1. Introduction
Metabolic syndrome (MetS), a complex disorder with high socio-economic costs, is considered a worldwide epidemic. MetS is defined by a cluster of interconnected factors that directly increase the risk of cardiovascular diseases (CVDs) and diabetes mellitus type 2 (Lakka et al., Reference Lakka, Laaksonen, Lakka, Niskanen, Kumpusalo, Tuomilehto and Salonen2002; Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, 2002). Its main components are dyslipidaemia (decreased HDL-cholesterol (HDL-C) and high triglyceride (TG) concentration) and elevation of arterial blood pressure (BP), with abdominal obesity also having attracted increasing attention as one of the core manifestations of the syndrome (Zhu et al., Reference Zhu, Wang, Heshka, Heo, Faith and Heymsfield2002).
The prevalence of the MetS varies among different ethnic groups (Meigs et al., Reference Meigs, Wilson, Nathan, D'Agostino, Williams and Haffner2003). The prevalence of MetS, based on the International Diabetes Federation (IDF) definition, among the Iranian population is 37·4% and this indicates that the Middle East ethnic groups are probably prone to the highest prevalence of CVDs in the near future (Delavari et al., Reference Delavari, Forouzanfar, Alikhani, Sharifian and Kelishadi2009). The prevalence of this syndrome is 24 and 42% in males and females, respectively, among the Tehranian population (Azizi et al., Reference Azizi, Salehi, Etemadi and Zahedi-Asl2003). The reasons for the racial/ethnic disparities of prevalence observed are not clear, but variations in environmental factors and increased genetic susceptibility could probably explain these.
Indeed, growing evidence suggests that a genetic basis for the MetS and its components is credible (Austin et al., Reference Austin, Edwards, McNeely, Chandler, Leonetti, Talmud, Humphries and Fujimoto2004). According to studies in twins, the individual components of the MetS, such as obesity, serum lipids, BP and fasting insulin levels are to some extent influenced by genetic factors (Stunkard et al., Reference Stunkard, Harris, Pedersen and McClearn1990; Iliadou et al., Reference Iliadou, Lichtenstein, de Faire and Pedersen2001). Several studies have also provided evidence for genetic influences on the combinations of components that characterize the MetS (Mahaney et al., Reference Mahaney, Blangero, Comuzzie, VandeBerg, Stern and MacCluer1995; Olswold & de Andrade Reference Olswold and de Andrade2003). Using factor analysis, a few studies combined these inter-correlated components into fewer independent factor structures to examine their heritability (Arya et al., Reference Arya, Blangero, Williams, Almasy, Dyer, Leach, O'Connell, Stern and Duggirala2002; Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005). Some investigators have already started to map susceptibility genes to the MetS (Tam et al., Reference Tam, Lam, So, Ma, Chan and Ng2010; Edwards et al., Reference Edwards, Wan, Hutter, Fong and Santorico2011). Thus, it is important to know whether this syndrome has appreciable heritability.
In this study, we used two different approaches to investigate the genetic structure of MetS. The first was to estimate the heritability of the metabolic syndrome score (MSS) and MetS components among families with MetS, who participated in the Tehran Lipid and Glucose Study (TLGS). The second was to obtain the structure underlying the MetS using principal component factor analysis of the six quantitative phenotypes and to calculate the heritability of the factor score for each independent factor.
2. Materials and methods
(i) Study population
This family-based study was conducted on 1106 families who participated in the TLGS, which is an ongoing prospective population-based longitudinal cohort study being conducted to determine the risk factors for non-communicable diseases among a representative urban population of Tehran (Azizi et al., Reference Azizi, Ghanbarian, Momenan, Hadaegh, Mirmiran, Hedayati, Mehrabi and Zahedi-Asl2009). The design of TLGS includes two major components, a cross-sectional prevalence study of CVD and associated risk factors and a prospective 20-year follow up in several phases: phase I in 1999–2001, phase II in 2002–2005 and phase III in 2006–2008 at approximately 3·6-year intervals. In the present study, the families were recruited from phase III of TLGS. All families with two biological parents, at least one offspring (nuclear family) and at least two members with MetS were eligible for the study (2350 parents and 3277 children at entrance). Subjects who lacked complete data for biochemical variables or family data were excluded as were those who used hypoglycaemic or anti-hypertensive medication. Consequently, 904 families (1565 parents and 2448 children, aged 3–90 years) remained. Participants provided informed consent and the study was approved by the institutional ethics committees of the Research Institute for Endocrine Sciences affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran. Information on age, sex, demographic and medication usage for treatment diabetes, hypertension and lipid disorders were collected with a standardized questionnaire. Waist circumference (WC) was measured at the umbilical site using an outstretched tape meter and was recorded to the nearest 0·1 cm; systolic BP (SBP) and diastolic BP (DBP) were measured in the sitting position with a standard mercury sphygmomanometer on the left arm after at least 10 min of rest. Blood samples were taken after 12–14 h of overnight fasting for biochemical analysis, in a standard sitting position and then centrifuged for 45 min after collection. All the assays including fasting blood sugar (FBS), TG and high density lipoprotein-cholesterol (HDL-C) were done on the day of sampling. Blood glucose was measured by the glucose oxidase method (Glucose kit; Pars Azmun, Tehran, Iran). TG levels of the samples were determined by the enzymatic colorimetric method (TG kit; Pars Azmun, Tehran, Iran); HDL-C samples were determined by precipitation and the enzymatic colorimetric method (HDL-C kit; Pars Azmun, Tehran, Iran).
