Catheter-related bloodstream infections (CRBSIs) are a significant cause of morbidity and mortality in hospitalized patients. Reference O’Grady, Alexander and Burns1 One mechanism of CRBSI infection is through intraluminal contamination via needleless catheter connectors. Reference Danzig, Short and Collins2–Reference Rupp, Sholtz and Jourdan4 Manipulation of the catheter connector during routine patient care can allow for bacterial colonization of the catheter connecter and subsequent intraluminal spread. Reference Liñares, Sitges-Serra, Garau, Perez and Martin5,Reference Mermel, McCormick, Springman and Maki6 Current guidelines for preventing intraluminal contamination of needleless connectors include vigorously disinfecting the catheter connector with 70% alcohol for at least 5 seconds. Reference Buetti, Marschall and Drees7,8 We previously showed that a 5-second manual disinfection with an alcohol pad is sufficient to disinfect a split-septum catheter connector. Reference Rupp, Yu and Huerta9 Despite compliance with disinfection guidelines, 20%–40% of catheter connectors can become colonized. Reference Hankins, Majorant and Rupp10
One method to reduce colonization of catheter connectors is to use antiseptic-containing port-protecting caps. An in vitro study of antiseptic-containing port-protecting caps demonstrated more effective prevention of microbial transmission compared to catheters disinfected manually for 5 seconds with wipes containing 70% isopropyl alcohol (ie, alcohol wipes). Reference Menyhay and Maki11 Bacterial colonization of catheters decreased with the use of antiseptic-containing caps. Reference Hankins, Majorant and Rupp10,Reference Nicolás, Casariego, Romero, García, Diaz and Perez12,Reference Merrill, Sumner, Linford, Taylor and Macintosh13 Two meta-analyses of quasi-experimental studies evaluating rates of bacterial colonization of catheters and CRBSI following the implementation of antiseptic-containing caps revealed decreased bacterial colonization and rates of bacteremia. Reference Voor, Helder and Vos14,Reference Tejada15 Although these studies highlight the effectiveness of antiseptic-containing port protectors in reducing bacterial colonization, the most effective method for utilizing alcohol wipes with port protectors remains unclear. The 2022 compendium of strategies to prevent CRBSI highlight the uncertainty of requiring manual disinfection when antiseptic-containing caps are used, recognizing this question as an “unresolved issue.” Reference Buetti, Marschall and Drees7 Therefore, we evaluated whether antiseptic-containing caps alone are effective at decreasing microbial colonization of catheter connectors compared to the current standard at our institution, which is antiseptic-containing caps plus a 5-second manual disinfection with an alcohol wipe prior to utilization.
Methods
Study design and setting
Quality improvement project was conducted at a 718-bed, tertiary-care, academic hospital. No patient-identifying information was gathered, and patients, in-room visitors, and nurses were made aware of the project. The project was approved by the local institutional review board as a quality improvement project, and individual written informed consent was not required. A convenience sampling of adult patients was performed across intensive care units (ICUs) and acute care wards over 5 consecutive days in August 2022. Patients without inactive intravascular lumens (all lumens in active use), those who were unavailable at the time of sampling, were actively dying, or were in airborne isolation (eg, COVID-19) were excluded.
Catheter groups
The standard-of-care group consisted of inactive catheter connectors with passive antiseptic-containing port caps cleaned with the institutional infection control protocol of a 5-second scrub with an alcohol wipe prior to culture. The comparison group consisted of inactive catheter connectors with passive antiseptic-containing port protectors without a 5-second scrub with an alcohol wipe prior to obtaining a sample for culture. When patients with intravenous catheter connectors without antiseptic-containing port caps in place were encountered, the connectors were cultured without alcohol-wipe disinfection to serve as a positive control (ie, determination of baseline connector contamination without antiseptic cap or alcohol wipe disinfection). We allotted 5 seconds after scrubbing with an alcohol wipe to allow the alcohol to dry and to prevent the alcohol from further affecting culture results.
