Introduction
The Asiatic frog Amolops wuyiensis Liu and Hu, 1975 (order Anura) is a species unique to China and mainly distributed in the Zhejiang, Anhui and Fujian provinces (Fei et al., Reference Fei, Ye and Jiang2012). Wang (Reference Wang1981) isolated the nematode Spironoura wuyiensis (order Kathlanidae) from the intestine of Staurois wuyiensis Liu and Hu, 1975 (synonym Amolops wuyiensis Liu and Hu, 1975) located in Wuyi mountain, Fujian province, China. Recently, we collected 148 Amolops wuyiensis Liu and Hu, 1975 frogs from four localities of the Anhui province in south-east China, and detected several nematode species, including Cosmocercoides wuyiensis n. sp. in the small intestine. To our knowledge, 25 species of Cosmocercoides have been reported so far, distributed across China (Baylis, Reference Baylis1927; Hsü, Reference Hsü1933; K'ung & Wu, Reference K'ung and Wu1945; Wang, Reference Wang1980; Wang et al., Reference Wang, Sun, Zhao and Zhang1981; Chen et al., Reference Chen, Zhang, Nakao and Li2018), India (Karve, Reference Karve1944; Khera, Reference Khera1958; Arya, Reference Arya1979, Reference Arya1991; Rao, Reference Rao1979; Rizvi, Reference Rizvi2009; Rizvi & Bursey, Reference Rizvi and Bursey2014), Japan (Wilkie, Reference Wilkie1930), Malaysia (Bursey et al., Reference Bursey, Goldberg and Grismer2015), Ceylon (Ogden, Reference Ogden1966), Vietnam (Tran et al., Reference Tran, Sato and Luc2015), Argentina (Ramallo et al., Reference Ramallo, Bursey and Goldberg2007), Brazil (Ávila et al., Reference Ávila, Strüssmann and da Silva2010), USA (Holl, Reference Holl1928; Harwood, Reference Harwood1930), Mexico (Martínez-Salazar et al., Reference Martínez-Salazar, Falcón-Ordaz, González-Bernal, Parra-Olea and de León2013), Ukraine (Ivanitzky, Reference Ivanitzky1940) and Italy (Ricci, Reference Ricci1987). These nematodes usually parasitize amphibians (17 species known so far) and reptiles (eight species), but are occasionally detected in land snails and slugs as well (Vanderburgh & Anderson, Reference Vanderburgh and Anderson1986).
Materials and methods
Collection of nematode samples and morphological examination
A total of 148 Amolops wuyiensis Liu and Hu, 1975 frogs were collected between June 2018 and July 2018 from Huangshan city, Jing county, Jingde county and Jixi county of Xuancheng city, Anhui province, China (table 1). The intestines and stomachs of frogs were cut longitudinally under a dissecting microscope, and the nematodes in the intestinal lumen or on the wall were picked and immediately immersed in hot water (60°C). The killed worms were then fixed in 70% alcohol and processed suitably for the downstream assays. For gross morphological observation, the fixed nematodes were cleared with glycerin and lactic acid, and examined under a light microscope. For scanning electron microscopy (SEM), the specimens were fixed in 2.5% glutaraldehyde, post-fixed in 1% OsO4, dehydrated though an ethanol gradient and dried till the critical point. The specimens were then coated with gold and examined using a FEI XL-30 environmental SEM (Amsterdam, Netherlands) at the accelerating voltage of 20 KV. The illustrations were drawn manually according to the light microscopy photographs. The preserved nematodes were deposited in the Medical Parasitology Department of Wannan Medical College.
Table 1. Recovery of Cosmocercoides wuyiensis n. sp. from Amolops wuyiensis in China.

