Transposition of great arteries (TGA) with intact ventricular septum (IVS) and left ventricular outflow tract obstruction (LVOTO) is an uncommon type of discordant ventriculo-arterial connection. According to Freedom and colleagues, its incidence comprises 5% of the total cases of d-TGA. Reference Freedom, Smallhorn and Trusler1 In another series by the Congenital Heart Surgeons’ Society of 829 patients with d-TGA, only one patient (0.1%) had TGA + IVS + LVOTO. Reference Williams, McCrindle, Ashburn, Jonas, Mavroudis and Blackstone2
Many surgical options can be used to manage patients with TGA + IVS + LVOTO. The type of operation depends on the timing of presentation, the type of intervention the patient previously underwent, and anatomic considerations including coronary artery pattern and type and severity of LVOTO. Early-era surgical treatment involved performing an atrial switch operation with resection of the LVOTO or placing a left ventricle-to-pulmonary artery conduit. However, the atrial switch operation does not provide an anatomic repair and reported early and late mortality were high in the presence of LVOTO. Reference Dasmahapatra, Freedom and Moes3,Reference Vejlstrup, Sørensen and Mattsson4 Another alternative that does provide an anatomic repair is performing an arterial switch operation (ASO) with or without LVOTO resection. Reference Raja, Kostolny and Oswal5,Reference Emani, Beroukhim and Zurakowski6 Another option is a modified Nikaidoh operation in cases where LVOTO is unresectable or where resection is associated with high risk of complications. Reference Honjo, Kotani and Bharucha7 A modified Rastelli operation with creation of a subaortic ventricular septal defect (VSD) in the intact ventricular septum may also be considered. Reference Jex, Puga, Julsrud and Weidman8 When cardiac anatomy precludes biventricular repair, then a functionally univentricular palliation provides a viable alternative. Reference Honjo, Kotani and Bharucha7,Reference Weyand, Haun and Blaschczok9
Due to the rarity of TGA + IVS + LVOTO and the scarcity of published literature on this topic, the outcomes of surgical repair of this disease are not well described. The purpose of this study is to identify practice patterns, surgical options, and short-term outcomes for patients with TGA + IVS + LVOTO undergoing surgical repair.
Patients and methods
All patients with a diagnosis of TGA + IVS + LVOTO in the European Congenital Heart Surgeons Association Congenital Database (ECHSA-CD) who underwent cardiac surgery during the 21-year time interval between January 2000 and February 2021, inclusive, were included. Preoperative variables including demographic data, operative data, and postoperative outcomes including Operative Mortality and complications were collected from ECHSA-CD. Records with obvious errors of entry of data into ECHSA-CD were excluded from analysis. Such excluded errors of data entry included patients with a primary or secondary diagnosis of VSD, complete atrioventricular septal defect (AVSD), or any of the various types of “single ventricle”; patients with procedures such as VSD closure or repair, double outlet right ventricle repair, or tetralogy of Fallot repair; and undefined diagnoses. In addition, ECHSA-CD entries where the primary diagnosis was not TGA + IVS + LVOTO were not considered.
The data in this analysis were provided by the ECHSA-CD after study review and approval by ECHSA-CD Committee regarding compliance with all ECHSA ethical and patient data protection policies; these data were verified in accordance with ECHSA-CD procedures and encompass fully anonymised information regarding patients who have undergone paediatric and/or congenital heart surgery in participating hospitals.
Results
Patient and operative characteristics
This analysis included 109 patients who underwent 176 operations from 2000 to 2021, inclusive, in ECHSA-CD. For all operations, median weight was 6.7 kg and median age was 7 months. For initial corrective and palliative operations, median weight was 6.29 kg and median age was 6.5 months (interquartile range from 13 days to 29 months). Figure 1 provides a flow diagram demonstrating the pathway of all 109 patients, starting with their first operation. Table 1 documents patient characteristics; preoperative, intraoperative and postoperative data; and overall outcomes. Table 2 presents the surgical pathway and outcome by patient. All 109 patients are classified by their evident surgical pathway, and Operative Mortality is provided.