Product and culture procedure
According to the institutional standard of care, central venous catheter (CVC) and peripheral vascular catheter (PVC) connectors (MaxZero, Becton Dickinson, Franklin Lakes, NJ) were both passively protected by an antiseptic-containing cap (Curos Disinfecting Port Protector [3M]) (Fig. 1). According to institutional policy, needleless connectors are changed every 96 hours. Antiseptic-containing caps are required on all needleless connectors not in use. These policies are the same for all units. All CVCs are inserted by trained individuals utilizing an insertion checklist, with chlorhexidine dressings. Daily chlorhexidine baths in both acute care wards and ICUs are used to reduce CRBSI and other healthcare-associated infections. Inactive CVC and PVC connectors were cultured by removing the antiseptic-containing caps and pressing the diaphragm of the needleless catheter connector gently onto sheep blood agar plates as previously described. Reference Hankins, Majorant and Rupp10 Each agar plate was divided into 4 labeled quadrants with each quadrant representing a single diaphragm culture (Fig. 2). After the needleless catheter connector was sampled, it was cleaned with an alcohol wipe for 5 seconds and covered with the passive antiseptic-containing port protector. The catheter type was recorded as either a PVC or CVC. For both PVCs and CVCs, sampling alternated between the standard-of-care group with manual disinfection and the comparison group without disinfection. Investigators participated in standardized training on the methodology for catheter connector culturing techniques.
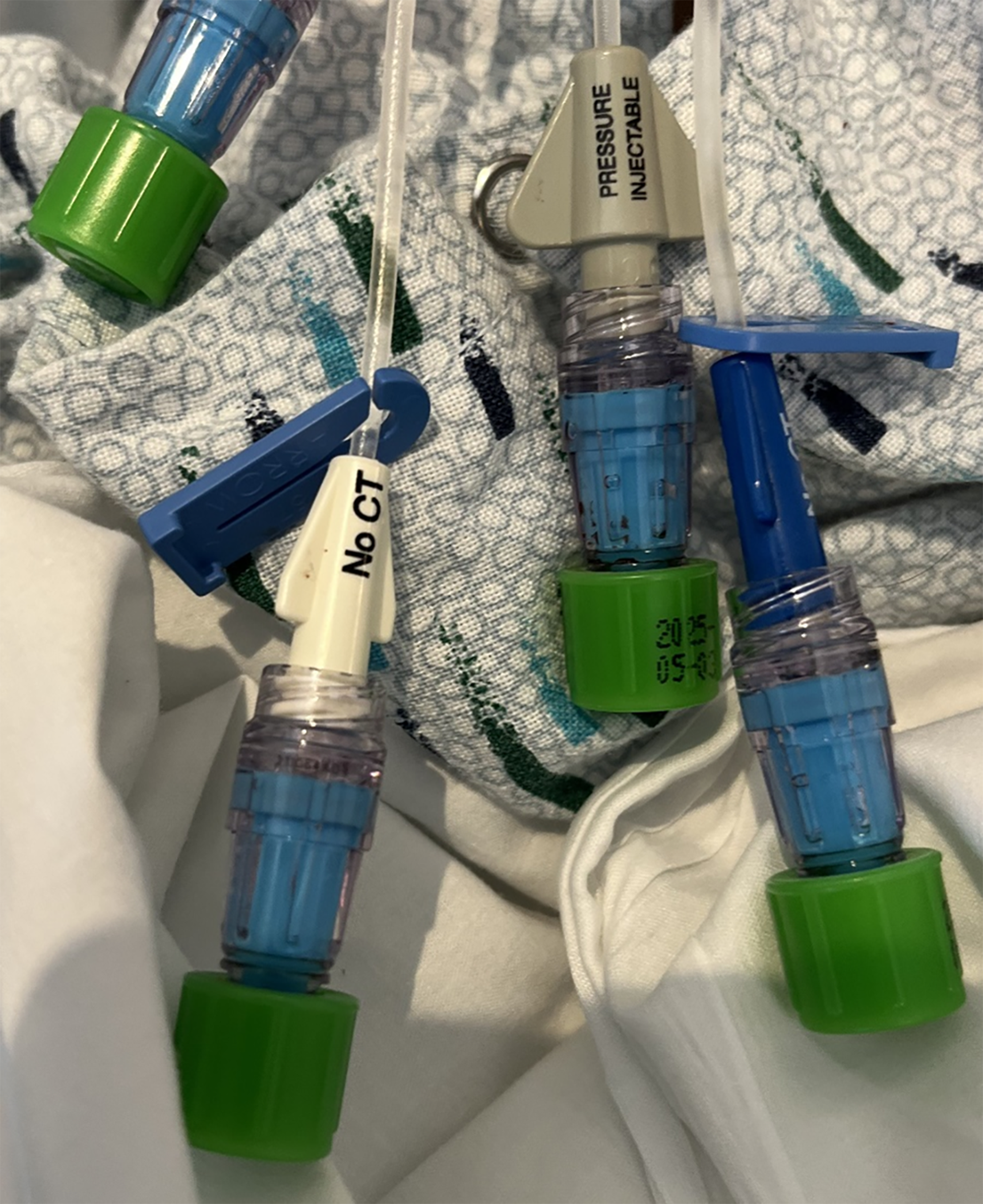
Figure 1. Antiseptic-containing caps on luer lock catheter connectors.

Figure 2. Agar plate with confluent bacterial growth in the impression zone from the catheter connector (a different catheter connector was assessed in each quadrant and sterile impression zones are evident in the other 3 quadrants).
Blood agar plates were incubated at 37°C and were assessed for growth after 48–72 hours by 2 investigators. These investigators evaluated the plates simultaneously to assess the growth of each plate, agreeing on the extent of growth. Each catheter-connector quadrant was assessed and recorded as exhibiting no growth, <15 separate colony forming units (CFUs), ≥15 CFUs, or confluent growth (Fig. 2).