Sequencing analyses
The internal transcribed spacers (ITS), partial 18S rDNA and the mitochondrial cytochrome c oxidase subunit 1 (COI) gene were sequenced. The target regions were first amplified by polymerase chain reaction (PCR) using the following primers: 18S rDNA – forward 5′-CGC GAA TRG CTC ATT ACA GC-3′ and reverse 5′-GGG CGG TAT CTG ATC GCC-3′ (Floyd et al., Reference Floyd, Rogers, Lambshead and Smith2005); ITS – forward NC5 5′-GTA GGT GAA CCT GCG GAA GGA TCA TT-3′ and reverse NC2 5′-TTA GTT TCT TTT CCT CCG CT-3′ (Gasser et al., Reference Gasser, Rossi and Zhu1999); COI – forward LCO1490 5′-GGT CAA ATC ATA AAG ATA TTG G-3′ and reverse HCO2198 5′- TAA ACT TCA GGG TGA CCA AAA AAT CA-3′ (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). The cycling conditions were as described previously (Li et al., Reference Li, Ali, Zhao, Lü and Xu2016). The amplified products were verified on GoldView-stained 1.5% agarose gels, purified with the Column PCR Product Purification Kit (Sangon Biotech, Shanghai, China), and sent to Sangon Biotech (Shanghai) Co. Ltd. for automated sequencing (ABI 3730, USA). The sequences were aligned using DNAMAN software (Lynnon Corporation, Canada) and adjusted manually, and compared (using the algorithm) with the available sequences in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov) using the BLAST program. All sequences of the new nematode species have been deposited in the GeneBank database (http://www.ncbi.nlm.nih.gov).
Results
General characteristics of C. wuyiensis n. sp.
The alcohol-fixed nematode bodies were white, opaque and cylindrical, with the middle third its widest part. Compared to the other Cosmocercoides species, C. wuyiensis n. sp. has a smaller average size. In addition, sexual dimorphism was highly evident, with males significantly smaller than the females. Both males and females showed fine transverse striations on their cuticles, along with lateral alae. The worms had three similar sized lips: dorsal lip with two large papillae, sub-ventral lips with one papilla each and one lateral amphid (figs 1I, 2B and 3B). The mouth cavity was shallow (7.27–12.73 µm) with a short pharynx, and each lip had one pharyngeal tooth, cylindrical corpus and valved bulb present. An excretory pore was present between the nerve ring and oesophageal bulb (fig. 1A, B). The tail-end of both sexes was sharply conical (figs 1F, G, 2E and 3D, E).
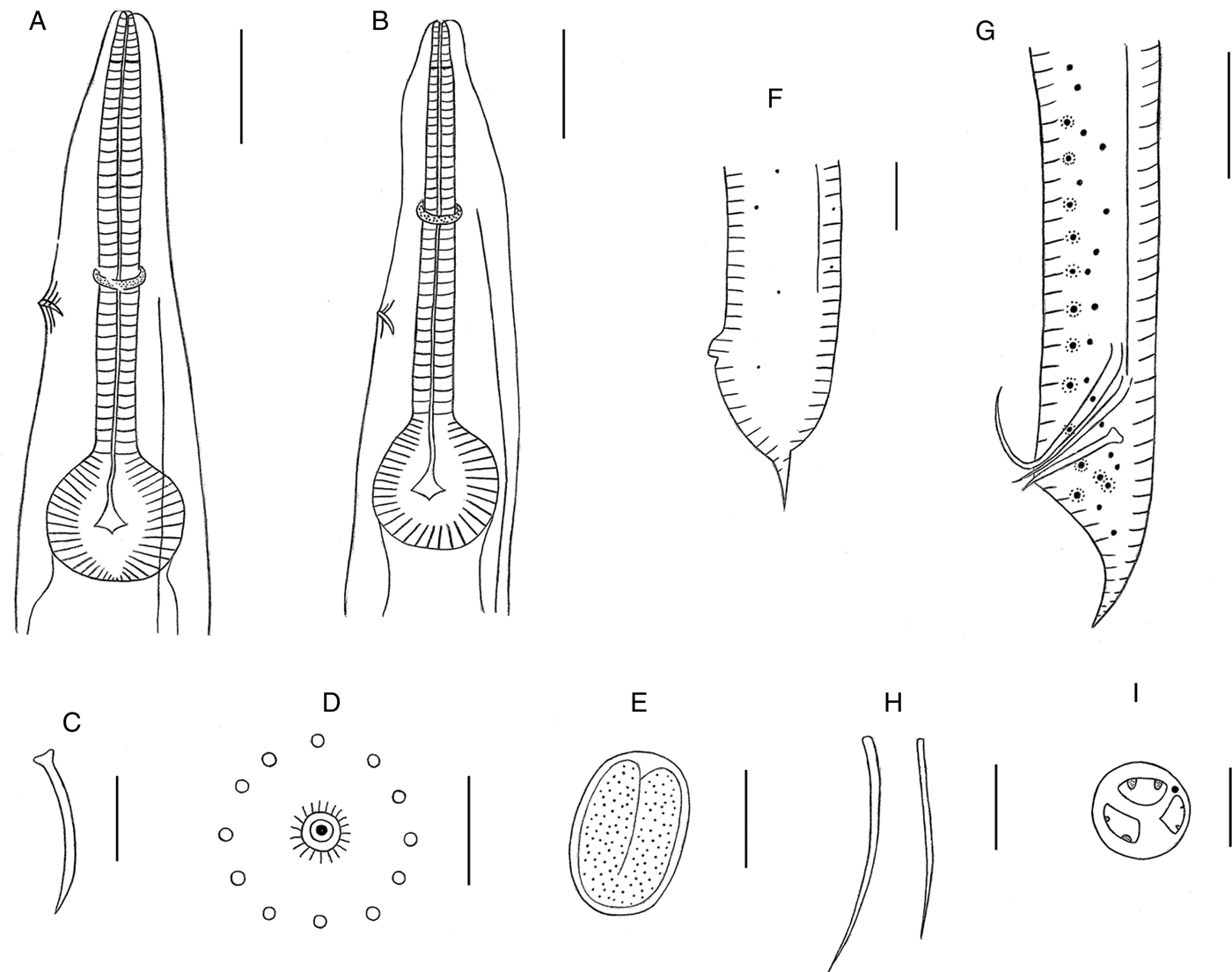
Fig. 1. Cosmocercoides wuyiensis n. sp. collected from the Asiatic frog Amolops wuyiensis (Amphibia: Anura) in China. (A) Anterior end of the female, lateral view, showing the oesophagus, nerve ring, excretory pore; (B) anterior end of the male, lateral view, showing the oesophagus, nerve ring, excretory pore; (C) gubernaculum; (D) magnified image of the caudal rosette; (E) embryonated egg in the uterus; (F) posterior of the female, lateral view; (G) posterior of the male, lateral view; (H) spicules; (I) en-face view. Scale bars: (A, B, F, G) 100 µm; (C, E, H) 50 µm; (D) 5 µm; (I) 10 µm.