(ii) Definition of MetS
MetS in adults was defined according to the Joint Interim Statement (JIS) criteria (Alberti et al., Reference Alberti, Eckel, Grundy, Zimmet, Cleeman, Donato, Fruchart, James, Loria and Smith2009) as the presence of three or more of the following features: TG ⩾ 150 mg/dl and drug treatment, HDL-C <40 mg/dl in men and <50 mg/dl in women and drug treatment, SBP ⩾ 130 mmHg and/or DBP ⩾ 85 mmHg and drug treatment, FBS ⩾ 100 mg/dl and drug treatment, and W ⩾95 cm for both genders based on the Guidelines of the Iranian National Committee for Obesity (Azizi et al., Reference Azizi, Khalili, Aghajani, Esteghamati, Hosseinpanah, Delavari, Larijani, Mirmiran, Mehrabi, Kelishadi and Hadaegh2010). For adolescents and children, MetS was defined according to the Cooks guidelines (Cook et al., Reference Cook, Weitzman, Auinger, Nguyen and Dietz2003). It defines the MetS as having three or more of the following: TG ⩾ 110 mg/dl, HDL-C ⩽ 40 mg/dl, waist ⩾90th for age and sex specified according to the national reference curve (Kelishadi et al., Reference Kelishadi, Gouya, Ardalan, Hosseini, Motaghian, Delavari, Majdzadeh, Heidarzadeh, Mahmoud-Arabi and Riazi2007). SBP and/or DBP ⩾90th for sex, age and height from National reference cut-off points (Kelishadi et al., Reference Kelishadi, Ardalan, Gheiratmand, Majdzadeh, Delavari, Heshmat, Gouya, Razaghi, Motaghian, Mokhtari, Barekati and Arabi2006), FBS ⩾ 100 mg/dl according to the recent recommendation of American Diabetes Association (Genuth et al., Reference Genuth, Alberti, Bennett, Buse, Defronzo, Kahn, Kitzmiller, Knowler, Lebovitz, Lernmark, Nathan, Palmer, Rizza, Saudek, Shaw, Steffes, Stern, Tuomilehto and Zimmet2003). To generate the MSS score for each of the subjects, we combined the standardized residuals for each component trait prior to the calculation of life-span averages as follows: [MSS = BP + TG + FBS + Waist – HDL]. HDL was subtracted from the score because it is protective against CAD, and tends to be inversely correlated with other component traits; thus, lower HDL values correspond to a higher MSS (McQueen et al., Reference McQueen, Bertram, Rimm, Blacker and Santangelo2003).
(iii) Statistical analysis
The normality of distribution was checked for all variables by Kolmogorov–Smirnov analysis. Normally distributed continuous variables are reported as the means±sd, whereas categorical variables were summarized as frequencies and percentage. If necessary a logarithmic transformation was performed to normalize the error distribution and stabilize the error variables. Means differences within generations between genders were assessed for statistical significance by Student's t test. The discrete variables were compared among groups using Chi-square analysis. Partial correlation coefficient (Pearson) adjusted for age and sex were used to compare the relationship between single MetS components. Statistical analysis was performed with the SPSS version 15 software.