Statistical analysis
Descriptive statistics included counts and percentages for the collected data. The primary objective of the study was to determine the noninferiority of a catheter connector without a 5-second scrub with an alcohol wipe compared to a connector with a 5-second scrub with an alcohol wipe in terms of microbial growth. Noninferiority analysis of the effect of not performing a 5-second scrub on the risk difference for growth was completed by comparing the risk difference to a noninferiority margin with the Farrington-Manning method. Reference Farrington and Manning16 The null hypothesis for this test was that the risk difference would be less than or equal to the negative margin. The Fisher exact test was used to determine associations of growth with positive controls, line type, and location. The Mann-Whitney test was used to compare the median line duration with culture results. Analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC), and P < .05 was considered statistically significant.
Sample sizes of 132 in the standard-of-care group and 132 in the comparison group achieved 80% power to detect a difference of 0.0 when the noninferiority difference was 0.036. The standard-of-care group proportion was 0.014, which aligned with the finding of our previous test of needleless connectors. The comparison group proportion was assumed to be 0.050 under the null hypothesis. The power was computed for the case when the actual comparison group proportion was 0.014. The significance level of the test was 0.05. Reference Rupp, Yu and Huerta9
Results
In total, 356 catheter connectors were cultured: 330 cultured connectors had antiseptic-containing caps, including 165 in the standard-of-care 5-second manual disinfection group and 165 in the comparison group without a 5-second disinfection. We found 26 catheters without an antiseptic-containing cap in place and designated them as additional controls. In total, 105 CVCs and 251 PVCs were sampled (Table 1).
Table 1. Description of Sampled Vascular Catheter Connectors

Note. ICU, intensive care unit; CVC, central venous catheter; PVC, peripheral vascular catheter.