Fig. 2. Scanning electron micrographs of Cosmocercoides wuyiensis n. sp. collected from the Asiatic frog Amolops wuyiensis (Amphibia: Anura) in China, male. (A) Lateral view of the full body; (B) sub-facial view of the anterior end showing sub-ventral lip (svl) and dorsal lip (dl); (C) lateral view of the anterior end showing lateral alae (la) and somatic papillae (sp); (D) lateral view of cloacal site showing spicules(s), rosette papillae (rp) and gubernaculum (g); (E) lateral view of the posterior end showing tail and phasmid (p); (F) magnified image of rosette papillae.

Fig. 3. Scanning electron micrographs of Cosmocercoides wuyiensis n. sp. collected from the Asiatic frog Amolops wuyiensis (Amphibia: Anura) in China, female. (A) Lateral view of the full body; (B) sub-facial view of the anterior end showing sub-ventral lip (svl) and dorsal lip (dl); (C) lateral view of the anterior end showing the somatic papillae (sp); (D) lateral view of the posterior end showing the narrow lateral alae (la); (E) ventral view of the posterior end showing the tail and anus (a); (F) lateral view of the middle body showing somatic papillae (sp) and lateral alae (la).
The male worms measured 1.94–3.22 mm in length and 0.146–0.266 mm in width (from five mature specimens). The oesophagus including the bulb was 0.533–0.596 mm long, and the pharynx was located 0.049–0.056 mm from the anterior end, while the nerve ring and excretory pore were located 0.246–0.447 mm and 0.742–0.844 mm, respectively, from the anterior end. The two spicules differed in shape and length: one was slim and measured only 0.151–0.163 mm in length, and the other was thick and longer at 0.189–0.206 mm (figs 1G, H and 2D). The gubernaculum, with well-sclerotized, conical and lateral projections, measured 0.054–0.105 mm in length (figs 1C, G and 2D). The ventral surface of the posterior fourth of body had 13–16 pairs of caudal rosettes, arranged in 9–12 sub-ventral pre-cloacal pairs in two columns, 1–2 outer column pairs, one ad-cloacal pair and three post-cloacal pairs, of which two were in the sub-ventral and one in lateral positions. Each rosette was composed of only one circle of about 11–16 punctations around the central papilla. The lateral alae (79.7–87.16% of the body length) began 0.187–0.193 mm from the anterior, and terminated 0.206–0.220 mm to the posterior (figs 1B, G and 2A, C, E). Small lateral phasmids were located midway between the dorsolateral pairs of post-cloacal papillae, about 0.114 mm to the tail tip. The tail was 0.061–0.220 mm in length.