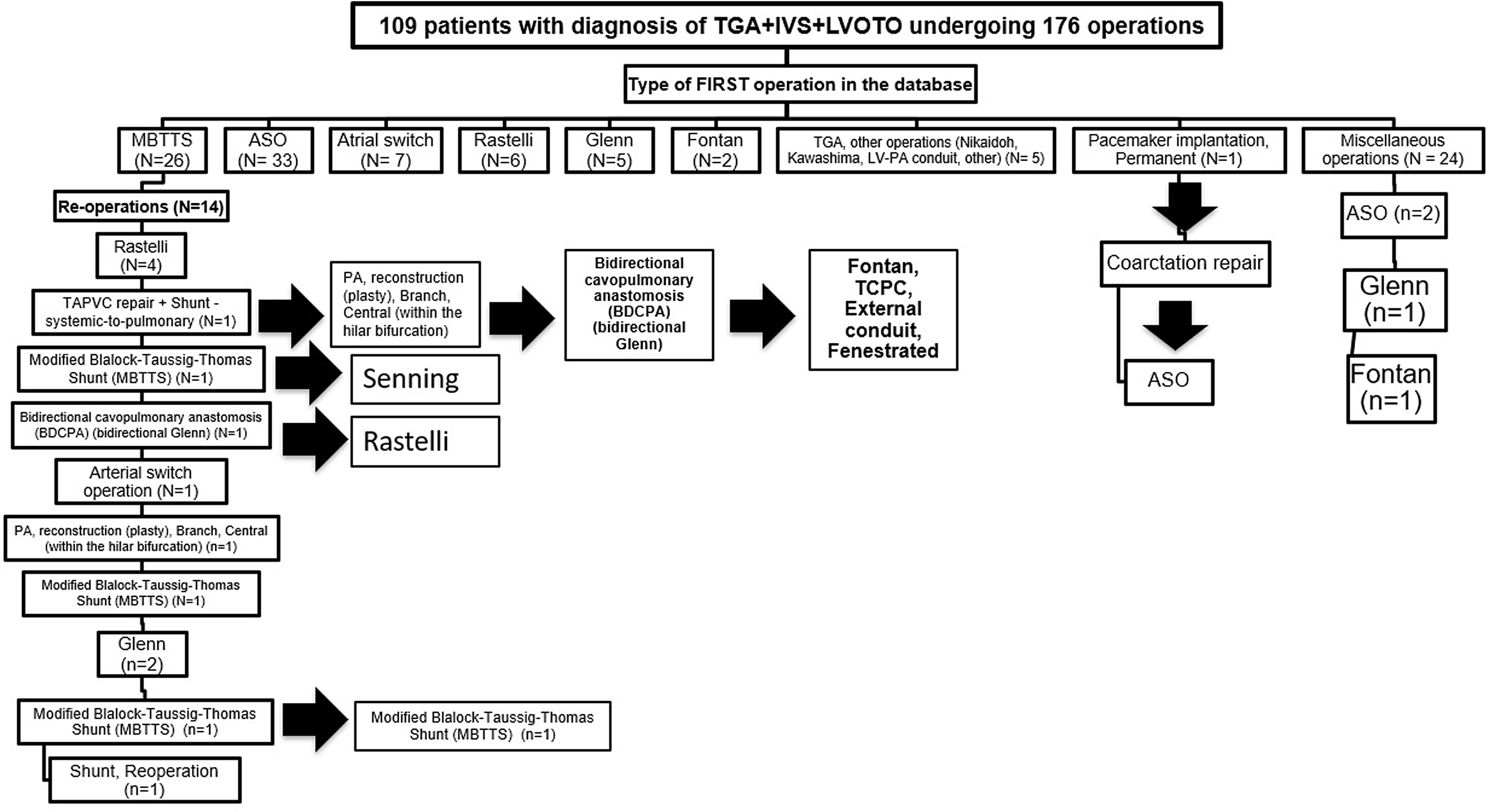
Figure 1. This figure is a flow diagram demonstrating the pathway of all 109 patients, starting with their first operation. Therefore, this figure displays the operative approach utilized in all 109 patients with TGA + IVS + LVOTO in this analysis. As documented in this figure, these 109 patients underwent 176 operations. Abbreviations: ASO, arterial switch operation; BDCPA, bidirectional cavopulmonary anastomosis; IVS, intact ventricular septum; LV, left ventricle; LVOTO, left ventricular outflow tract obstruction; MBTTS, modified Blalock-Taussig-Thomas shunt; PA, pulmonary artery; TAPVC, total anomalous pulmonary venous connection; TCPC, total cavopulmonary connection; TGA, transposition of great arteries.