Genetic analysis was carried out using SOLAR (Sequential Oligogenic Linkage Analysis Routines) software package to assess the heritability estimates for the MSS and MetS components as defined in the JIS. We estimated heritability for each continuous component of the Mets with adjustment for age and sex. Heritability (h 2) of continuous variables was calculated using a standard quantitative genetic variance-components model implemented in SOLAR; h 2 is defined as the proportion of phenotypic variance that is attributed to additive genetic causes according to covariates. The heritability of discrete traits was assessed using a threshold model in SOLAR. The assumed method was that an individual belongs to a specific affected status if an underlying genetically determined risk exceeds a certain cut-off point (Duggirala et al., Reference Duggirala, Williams, Williams-Blangero and Blangero1997). The null hypothesis of no genetic effect (h 2 = 0) is tested by comparing the likelihood of the restricted model, where h 2 is constrained to zero with a general model in which the same parameter is estimated. Evidence of a non-zero estimate for a given parameter was considered statistically significant at P-values <0·05. For factor analysis, continuous traits were first adjusted for age and sex by a multiple regression model, and standardized residuals were used in the analysis. Factor analysis consisted of principal component analysis, a varimax rotation and identification of the variables to facilitate interpretation. Factor numbers were determined using the eigenvalue-one criterion and the Scree test. All factor analyses were performed using SPSS version 15.
3. Results
Participants of the current cross-sectional study included 4013 individuals (703 fathers, 862 mothers, 1292 sons and 1156 daughters). The mean age for parents and offspring was 54·2 ± 12·5 and 27·1 ± 14·1 years, respectively. In the parents’ group, females were younger than males and had a higher prevalence of MetS (58·6 versus 52·9%). No significant differences were detected in serum concentration of the TG and FBS between genders. In the offspring group, in spite of low concentration of HDL-C in males, the levels of other MetS components were higher compared with females (Table 1).
Table 1. Characteristics of the study participants

Unless otherwise stated, values are means±sd.
a High WC: adult: ⩾95 cm in both gender, adolescents and children: ⩾90th percentile for age and sex.
b Low HDL-C: adult: men < 40 mg/dl, women < 50 mg/dl; adolescent: ⩽40 mg/dl.
c High TG: adult: ⩾150 (mg/dl); adolescent: >110 mg/dl.
d High fasting-glucose: adult: ⩾100 (mg/dl); adolescent: ⩾100 mg/dl.
e High BP: adult: SBP ⩾ 85 mmHg and/or DBP ⩾ 130 mmHg; adolescent and children: ⩾90th percentile for age and sex.
* P < 0·05.
To determine the most prevalent contribution of combinations of the trait for MetS, we calculated Pearson partial correlation between MetS components of individuals, adjusted for age and gender. According to the above-reported observations on the most prevalent MetS subtypes, SBP showed a strong direct correlation with DBP (r = 0·526) and TG level showed an inverse correlation with HDL-C (r = − 0·368). By contrast, SBP and HDL-C demonstrated the lowest correlation (r = − 0·007) (Table 2).
Table 2. Correlation coefficients between variables used in factor analysis
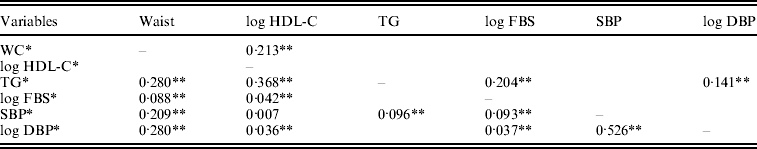
* Adjusted for age, sex by regression analysis.
** P < 0·05.
After adjusting for age and gender, the MSS had a heritability of 15% (P = 1 × 10−7). When the components were treated as continuous traits, the estimates of heritability varied from 25% (P = 1·01 × 10−16) for SBP to 46% (P = 1·15 × 10−55) for HDL-C after adjusting for age and gender. When the MetS components were analysed as discrete traits according to the JIS, the estimates of age and gender adjusted heritability varied from 22% (P = 3 × 10−5) for high WC to 40% (P = 4·68 × 10−16) for low HDL-C levels (Table 3).
Table 3. Heritability estimates for the MetS and its components treated as continuous and discrete traits

a Adjusted for age, sex, age2, age × sex, age2 × sex.
b Treated as continuous traits.
c Variation explained by covariates as suggested by the Kullback–Leibler R 2 value.
d Treated as discrete traits.
e Log transformation.
The factors and patterns of factor loadings were obtained in this study. Three factors were extracted from six continuous traits of the MetS. The first factor, which explained 17·1% of the total variance in the dataset, was dominated by WC and FBS, the second factor by TG and HDL-C that contained 19·4% of total variance in the dataset, and the third factor by BP, which explained 22·5% of variance (Table 4). Heritability estimations for the three factors were 6, 14 and 7%, respectively (P < 0·05).