Of 165 catheter connectors with an antiseptic-containing cap alone, 2 had bacterial colonization, compared with 1 of 165 catheter connectors that was colonized after utilizing both an antiseptic-containing cap as well as a 5-second scrub (1.21% vs 0.61%; absolute difference, 0.61%; 95% confidence interval [CI] for the difference, −2.70 to 3.91) (Table 2).
Table 2. Colonization of Standard-of-Care Versus Comparison Group

Note. CI, confidence interval.
In total, 157 catheter connectors were cultured from patients located on ICUs, and 173 were from general medicine floors (Table 1). Moreover, 18 connectors (5.06%) yielded microbial growth. Among ICU catheters, 6 (3.8%) cultures had bacterial growth. In catheters from the acute care ward, 12 (6.9%) had cultures that showed bacterial growth. Of the cultures with growth collected in the ICUs, 4 (66.7%) were controls without an antiseptic-containing cap. Of the cultures with growth collected from catheters on the acute care wards, 11 (91.7%) were controls without an antiseptic-containing cap.
Of the 26 controls without an antiseptic-containing cap, 15 (57.7%) were positive for bacterial colonization. Comparatively, only 3 connector cultures (0.9%) had bacterial growth when an antiseptic-containing cap was in place. The connectors that were covered with an antiseptic-containing cap were also compared with the connectors without antiseptic-containing caps. Microbial colonization was significantly higher on the catheter connectors that were not covered by antiseptic-containing caps, compared to the standard-of-care group (P < .0001) (Table 3) and to the comparison group (P < .0001) (Table 4).
Table 3. Colonization of Standard-of-Care and Connectors without Antiseptic-Containing Caps

Table 4. Colonization of Comparison Group and Connectors Without Antiseptic-Containing Caps

Of the total positive cultures, 11 had <15 CFU, 5 had ≥15 CFU, and 2 had areas of confluent growth. Among the positive cultures, 9 (82%) of 11 <15 CFU, 4 (80%) of 5 >15 CFU, and 2 (100%) of 2 confluent growth cultures were in controls without an antiseptic-containing cap. The standard-of-care group had 1 culture with growth of <15 CFU, and the comparison group had 1 culture each with growth of <15 CFU and ≥15 CFU. Line duration was monitored. The single catheter connector that had bacterial growth in the standard-of-care group was in place for 14 days before it was assessed. The 2 catheter connectors that were positive in the comparison group were in place for 3 and 13 days, respectively, prior to being assessed. In the connectors without antiseptic containing caps, 6 had growth in <24 hours, 3 had growth between 24 hours and 48 hours, 3 had growth between 48 hours and 72 hours, and 3 had growth at >72 hours after line insertion. The average duration of intravenous catheters that had connectors with bacterial colonization was 5.1 days, and the average duration of intravenous catheters that had connectors without bacterial colonization was 9.6 days (P = .177).
Discussion
Noninferiority analysis demonstrated no risk difference in positive growth between catheter connectors with only an antiseptic-containing caps without an additional 5-second scrub with an alcohol wipe and catheter connectors disinfected with antiseptic-containing caps and the additional 5-second scrub. The percent difference was 0.61% (95% CI, −2.70%–3.91%; P = .0063) and the a priori noninferiority margin was −3.60%. Our findings contribute to previous knowledge that antiseptic-containing caps significantly reduce bacterial colonization compared to catheter connectors that are not covered with an antiseptic-containing cap. Reference Buetti, Marschall and Drees7 With the significant increase in CRBSIs in association with the COVID-19 pandemic, identifying effective methods of reducing bacterial colonization, and potential CRBSIs is paramount. Reference Fakih, Bufalino and Sturm17 By removing this small, seemingly insignificant step of nurses performing an additional 5-second scrub every time they access a vascular catheter, nurses potentially save a substantial amount of time in simplifying the care they provide and decreasing unnecessary use of alcohol wipes. Infection control efforts can also be better focused on making sure that every catheter connector has an antiseptic-containing cap, rather than on performing the additional step of performing a 5-second scrub. Because our institution showed a 7.3% rate of nonadherence with catheter connectors without an antiseptic-containing cap, opportunities remain for improvements in patient safety.