The female worms were larger than males, and measured 3.34–4.37 mm in length and 0.192–0.382 mm in width (from five mature specimens). The oesophagus including bulb measured 0.608–0.629 mm in length. The pharynx was located 0.039–0.054 mm from the anterior end, and the nerve ring and excretory pore were respectively located 0.247–0.253 mm and 0.370–0.432 mm from the anterior end. The vulva was located 1.919–2.564 mm from the anterior end, corresponding to 1.35–1.42:1 from the posterior end. The narrow lateral alae (89.19–89.75% of the total body length) began 0.265–0.294 mm from anterior end and ended 0.096–0.107 mm to posterior end (figs 1A, F and 3A, D, F). The embryonated eggs in the uterus were oval, thin-shelled and smooth surfaced, measuring 0.063–0.068 mm in length and 0.034–0.046 mm in width (n = 7) (fig. 1E). The tail was 0.077–0.156 mm long.
Taxonomic summary
Type host. Amolops wuyiensis Liu and Hu, 1975, family Amphibia, order Anura.
Type locality. Zhanghe (30°6′N, 118°47′E), Jixi county, Yiwanling (30°23′N, 118°43′E), Jingde county, Taoling (30°31′N, 118°38′E), Jing county, Fuxi (30°4′N, 118°9′E), Huangshan city, Anhui province, China.
Site of infection. Small intestine.
Level of infection. Total rate 19.6%, the average and range of infection intensity 0.72 (0, 32) (table 1).
Specimens deposited. Holotype, male (WNMC-2018001); allotype, female (WNMC-2018002); paratypes, males (WNMC-2018003) and females (WNMC-2018004). Deposited in the College of Life Sciences, Anhui Normal University, Anhui province, China.
Etymology. Based on the name of the host.
Remarks
Cosmocercoides wuyiensis n. sp. is the 26th species of this genus, the seventh species from China and the first species of its genus to be isolated from Amolops wuyiensis Liu and Hu, 1975 in China. Cosmocercoides wuyiensis n. sp. shows a distinct arrangement and morphology of the caudal rosette papillae, in addition to the presence of spicules, lateral alae and somatic papillae in the males – features that are also shown by C. multipapillata Khera, 1958, C. himalayanus Rizvi & Bursey, 2014, C. nainitalensis Arya, 1979, C. rickae Ogden, 1966, C. tonkiensis Tran BT, Sato H and Luc PV, 2015 C. tridens Wilkie, 1930, C. qingtianensis Chen HX, Zhang LP, Nakao M and Li L, 2018 and C. skrjabini Ivanitzky, 1940. The male of the new species is easily distinguished by the pre-cloacal, para-cloacal and post-cloacal papillae arranged in the ratio of 18–24:2:6. However, the ratio of the caudal rosette papillae arrangement is 24–28:4:2, 24:2:4 and 26:2:6 in C. qingtianensis, C. himalayanus and C. malayensis, respectively. The number of punctations per rosette is 11–16 in the new species, 13–16 in C. tonkinensis and 13 in C. malayensis. The new species differs from C. multipapillata, C. himalayanus, C. rickae, C. tonkinensis, C. tridens, C. qingtianensis and C. skrjabini in terms of having unequal spicules length in males (151–163 µm and 189–206 µm). Three other species have unequal spicules in males too – C. nainitalensis Arya, 1979 (130–151 µm and 112–134 µm), C. kumaoni Arya, 1991 (100–160 µm and 90–125 µm) and C. barodensis Rao, 1979 (240 µm and 230 µm), but somatic papillae are absent in the males of C. barodensis. The caudal rosette papillae arrangement ratio in male C. wuyiensis n. sp. is different from C. nainitalensis (26–32:2:12), C. kumaoni (24:2:10) and C. barodensis (16:0:4).