Table 1. Patient characteristics, preoperative, intraoperative and post-operative data, and outcomes

Table 2. Surgical pathway and outcome by patient (All 109 patients are classified by their evident surgical pathway)

TGA = transposition of great arteries.
Note: “TGA, Other” is a procedural term that was in ECHSA-CD that includes Nikaidoh, Kawashima, and LV-PA conduit procedures.
Initial systemic-to-pulmonary artery shunts were performed in 26 patients. Table 3 lists the operations performed after a systemic-to-pulmonary artery shunt.
Table 3. Operations after a systemic-to-pulmonary artery shunt

PA = pulmonary artery.
There were 37 ASOs, and 22 of those operations had a concomitant procedure reported. Table 4 lists procedures concomitant with ASO. The most common procedures concomitant to ASO were atrial septal defect (ASD) repair with primary closure (n = 8), patent foramen ovale (PFO) closure (n = 8), and subvalvar aortic stenosis repair (n = 7).
Table 4. Procedures concomitant with ASO

ASO = arterial switch operation; PVR = pulmonary valve replacement.
* The degree of importance of the LVOTO is unknown from ECHSA-CD.
Outcomes
As detailed in Table 2, there were nine operative mortalities (8.2%), including three following ASO, two following “Nikaidoh, Kawashima, or LV-PA conduit procedures”, and two following Rastelli. Perioperative complications occurred after 39 operations (20.4%). Table 5 provides information about in-hospital complications. The most common complications were delayed sternal closure (n = 11), respiratory insufficiency requiring prolonged mechanical ventilation (n = 9), and renal failure requiring temporary dialysis (n = 8). Other complications included arrhythmias (n = 5), perioperative cardiac arrest (n = 5), postoperative cardiac dysfunction (n = 5), pleural effusion requiring drainage (n = 5), and postoperative mechanical circulatory support (n = 5).
Table 5. In-hospital complications

IABP = intra-aortic balloon pump; VAD = ventricular assist device; ECMO = extracorporeal membrane oxygenation; CPS = cardiopulmonary support
There were 68 reoperations performed in 41 patients during the 21 years of this study. There were 15 patients who had more than 1 reoperation. The most common reoperations were pacemaker procedure, ASO, Glenn, Rastelli, and extracorporeal membrane oxygenation (ECMO) operations.
Discussion
This study reports the surgical options and short-term outcomes of TGA + IVS + LVOTO from ECHSA-CD. In this analysis, 109 patients were identified in ECHSA-CD with the diagnosis of TGA + IVS + LVOTO, and these patients underwent 176 operations over 21 years (01/2000 to 02/2021). This study is therefore the largest analysis of patients with TGA + IVS + LVOTO ever performed. In this study, the most common corrective operation for TGA + IVS + LVOTO was ASO, which is similar to the Toronto experience reported by Honjo and colleagues on the surgical repair of TGA + VSD + LVOTO. Reference Honjo, Kotani and Bharucha7 In contrast, a large multicentre study by Hazekamp and colleagues showed that the most common operation of TGA + VSD + LVOTO was a Rastelli procedure. Reference Hazekamp, Gomez and Koolbergen10 This highlights the differences in surgical repair of TGA + LVOTO patients with and without a VSD. An intact ventricular septum in this setting represents a surgical challenge since repair options are limited, and the studies reporting the outcomes of this repair are limited. In this cohort, Operative Mortality was highest in the ASO population; however, due to the small number of other corrective procedures in the study as well as the lack of detailed anatomic data, one cannot perform meaningful statistical comparisons between the various operations. Reoperations occurred most commonly after modified Blalock-Taussig-Thomas shunts (MBTTS) (14 patients) and ASO procedures (6 patients), which partially reflects the higher frequency of these procedures in this cohort in comparison with other procedures. Furthermore, MBTTS is obviously a palliative procedure performed with plans for subsequent surgical intervention.