More controls without an antiseptic-containing cap were PVCs compared to CVCs despite institutional policy that all unused catheter connectors be covered with an antiseptic-containing cap. There was also an increase in controls without an antiseptic-containing cap on acute care units compared to ICUs. This finding may be explained by differences in nursing practices (eg, nurse-to-patient ratio) involving catheter-connector maintenance and higher frequency of PVCs on acute care units. In addition, 15 of 18 catheters exhibiting bacterial growth were catheters without an antiseptic-containing cap, with an overall bacterial contamination rate of 57.7%. Given that catheters with an antiseptic-containing cap only had a contamination rate of 0.95%, these data reinforce the utility of antiseptic-containing catheter connector caps in reducing contamination.
This study was a prospective assessment of multiple catheter types across multiple units of a tertiary-care center. We documented intravascular catheter-connector colonization in multiple active hospital units, in patients with intravenous catheters in place, and in clinical use. Catheter duration was monitored. However, we were unable to adequately evaluate the correlation between line duration and connector colonization due to the low rate of colonization of connectors with antiseptic-containing caps. Positive controls were evaluated with the Cochran-Armitage trend test, and a trend between time and positive bacterial colonization was not found.
This study had several limitations. Data collection was performed at a single site, which allowed hospital-specific nursing practices to potentially influence the results and raises questions regarding generalizability. Additionally, no patient-specific outcomes were collected; therefore, no correlation of colonization and bloodstream infections can be concluded. For catheter connectors that received a 5-second alcohol disinfection scrub prior to culture, the possibility the alcohol was not given enough time to dry and thus affected the culture results is possible. This factor was mitigated by waiting 5 seconds after swabbing the catheter connector with alcohol and ensuring that the tip was dry before culturing. Also, catheter connector could have been contaminated by the investigators (during manipulation) after the antiseptic-containing cap was removed, skewing results. However, investigators performing the cultures were trained, and no instances of a break in aseptic procedures were noted. Another limitation of the study was that the duration that the antiseptic-containing caps were in place since the connector was last accessed was unknown. We only assessed a single brand of needleless catheter connectors and antiseptic-containing caps, which may limit the generalizability of our findings to other products.
We noted that 42.3% of catheters without an antiseptic-containing cap were found in a single unit, which may suggest clustering due to differences in nursing practices. Also, excluding unavailable patients and patients in airborne isolation may have resulted in selection bias. Because there has been a significant increase in CRSBI in association with the COVID-19 pandemic, a comparison of connector colonization between patients in COVID-19 isolation and the rest of the clinical population would have been of value. Reference Fakih, Bufalino and Sturm17
We conducted this study to address a stated unresolved issue regarding the care of patients with vascular catheters. Reference Buetti, Marschall and Drees7 Bacterial colonization rates were noninferior between the catheter connectors cultured with an antiseptic-containing cap alone and catheter connectors with an antiseptic-containing cap cultured after a 5-second scrub with an alcohol wipe. This finding suggests that the 5-second alcohol-wipe disinfection step is unnecessary when an antiseptic-containing cap is in place and that the use of an antiseptic-containing cap reduces the risk of catheter connector colonization independent of an alcohol scrub.
Acknowledgements
Financial support
No financial support was provided relevant to this article.
Competing interests
Mark E. Rupp notes having received clinical research support from 3M (research contract with institution) and having served as a consultant for 3M. He has served as a consultant or on advisory boards for Teleflex, Becton Dickinson, and Citius. 3M had no role in this project including the design, conduct, funding, data analysis, or manuscript, preparation. All other authors report no conflicts of interest relevant to this article.