Phasmids are present in the males of seven Cosmocercoides species (table 2); C. wuyiensis n. sp. is closest to C. qingtianensis, C. himalayanus and C. malayensis, with present phasmids in the males (Rizvi & Bursey, Reference Rizvi and Bursey2014; Bursey et al., Reference Bursey, Goldberg and Grismer2015; Chen et al., Reference Chen, Zhang, Nakao and Li2018). The body size of both sexes of the new species are smaller than that of C. qingtianensis and C. himalayanus. Finally, the main hosts of C. qingtianensis and C. himalayanus are toads, the host of C. malayensis is lizard, while that of the new species is frog.
Table 2. Morphological comparison of Cosmocercoides wuyiensis n. sp. with related species.

Caudal papillae: rosette*/simple* pre-cloacal: para-cloacal: post-cloacal/subventral papillae: lateral papillae: medioventral.
Cosmocercoides wuyiensis n. sp. represents the first record of the genus Cosmocercoides in China infecting a ranid frog.
Molecular evidence
Sequences from three different C. wuyiensis n. sp. specimens were identical (100% homology) for ITS, 18S rDNA and COI gene amplified fragments. Excluding primers, 868, 888, 655 base pairs in length were obtained herein for the ITS, 18S rDNA and COI gene of C. wuyiensis n. sp., and have been deposited in the GenBank database (accession nos MK110871, MK110872 and MK138680), respectively.
Prior to the present study, there were three species of the Cosmocercoides sequence registered in GenBank for ITS (C. pulcher: LC018444, MH178314, MH178315, MH178316, MH178317, MH178318; C. tonkinensis: AB908160, AB908161; and C. qingtianensis: MH032772, MH032773, MH032774, MH178311, MH178312, MH178313). The maximum score, query coverage and maximum identity in BLAST analysis were 1563, 100%, 99.19% (C. pulcher); 1598, 100%, 99.88% (C. tonkinensis); 1592, 100%, 99.77% and 1587, 100%, 99.65% (C. qingtianensis) for ITS gene sequence, respectively.
The 18S rDNA sequences of four other species of Cosmocercoides are currently registered in GenBank (C. pulcher: MH178322, MH178323, MH178324, MH178325, MH178326, LC018444; C. tonkinensis: accession no. AB908160; C. qingtianensis: MH178319, MH178320, MH178321, MH032769, MH032770, MH032771; and C. dukae: accession no. FJ516753). The maximum score, query coverage and maximum identity in BLAST analysis were 1628, 100%, 99.77% (C. pulcher, C. qingtianensis); 1633, 100%, 99.89% (C. tonkinensis); 1609, 100%, 99.44% (C. dukae) for 18S rDNA sequence, respectively.
The COI gene sequences of two other species of Cosmocercoides are currently registered in GenBank (C. pulcher – accession no. LC052771, C. qingtianensis – accession no. MH178305). Pairwise comparison between C. wuyiensis n. sp. and C. pulcher showed 16.36% nucleotide divergence; moreover, pairwise comparison between C. wuyiensis n. sp. and C. qingtianensis showed 47.99% nucleotide divergence.
Discussion
Members of Cosmocercoides Wilkie, 1930 (Syn. Trionchonema Kreis, 1932) and its related genus Cosmocercella Steiner, 1924 are characterized by caudal ‘rosette papillae’, where the latter are surrounded by punctations, and not ornamented with ‘plectanes’ (cuticular supports around caudal papillae) as seen in Cosmocerca Diesing, 1861 (Syn. Paracosmocerca K'ung & Wu, 1945). Unlike the species of Cosmocercoides, the caudal rosette papillae of Cosmocercella are raised on the surface of clear vesicle (Anderson et al., Reference Anderson, Chabaud and Willmott2009). The species isolated from the Sino-Japanese regions only infect toads and frogs, with the exception of C. oligodentis Wang PQ, Sun YL, Zhao YR and Zhang WH, 1981 and C. tridens Wilkie, 1930, which respectively infect the snake Oligodon chinensis and the salamander Tylototriton andersoni. The species isolated from other geographical areas have been detected in frogs, toads, lizards and salamanders.