The aetiology of LVOTO in TGA is diverse, and the obstruction can be dynamic or fixed. Dynamic obstruction is caused by high right ventricular pressure and low pulmonary vascular resistance, which lead to ventricular septal bulging towards the left ventricle. Reference Robinson, Wyse and Macartney11 Fixed obstruction may be valvar, subvalvar, or multi-leveled. Subvalvar obstruction can be caused by posterior deviation of the outlet septum, fibrous membrane, fibromuscular tunnel, accessory atrioventricular valve tissue, abnormal attachment of atrioventricular valve apparatus to the ventricular septum, or protrusion of tricuspid valve leaflet through an essentially intact septum. Reference Freedom, Smallhorn and Trusler1,Reference Hazekamp, Portela and Bartelings12
ASO provides an anatomical repair that results in concordant ventriculoarterial connections, a systemic morphologically left ventricle, and correct pressures within the ventricles. Reference Sharma, Choudhary and Bhan13 Other major benefits of ASO include the absence of prosthetic conduits and the associated need for conduit reinterventions. Raja and colleagues reported excellent outcomes with ASO in 13 patients with TGA + IVS + LVOTO, with no mortalities or reinterventions at mid-term follow-up. Reference Raja, Kostolny and Oswal5 A possible downside of this operation in patients with TGA + IVS + LVOTO is the higher incidence of neoaortic regurgitation in patients undergoing ASO compared to patients undergoing other biventricular repairs. This neoaortic regurgitation is possibly due to the need for resection of subvalvar tissue, which may distort supporting muscular tissues or injure pulmonary valve leaflets. A structurally abnormal pulmonary valve may also contribute to the development of neoaortic regurgitation. Left ventricular outflow tract (LVOT) intervention has been shown to increase the risk of LVOT reintervention and neoaortic regurgitation. Reference Nakayama, Shinkawa, Matsumura, Hoki, Kobayashi and Niinami14–Reference Vida, Zanotto and Zanotto16 Nevertheless, the risk of clinically significant neoaortic regurgitation requiring neoaortic valve intervention is low. Reference Nakayama, Shinkawa, Matsumura, Hoki, Kobayashi and Niinami14,Reference Lange, Cleuziou and Hörer15
Ideally, candidates for ASO + LVOTO resection have a normal pulmonary annulus size, repairable pulmonary valve, and a resectable LVOTO. Reference Weyand, Haun and Blaschczok9 On the other hand, ASO is unfavourable in the following conditions: significant pulmonary annular hypoplasia, unresectable or multilevel LVOTO, and severely dysplastic or unicommissural pulmonary valve. Reference Emani, Beroukhim and Zurakowski6 It is thought that ASO with LVOT intervention should ideally be performed during the neonatal period as the operation may not be feasible later in life due to progression of severity of most types of LVOTO over time (e.g., decrease in pulmonary annulus size or decrease in LVOT size due to reduction of blood flow). Reference Emani, Beroukhim and Zurakowski6,Reference Weyand, Haun and Blaschczok9,Reference Sohn, Brizard, Cochrane, Wilkinson, Mas and Karl17
The Nikaidoh procedure (aortic translocation) results in a better anatomical alignment of the outflow tracts in comparison to the Rastelli operation, thus decreasing the risk of subsequent LVOT and right ventricular outflow tract (RVOT) reintervention. Reference Emani, Beroukhim and Zurakowski6 Aortic translocation is appropriate in patients who are not candidates for ASO with LVOTO resection. Traditionally, the Nikaidoh procedure is performed in patients with TGA + VSD + LVOTO. However, a modified Nikaidoh operation with Konno incision into the intact ventricular septum to enlarge the LVOT has been reported by Honjo and colleagues as an option in cases where the LVOTO is unresectable or multilevel, or where resection is associated with high risk of complications. Reference Honjo, Kotani and Bharucha7 A similar technique was described by Bautista-Hernandez and colleagues in a single patient with intact ventricular septum and LVOTO, where a modified Nikaidoh that includes detachment and mobilisation of the coronary buttons was performed with good intermediate outcomes. Reference Bautista-Hernandez, Marx, Bacha and del Nido18 There were no Operative Mortalities in these two cases. In our study, there were two Operative Mortalities following five “Nikaidoh, Kawashima, or LV-PA conduit operations”. Further experience is needed to understand the role of aortic translocation in treatment of TGA + IVS + LVOTO. Of note, the Nikaidoh procedure requires mobilisation of the aortic root with the coronary arteries, which may not be possible with certain coronary artery anomalies without increasing the risk of coronary artery injury. Reference Honjo, Kotani and Bharucha7,Reference Yeh, Ramaciotti, Leonard, Roy and Nikaidoh19 In addition, the long-term outcomes of the Nikaidoh operation have not been reported yet in patients with TGA + IVS + LVOTO.
