reported by Sridhar et al. (Reference Sridhar, Vinayagamoorthi and Arul Suyambunathan31). BG improved both glucose tolerance and insulin sensitivity significantly (P ≤ 0·05). Western blot analysis showed a significant (P ≤ 0·001) increase in insulin-stimulated tyrosine phosphorylation of insulin receptor substrate 1 in the calf muscle.
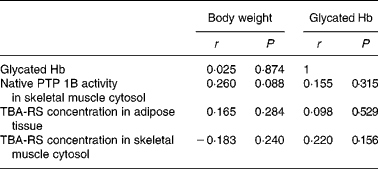
In the present study, administration of BG extracts reduced body weight gain (P < 0·0001) and glycated Hb levels (P = 0·02) in growing db/db mice. Greer et al. (Reference Greer, Ware and Lefer32) published data obtained from 10-week-old db/db mice that not only had increased blood glucose levels but also had increased insulin levels compared with the age-matched control mice, indicating that the mice suffered from insulin resistance. Similar to our control mice, db/db mice used in the study done by Greer et al. (Reference Greer, Ware and Lefer32) had a mean glycated Hb level of 9·2 %, whereas healthy mice had a mean glycated Hb level of 4·5 %. In the present study, 9-week-old control mice had a mean glycated Hb level of 9·3 %. Thus, reduced glycated Hb levels after BG treatment are the result of increased insulin sensitivity. Hence, the present study provides further evidence for the insulin-sensitising and antidiabetic effects of BG in type 2 diabetes. As there was no correlation between body weight and glycated Hb levels (Table 4), the antidiabetic effect of BG cannot be explained by reductions in body weight after BG administration. However, the inhibition of PTP 1B activity in the skeletal muscle cytosol after the administration of the saponin or the lipid fraction of BG provides information on the biochemical mechanism of this antidiabetic effect. Reduced PTP 1B activity is directly associated with increased insulin sensitivity. The insulin signalling pathway and tyrosine phosphorylation of the insulin receptor and its substrates and finally glucose uptake into the cell via GLUT4 can be amplified by the inhibition of PTP 1B(Reference Müller, Bosse and Most9, Reference Nieto-Vazquez, Fernández-Veledo and de Alvaro33). Thus, the down-regulation of PTP 1B activity by BG saponins and lipids may provide mechanistic information for the increased insulin sensitivity and the above-mentioned results(Reference Nerurkar, Lee and Motosue7, Reference Miura, Itoh and Iwamoto8, Reference Sridhar, Vinayagamoorthi and Arul Suyambunathan31). Although saponins are soluble in water, we separated the saponin fraction from the hydrophilic residue using n-butanol. Some authors(Reference Miura, Itoh and Iwamoto8, Reference Roffey, Atwal and Johns27) mentioned above used aqueous BG extracts presumably containing saponins(Reference Ojewole, Adewole and Olayiwola34). The insulin-sensitising effect of the aqueous BG extracts of these studies may have been at least partly induced by saponins. In view of the present results, hydrophilic substances other than saponins do not seem to be as effective as the saponin fraction in increasing insulin sensitivity. In the present study, mice treated with the hydrophilic residue did not show significant effects with regard to glycated Hb level or PTP 1B activity. The present study indicates that saponins and the saponin fraction are the most effective water-soluble compounds of BG when treating type 2 diabetic mice. Saponins isolated from Argania spinosa were also shown to enhance insulin signalling via insulin-dependent activation of protein kinase B in hepatoma tissue culture cells(Reference Samane, Noël and Charrouf35). Possibly, these findings can also be explained by PTP 1B inhibition, and the insulin-sensitising effect of saponins via PTP 1B inhibition is not limited to specific BG saponins, but it is the same for other saponins.
Table 4 Correlations between body weight, glycated Hb levels, protein tyrosine phosphatase 1B (PTP 1B) activity and concentrations of thiobarbituric acid-reactive substances (TBA-RS) in male db/db mice after 5 weeks of bitter gourd treatment and in the control mice
r, Correlation coefficients.
Nevertheless, BG saponins or other substances in the saponin fraction may also improve insulin sensitivity or reduce body weight gain via other mechanisms. For example, it is known that the saponin fraction of BG inhibits intestinal disaccharidases and pancreatic lipase dose dependently(Reference Oishi, Sakamoto and Udagawa6, Reference Mahomoodally, Fakim and Subratty36).
The lipid fraction of BG also tended to reduce PTP 1B activity of the skeletal muscle cytosol (P = 0·07). In contrast to the saponin fraction, the lipid fraction led to an inhibition that was not reversible by dithiothreitol. Contrary to our initial assumption that BG lipids reduce PTP 1B expression at either the gene or protein level, the present results show no regulation of PTP 1B expression in the mice treated with BG lipids compared with the control mice. The mechanism by which BG lipids inhibit PTP 1B activity has not been clarified so far. A possible explanation is that BG lipids inhibit PTP 1B activity irreversibly, which might lead to the slight (NS) up-regulation of the enzyme at the protein level. The cysteine residue 215 at the active site of the enzyme is usually oxidised to sulphenic acid, which leads to a reversible inhibition of PTP 1B. However, the sulphenic acid can be further oxidised to sulphinic and sulphonic acids. These oxidations and the resulting inhibition are irreversible(Reference Denu and Tanner37). For BG lipids, an irreversible inhibition of PTP 1B via oxidation or other mechanisms is most likely to occur.