Wilkie (Reference Wilkie1930) deduces C. dukae Holl, 1928 should not have been placed in the genus Cosmocerca, but is a species of the new genus Cosmocercoides. Two new species of the genus Cosmocercoides, – C. pulcher (type species) and C. tridens – are reported by Wilkie (Reference Wilkie1930). Diagnosis of the genus Cosmocercoides includes males furnished with complex caudal papillae (large papillae, each of which is surrounded by a ring of cuticular tubercles); no lateral alae; no vesiculate bursa; and the vulva of the female behind the middle of the body (Wilkie, Reference Wilkie1930).
The form of the rosette papillae is used by Ivanitzky (Reference Ivanitzky1940) as a diagnostic character of C. skrjabini, since each papilla is surrounded by two rings of tubercules instead of the normal one, similar to C. kiliwai Martínez-Salazar, 2013 and C. tonkinensis Tran et al., 2015. However, there is one ring of cuticular tubercles around each rosette papillae on C. himalayanus Rizvi AN and Bursey CR, 2014, C. qingtianensis Chen HX, Zhang LP, Nakao M and Li L, 2018 and the study C. wuyiensis n. sp., while the number of punctations per rosette is 11–16 in C. wuyiensis n. sp., 12 in C. himalayanus and 15–18 in C. qingtianensis.
Ogden (Reference Ogden1966) confirms the generic diagnosis of Cosmocercoides should be amended to include the presence of narrow lateral alae and simple body papillae. With narrow lateral alae and simple body papillae (syn. somatic papillae), C. wuyiensis n. sp. is similar to the following species: C. multipapillata Khera, 1958, C. dukae Anderson, 1960, C. rickae Ogden, 1966, C. nainitalensis Arya, 1979, C. tridens Hasegawa, 1989, C. kumaoni Arya, 1991, C. himalayanus Rizvi & Bursey, 2014, C. tonkinensis Tran et al., 2015 and C. qingtianensis Chen HX, Zhang LP, Nakao M and Li L, 2018. The new species differs from C. multipapillata, C. himalayanus, C. tonkinensis and C. qingtianensis by having a smaller size in males (1.9–3.2 mm long in former vs. 4.2–8.0 mm long in the latter four species) and a shorter tail in males (54–105 µm long in former vs. 113–175 µm long in the latter four species).
Cosmocercoides spp. has unstable characters or overlapping ranges of morphological features affected by processing; therefore, molecular genetic characterization of parasites can be further used as molecular evidence for species identification. In the present study, the ITS gene sequence of Cosmocercoides spp. registered in GenBank by BLAST analysis shows the lower nucleotide divergence (0.12% vs. C. tonkinensis, 0.81% vs. C. pulcher, 0.23–0.35% vs. C. qingtianensis), and that of the 18S rDNA sequence shows slight lower nucleotide divergence (0.11% vs. C. tonkinensis, 0.23% vs. C. qingtianensis and C. pulcher, 0.56% vs. C. dukae). Molecular analysis using the 18S rDNA and ITS sequences probably validates that the new species belongs to the genus Cosmocercoides, while the relatively lager nucleotide divergence (16.36% vs. C. pulcher, 47.99% vs. C. qingtianensis) of the COI sequences between C. wuyiensis n. sp. and the other two sequences registered in GenBank indicates that C. wuyiensis is a new species of the genus Cosmocercoides. The findings of our molecular analysis provide new insights into phylogenetic and population genetic studies. To conclude, the isolation of C. wuyiensis n. sp. from the Asiatic frog in China indicates a new geographical location for these parasites and adds to the number of helminths known to infect this species of frog.
Author ORCIDs
Y. Liu, 0000-0003-2249-2045
Acknowledgements
We are greatly indebted to Professor Chaopin Li (Wannan Medical College) for providing his expertise about nematode identification. We thank Jun He and Lisong He for their collection and identification of amphibian hosts, and Shuaitao Deng, Huijuan Zhang and Ting Liu who dissected the frogs and classified the nematodes. The authors’ thanks are also attributed to the staff of the Laboratory of Electron Microscopy, Testing Centre of Yangzhou University, for their SEM technical assistance.
Financial support
This study was supported by the National Natural Science Foundation of China (grant number 31370537); the Doctoral Fund of the Ministry of Education of China (grant number 20133424110006); and the Natural Science Research Fund Project of Institutions of Higher Learning, Education Department of Anhui Province (grant numbers KJ2012B207, KJ2012Z426).
Conflicts of interest
None.