Atrial switch operations (Mustard or Senning) result in a non-anatomical repair of TGA with a systemic morphologically right ventricle. Atrial switch procedures are associated with short-term and long-term complications such as right ventricular failure, baffle obstruction, and arrhythmias. Reference Vejlstrup, Sørensen and Mattsson4 Atrial switch procedures in the setting of LVOTO also have an important reported Operative Mortality. Reference Vejlstrup, Sørensen and Mattsson4,Reference Moons, Gewillig and Sluysmans20 Also, if LVOTO is not treated, it can lead to increased severity of obstruction over time, which is secondary to both ventricular hypertrophy with increased anatomic obstruction, as well as dynamic obstruction caused by high systemic right ventricular pressure causing septal bulging into the LVOT. The fact that the eight patients in this current analysis who were managed with atrial switch operations experienced zero Operative Mortality must be contextualised by our knowledge about the long-term problems associated with the atrial switch operations (e.g., late development of arrhythmias, baffle problems, and, particularly, progressive failure of the systemic morphological right ventricle). These late problems associated with the atrial switch operations have resulted in the replacement of the atrial switch operation with the arterial switch operation (ASO) for the overwhelming majority of patients with TGA. Furthermore, these late problems associated with the atrial switch operation certainly remain potential adverse sequalae of the atrial switch when it is performed in the setting of TGA + IVS + LVOTO.
A Rastelli operation is an alternative option in TGA + VSD + LVOTO. However, this operation is technically not feasible in patients with an intact septum, unless a VSD is created. Thus, a “modified” Rastelli with creation of a VSD in the intact infundibular septum was reported 32 years ago in a patient with an intact ventricular septum. Reference Jex, Puga, Julsrud and Weidman8 However, there has yet to be a series reporting on the short-term and long-term outcomes of this modified technique. The creation of a VSD in this setting is likely to be associated with considerable risk of injury to the conduction system, as well as risk of the need for repeat reintervention on the LVOT if the VSD that was created is small. In our study, the Rastelli operation was one of the most common operations performed in TGA + IVS + LVOTO. The Rastelli operation was performed in 11 patients in our cohort, with an Operative Mortality of 18% (n = 2). In patients with LVOTO, the Rastelli operation has been shown to have suboptimal long-term survival and reintervention rates, and specifically, high rates of reintervention on the RVOT and LVOT, compared to more contemporary corrective procedures. Reference Emani, Beroukhim and Zurakowski6,Reference Hazekamp, Gomez and Koolbergen10,Reference Hörer, Schreiber and Dworak21 The Rastelli operation is however, still considered as an alternative when ASO and Nikaidoh are not possible.
Finally, the last option that can be considered if cardiac anatomy precludes biventricular repair (e.g., abnormal chordal attachment in LVOT) is a functionally univentricular pathway. Although the operative techniques, Operative Mortality, and long-term survival of the Fontan procedure have been improving over the last few decades, it still carries risk of long-term complications such as Fontan failure, thromboembolism, tachyarrhythmias, bradyarrhythmias, protein losing enteropathy, and plastic bronchitis. Reference Stamm, Friehs and Mayer22,Reference D’udekem, Iyengar and Galati23
Limitations
The limitations of this current study include the following:
-
1. Because this study collects data from ECHSA-CD, the precise details related to the anatomical features of the specific cardiac malformation are not available beyond the variables already present in ECHSA-CD.
-
2. As this study collects data from ECHSA-CD, the details of the operations and outcomes are limited by the degree of details in ECHSA-CD.