Table 4. Results of factor analysis, variance components and heritability estimation
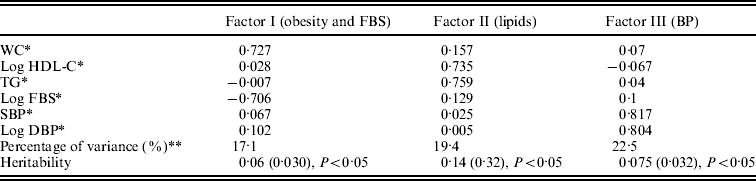
* Adjusted for age, sex by regression analysis.
** Percentage of variance.
4. Discussion
In this study, we assessed the heritability for the MetS components and MSS among TLGS families. Our results confirm that genetic factors contribute to the familial aggregation of the MetS components. Among the MetS components analysed, HDL-C had the highest heritability estimation of 46%, while high WC had the lowest heritability estimation (22%). Moreover, heritability estimation for MSS was even lower than WC (15%). Using factor analysis, the heritability of factor I (obesity and FBS) was 6% and that of factor II (lipids) and III (BP) were 14 and 7%, respectively. Although the heritability of MSS and factor analysis was not directly comparable, because they represent different phenotypes, both approaches suggested different estimates of heritability for each phenotype. However, because most clinical and epidemiological studies investigate a consequence of the MetS, genetic studies on the syndrome may provide more direct and valuable information to healthcare providers and the general population. It is not clear whether the co-occurrence of the MetS traits may be described by familial genetic factors or by environmental effects such as lifestyle factors including calorie intake and physical activity. Considering this complexity of gene–environment interactions, the opportunity of studying an isolated population presented a potentially powerful strategy to identify new genetic variants.
The heritability of MetS is unknown. It would be of interest to determine whether the MetS per se can be inherited. Some different studies have investigated the heritability of MetS components but not for the MetS (Herbeth et al., Reference Herbeth, Samara, Ndiaye, Marteau, Berrahmoune, Siest and Visvikis-Siest2010; Jermendy et al., Reference Jermendy, Littvay, Steinbach, Jermendy, Tarnoki, Tarnoki, Metneki and Osztovits2011; Vattikuti et al., Reference Vattikuti, Guo and Chow2012). One previous study has reported the heritability of MetS (24%) (Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005), and one another study has stated an estimation of heritability for the MetS in which the author defined a novel variable called the MSS (McQueen et al., Reference McQueen, Bertram, Rimm, Blacker and Santangelo2003); in this study, the heritability obtained for MSS was 61%. We could not estimate the heritability for the MetS, so we estimated the heritability of MSS in this study. Our result, on the contrary, showed a lower heritability for MSS, i.e. 15%. However, it is not clear whether the MSS can also be highly associated with complications related to the MetS.
Our study results of heritability for the MetS components are generally consistent with those of previous similar studies conducted in different populations (Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005; Bellia et al., Reference Bellia, Giardina, Lauro, Tesauro, Di Fede, Cusumano, Federici, Rini, Novelli, Lauro and Sbraccia2009). A number of papers have been published regarding the heritability of MetS components among different ethnic groups (Austin et al., Reference Austin, Edwards, McNeely, Chandler, Leonetti, Talmud, Humphries and Fujimoto2004; Bellia et al., Reference Bellia, Giardina, Lauro, Tesauro, Di Fede, Cusumano, Federici, Rini, Novelli, Lauro and Sbraccia2009). In a recent study on 403 individuals from 156 consenting Saudi families (123 adults and 131 children) 21% of the variation was most strongly loaded on levels of leptin, tumour necrosis factor α (TNFα), insulin and aPAI1, and inversely with adiponectin. It was significantly associated with body mass index (BMI) and phenotypically stronger in children, and showed a heritability of 50%, after adjustment for age, gender and generational effects (Al-Daghri et al., Reference Al-Daghri, Al-Attas, Alokail, Alkharfy, Yakout, Sabico, Gibson, Chrousos and Kumar2011). The ERF study reported heritability of the MetS (13·2%) in an isolated Dutch population, with a mean age of 48 years (Henneman et al., Reference Henneman, Aulchenko, Frants, van Dijk, Oostra and van Duijn2008). A study of Mexican-Americans analysed the heritability of MetS components and indicated a heritability of 40% for TG, 46% for HDL-C, 18% for SBP, 42% for BMI and 18% for FBS (Mitchell et al., Reference Mitchell, Kammerer, Blangero, Mahaney, Rainwater, Dyke, Hixson, Henkel, Sharp, Comuzzie, VandeBerg, Stern and MacCluer1996). Another study of 1277 healthy Arabs