We found a positive, but NS correlation between PTP 1B activity and body weight (Table 4). Thus, BG lipids and saponins may be able to inhibit PTP 1B independent of the body weight. Although the mechanism is not clarified, it is an important finding that BG compounds are able to regulate PTP 1B in the skeletal muscle of db/db mice. Delibegovic et al. (Reference Delibegovic, Bence and Mody38) could prove that a complete or 50 % deletion of muscle-specific PTP 1B in mice fed a high-fat (55 % (w/w) fat) or a normal diet leads to decreased blood glucose and insulin levels, ameliorated glucose clearance and increased insulin sensitivity without lowering the body weight. These results show that decreased PTP 1B levels of skeletal muscle are able to increase whole body insulin sensitivity independently of the body weight. However, correlation between PTP 1B activity of the skeletal muscle cytosol and glycated Hb levels was positive, but NS (Table 4). This leads to the conclusion that other mechanisms might also be involved in the antidiabetic effects of BG.
Apart from their effect on PTP 1B regulation, BG lipids and saponins reduced lipid peroxidation of adipose tissue significantly. The amount of TBA-RS was tenfold higher in the adipose tissue than in the skeletal muscle, and might be more relevant in the prevention of diabetic complications associated with oxidative stress. This protection against oxidative stress is independent of lower blood glucose levels, as there was no significant correlation between glycated Hb levels and TBA-RS concentrations in the adipose tissue (Table 4).
The lipid fraction of BG contains a high amount of conjugated linolenic acids such as cis-9, trans-11, trans-13-18 : 3(Reference Suzuki, Arato and Noguchi39), which might be responsible for the protection against lipid peroxidation in the mice treated with the lipid fraction. In vitro, the addition of 0·05 % of cis-9, trans-11, trans-13-18 : 3, extracted from BG seeds, effectively prevented lipid peroxidation in the plasma and LDL and VLDL molecules as well as in the membranes of erythrocytes of diabetic and healthy patients. The protection against lipid peroxidation could even be enhanced by increasing the concentration of cis-9, trans-11, trans-13-18: 3 to 0·1 % of the plasma(Reference Dhar, Chattopadhyay and Bhattacharyya40).
Although the saponin and the lipid fractions of BG showed the clearest antidiabetic effects, the mice treated with the hydrophilic residue also had lower levels of glycated Hb and a lower PTP 1B activity in comparison with the control mice. These effects were NS, but may be the result of the significantly (P = 0·05) lower body weight of these mice compared with that of the control mice. As the concentration of the hydrophilic residue (12·2 %) in BG is higher than that of the saponin (4·2 %) or the lipid (3·2 %) fraction, the effect of the hydrophilic residue seems to be very important, considering the effect of BG fruit. However, the body weight-lowering effect of the hydrophilic compounds is not clear. According to the literature, BG contains water-soluble vitamins(Reference Njoroge, van Luijk, Grubben and Denton41), which might exert positive effects.
The moderate dosage of 150 mg/kg body weight used in the present study was derived from the study done by Sathishsekar & Subramanian(Reference Sathishsekar and Subramanian42). Especially, for the whole fruit powder, this dosage was very low compared with the dosages used by other authors in their studies, up to 10 % (w/w) of the diet(Reference Kumar Shetty, Suresh Kumar and Veerayya Salimath43), which means a daily dosage of about 5000 mg/kg body weight. It is very likely that the effect of the whole fruit powder will be more pronounced with a higher dosage. But in particular for isolated fractions such as the saponin fraction, the lipid fraction or the hydrophilic residue, there seems to be an upper limit for the most effective dosage(Reference Cheng, Huang and Chang30). At high insulin concentrations (50 or 100 nmol/l), BG juice and water or chloroform extracts of BG only show insulin-sensitising effects in vitro at low concentrations (10 μg BG/ml medium) and inhibit glucose uptake into L6-myocytes or 3T3-L1 adipocytes at concentrations higher than 50 μg BG/ml medium dose dependently(Reference Cummings, Hundal and Wackerhage2, Reference Singh, Adeghate and Cummings3, Reference Roffey, Atwal and Johns27). Therefore, in future studies, it is important to define the optimal dosage of BG for rodents that is also a realistic dosage for patients, which is calculated as amount per kg0·75 body weight.
Conclusion
The present study proves the effectiveness of BG in decreasing body weight gain, glycated Hb levels and oxidative stress in type 2 diabetic db/db mice. It also indicates that there are a number of different bioactive compounds that are responsible for the different metabolic effects exerted by BG.
The identification of the substance groups with the highest effects, namely saponins and lipids, is important for developing supplements for the prevention and treatment of diabetes mellitus. Particularly in developing countries, where nutrition and dietary measures play a crucial role in the treatment of diabetes mellitus, BG represents a possible means for preventing and treating diabetes mellitus. BG is a cheap vegetable that is available the whole year at local markets in southern and eastern Asia and tropical Africa(Reference Sridhar, Vinayagamoorthi and Arul Suyambunathan31, Reference Sekar, Sivagnanam and Subramanian44). BG could also make a positive contribution to the medical treatment of diabetes in industrialised countries.
Acknowledgements
This work was funded by the Federal Ministry for Economic Cooperation and Development (Germany) and the Dannon Institute Nutrition for Health (Germany). There are no conflicts of interests. The study was planned and performed by S. D. K. and M. B. K. The animal trial including preparation of the diet was performed in collaboration with J. P.; Western blot analyses were performed in collaboration with A. S. M. All authors were involved in the preparation of the paper.