-
3. The outcomes are limited to short-term outcomes because ECHSA-CD records outcomes only until hospital discharge and 30 days after the operation.
-
4. Not all patients with TGA + IVS + LVOTO have their initial surgical repair or reoperations recorded in ECHSA-CD, because the repair or reoperation may have been performed in a centre that does not contribute to ECHSA-CD. This limitation means that this study may underestimate or overestimate the outcomes presented.
-
5. ECHSA-CD does not contain detailed information regarding the cause, level, or severity of LVOTO, or the operation performed for LVOTO.
-
6. In addition, the nature of LVOTO (i.e., dynamic or fixed obstruction) is not described in ECHSA-CD. It is likely that a portion of the patients in this series who underwent ASO had dynamic LVOTO; but unfortunately, ECHSA-CD does not allow differentiation between dynamic LVOTO and fixed LVOTO. Indeed, the management of patients with dynamic LVOTO is different from the management of patients with fixed, or structural, LVOTO; however, ECHSA-CD does not allow differentiation of these variables.
-
7. Finally, the Rastelli operation is one of the most common corrective procedures performed in this study (11 Rastelli operations were performed over the 21 years of this analysis of ECHSA-CD, representing 11/176 operations, or 6.25% of all operations in this analysis). Nevertheless, details regarding the performance of a procedure concomitant to Rastelli such as creation of a VSD were not recorded. This limitation might mean that some of these data entered were actually for patients with TGA + VSD + LVOTO. It is a fact that each of 11 patients entered into ECHSA-CD with the diagnosis of TGA + IVS + LVOTO and the operation of Rastelli either underwent a concomitant VSD creation or was miscoded and actually had the diagnosis of TGA + VSD + LVOTO.
-
8. As with any analysis that spans 21 years of time, it is likely that this study is impacted and potentially confounded by an “era effect” over the 21 years of this analysis. For example, the atrial switch operation was more commonly performed during the earlier years of this 21-year study.
Although this analysis provides information about the operations performed on these 109 patients with TGA + IVS + LVOTO and their early outcomes, detailed patient-specific or institutional-specific insights were not possible secondary to the limitations of ECHSA-CD—limitations which exist in many multi-institutional surgical databases and registries. For example, one could not ascertain the rationale for a patient to undergo ASO versus any of the other potential operations. Furthermore, the dataset did not allow detailed evaluation of patients undergoing the Nikaidoh operation because early versions of ECHSA-CD included the following non-specific term: “TGA, Other procedures (Nikaidoh, Kawashima, LV-PA conduit, other)”, while in 2010, this term was separated into the following two more specific terms: “Aortic root translocation over left ventricle (Including Nikaidoh procedure)” and “TGA, Other procedures (Kawashima, LV-PA conduit, other)”. (Of note, these terms refer to the Repair of Kawashima that is an intraventricular tunnel repair with a posterior straight tunnel from the left ventricle to the aorta; these terms do not refer to the Kawashima procedure that is a superior cavopulmonary connection in the setting of interrupted inferior vena cava [IVC] with azygous continuation.) Finally, similar to most multi-institutional registries, analyses of ECHSA-CD do not allow a detailed assessment of the cause of death of any patients and do not allow longitudinal assessment of survival and reinterventions.
Value of this analysis
Despite these limitations, this study provides important information about the rareness of TGA + IVS + LVOTO, the variety of operations utilised to palliate and treat patients with this diagnosis, and the short-term outcomes associated with these operations. This manuscript reports the largest series of patients ever collected who underwent surgery with the diagnosis of TGA + IVS + LVOTO and provides unique and valuable information about practice of patterns and outcomes associated with this rare lesion. This manuscript also provides evidence about the limitations of the dataset and justification for the creation of a more detailed multi-institutional longitudinal study of this rare lesion.
This study provides clear evidence that patients with TGA + IVS + LVOTO are rare and that these patients are managed with multiple pathways of treatment. Perhaps the most valuable lesson from this analysis is that the limitations of the current source of data utilised for this analysis justify the creation of a multi-institutional longitudinal study to obtain longer term follow-up to understand the true outcomes associated with various anatomical variables and treatment approaches. Furthermore, additional imaging data would be extremely useful to stratify patients based on the morphology of their LVOTO.