of the Oman family study demonstrated heritability of 63% for HDL-C, 43% for TG, 40% for WC, 28% for SBP and 38% for DBP (Bayoumi et al., Reference Bayoumi, Al-Yahyaee, Albarwani, Rizvi, Al-Hadabi, Al-Ubaidi, Al-Hinai, Al-Kindi, Adnan, Al-Barwany, Comuzzie, Cai, Lopez-Alvarenga and Hassan2007). These differences in heritability could be related to the diversity of genetic backgrounds and/or the level of environmental effects in the population. In addition, the discrepancy may be attributable to different sample sizes, varying composition of study populations and covariates included in the analyses. In a recent review that considered the shared genetic variance between the features of the MetS, from nine twins and 19 family studies, genetic correlations varied. It was stronger between WC and HOMA-IR (r 2: 0·36–0·79), HDL-C and TG (r 2: −0·05 to −0·59), adiponectin and MetS (r 2: −0·32 to −0·43), adiponectin and insulin (r 2: −0·10 to −0·60) and between adiponectin and HDL-C (r 2: −0·22 to −0·51) and showed that the heritability studies suggest that genetic pleiotropy exist especially between certain MetS features (Povel et al., Reference Povel, Boer and Feskens2011).
To provide new insights into the underlying pathophysiological mechanisms, some studies used factor analysis to break the related MetS components into two (Loos et al., Reference Loos, Katzmarzyk, Rao, Rice, Leon, Skinner, Wilmore, Rankinen and Bouchard2003), three (Austin et al., Reference Austin, Edwards, McNeely, Chandler, Leonetti, Talmud, Humphries and Fujimoto2004) or four factors (Lakka et al., Reference Lakka, Laaksonen, Lakka, Niskanen, Kumpusalo, Tuomilehto and Salonen2002). Most previous studies included various definitions for MetS defined variables for factor analysis and yielded fewer or more factors than our study (Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005; Herbeth et al., Reference Herbeth, Samara, Ndiaye, Marteau, Berrahmoune, Siest and Visvikis-Siest2010). It has been suggested that the number of factors will be equal to two or less than the number of original variables divided by three (Meigs, Reference Meigs2000), therefore, our study had three factors extracted from six JIS-defined variables. Regardless of how many factors are extracted, our study shared common patterns with previous studies that indicated glucose and abdominal obesity load were part of the same factor, dyslipidaemia profiles load was a separate factor, and BP variables were determined as the third factor (Chien et al., Reference Chien, Hsu, Chen, Chen, Su and Lee2007). Also, the total variance explained by the three factors extracted in this study was 59%, which is similar to variance found by previous studies (ranging from 55 to 68%) (Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005). In contrast, among Caribbean-Hispanic families, heritability of the lipids/glucose/obesity factor (44%) and BP factor (20%) were higher than the current study (Lin et al., Reference Lin, Boden-Albala, Juo, Park, Rundek and Sacco2005). Taken together, these results demonstrate an important underlying genetic susceptibility to MetS components.
This study had a few limitations: first, we did not include all family members such as grandparents in the study, which could improve the validity of study because large pedigree structure provides more genetic transmission information than the nuclear family. Second, we did not take into account the puberty information of the participants and the role of some common environmental factors such factors as physical activity, nutrition and other lifestyle from pure genetic ones. Last but not least, the SOLAR program may underestimate the heritability that is caused by the genetic effects because of gene–environment interaction. However, to our knowledge, this is the first report of heritability of the MetS components as defined by the JIS in Iranian populations.
In conclusion, we clearly demonstrated a significant heritability of MetS components and MSS among TLGS families, with the highest heritability demonstrated for lipids (HDL-C and TG). These findings on the heritability of the MetS components and MSS might encourage researchers to conduct a genome-wide linkage study to identify the underlying susceptibility genes, given the potential advantage of analysing pedigrees in Iranian populations.
We would like to acknowledge Mrs Nilufar Shiva for language editing of this manuscript. This work was funded and supported by the Research Institute for Endocrine Sciences of Shahid Beheshti University.
Declaration of Interest
There is no conflict of interest.