It is a fact that TGA + IVS + LVOTO is a rare defect, and this lesion clearly represents a challenging constellation of anomalies to manage. TGA + IVS + LVOTO is further complicated by the varying degree of these anatomic challenges, including a wide spectrum of degree of, and aetiology for, the LVOTO, along with the variable coronary arterial arrangements. Because of these factors, agreement about a uniform treatment strategy or algorithm does not yet exist in the field, and as seen in this report, a wide practice variation exists (at least within the institutions submitting data to the ECHSA-CD). Therefore, there are many important questions yet to be answered in the treatment of patients with TGA + IVS + LVOTO. Although this manuscript does not answer all of these questions, this analysis does provide valuable information about patients with TGA + IVS + LVOTO and does inform potential future studies of these challenging patients.
Future studies
This analysis of ECHSA-CD provides insight into the patterns of practice and outcomes in ECHSA-CD for patients with the rare lesion of TGA + IVS + LVOTO. Over the 21 years of this analysis (01/2000-02/2021, inclusive), only 109 patients were identified in ECHSA-CD with the rare diagnosis of TGA + IVS + LVOTO, and these 109 patients underwent 176 operations. The important contributions of this analysis include:
-
1. This analysis provides information about what operations are performed for this rare lesion and the associated outcomes.
-
2. This analysis provides information about the limitations of ECHSA-CD (and consequently the Society of Thoracic Surgeons Congenital Heart Surgery Database [STS-CHSD], which uses identical fields of data). These limitations include (1) lack of granularity of diagnostic data (e.g., inability to differentiate dynamic obstruction from fixed obstruction); (2) lack of granularity of procedural data (e.g., inability to ascertain consistently whether a VSD creation was performed concomitantly with a Rastelli procedure); (3) inability to consistently follow a given patient over time and link operations across time for a given patient who had operations at different centres; and (4) absence of data about longitudinal long-term outcomes.
-
3. These limitations do impact our ability to use these data to make recommendations about the management of these challenging patients.
-
4. Importantly, knowledge of these publications can inform subsequent analyses of the rare lesion of TGA + IVS + LVOTO, as well as similar analyses of other rare lesions.
Two future studies are now being planned to study patients with the rare lesion of TGA + IVS + LVOTO. First, a study is now underway using data from STS-CHSD to provide insight into the patterns of practice and outcomes for patients with the rare lesion of TGA + IVS + LVOTO. Second, a longitudinal multi-institutional registry of patients with TGA + IVS + LVOTO is being operationalised under the leadership of World Society for Pediatric and Congenital Heart Surgery (WSPCHS), in collaboration with ECHSA and the Congenital Heart Surgeons’ Society (CHSS). Both of these future studies will be informed by this current analysis, and the construct of the WSPCHS TGA + IVS + LVOTO dataset will address the limitations of the dataset identified in the current analysis. Thus, the value of our current analysis is not only that it provides information about the rare lesion of TGA + IVS + LVOTO but also that it provides information about how to make our multi-institutional databases and registries better. Value exists in publishing and documenting not only the strengths of our current datasets but also the weaknesses and opportunities for improvement of our current datasets.
Conclusion
This study provides insight into the surgical options and short-term outcomes of patients undergoing surgical repair for TGA + IVS + LVOTO. TGA + IVS + LVOTO is a rare disease with multiple complex pathways of treatment; the rareness of this lesion combined with the limitation of the current dataset necessitate an international multi-institutional longitudinal study to obtain a large sample size, longer duration of follow-up, and more detailed data to better delineate the outcomes, with the goals of determining the optimal surgical strategy and guiding surgical decision-making for these challenging patients.
Acknowledgement
We thank the European Congenital Heart Surgeons Association (ECHSA) for providing access to data from the ECHSA Congenital Database (ECHSA-CD).
Disclosures
Jeffrey P. Jacobs, MD, Professor of Surgery and Pediatrics at University of Florida, is Editor-in-Chief of Cardiology in the Young and a consultant for SpecialtyCare and The American Academy of Dermatology.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









