Pseudomonas aeruginosa producing metallo-β-lactamase (MBLs) have been recognized in several parts of the world, e.g. Poland, Southern Brazil, Taiwan, and Europe [Reference Livermore1]. To date, at least 26 IMP and 30 VIM variants have been reported in several countries (http://www.lahey.org/Studies/other.asp#table1). Genetic determinants of MBLs (bla genes) are mostly carried on class 1 and class 3 integrons [Reference Shibata2] and can spread rapidly in hospitals via the integron system. In Thailand, Boonkerd et al. reported the first case of a bla IMP-1 gene in carbapenem-resistant P. aeruginosa in 2009; bla VIM-type MBL was not found in their study [Reference Boonkerd3]. We report here the prevalence of bla IMP and bla VIM genes associated with class 1 integrons and also investigated possible clonal dissemination of P. aeruginosa by pulsed-field gel electrophoresis (PFGE) based on observations in a tertiary-level teaching hospital in Thailand during March–April 2009.
Non-duplicate, clinical isolates of P. aeruginosa were randomly selected and species identity was confirmed by standard techniques. Isolates were tested for susceptibility to 11 antimicrobial agents [amikacin (AMK), ceftazidime (CAZ), ciprofloxacin (CIP), colistin (CL), cefepime (FEP), gentamicin (GEN), imipenem (IPM), levofloxacin (LVX), meropenem (MEM), cefoperazone/sulbactam (SCFP) and piperacillin/tazobactam (TZP) (Oxoid, UK) by the disk diffusion assay according to Clinical and Laboratory Standards Institute (CLSI) guidelines [4]. Minimum inhibitory concentrations (MICs) of IPM were determined by an IPM E-test strip and isolates were screened for the presence of MBL enzymes by the E-test MBL strip (AB Biodisk, Sweden). A MIC ratio (MIC of IPM alone/MIC of IPM plus EDTA) of ⩾8 was interpreted as suggestive of MBL production. Genomic DNA of P. aeruginosa was extracted and purified by phenol/chloroform as described previously [Reference Goldberg and Ohman5]. PCR amplification for MBL genes (bla IMP, bla VIM) was performed as described previously [Reference Ellington6]. Class 1 integron genes were amplified by multiplex PCR with the following primers: intI-1 primers (forward: 5′-GGC GCG CTG AAA GGT CTG GT-3′; reverse: 5′-CCG CTG CGT TCG GTC AAG GT-3′); qacEΔ1 primers (forward: 5′-TTG CCC CTT CCG CCG TTG TC-3′; reverse: 5′-CCT CCG CAG CGA CTT CCA CG-3′); sul1 primers (forward: 5′-GAC GCG AGG CCT GTA TCG CC-3′; reverse: 5′-TCC GTC GCA AGG CGG AAA CC-3′). PCR products were purified and directly sequenced using IMP-F, IMP-R, VIM-F and VIM-R primers (1st BASE Pte Ltd, Thailand). DNA sequences were analysed by the BLAST program (http://blast.ncbi.nlm.nih.gov/). P. aeruginosa IMP-1-producing strains were kindly provided by Dr Neil Woodford as positive controls for MBL detection. Genotyping of isolates carrying bla IMP or bla VIM was performed by PFGE with the restriction enzyme SpeI in a CHEF Mapper system (Bio-Rad Laboratories, USA).
The 75 P. aeruginosa isolates were from sputum (n=33, 44%), urine (n=21, 28%), pus (n=18, 24%) and blood (n=3, 4%). Most isolates were resistant to LVX and CIP (54·7%), followed by CAZ (50·7%), SCFP (42·7%), TZP (42·7%), MEM (38·7%), and IPM (33·3%); all were susceptible to CL. Thirteen isolates (17·3%) were positive for MBLs by E-test and were multidrug resistant with a high-level of resistance to IPM (MIC >256 μg/ml). Thirteen isolates were also positive for the bla IMP allele and one was positive for the bla VIM allele. Nucleotide sequencing identified the bla IMP and bla VIM genes to be IMP-14 (GenBank EMBL accession no. GQ302617) and VIM-2 (accession no GQ853417). Twenty-four isolates (32·0%) were positive for class 1 integron genes (intI-1, qacEΔ1, sul1; Fig. 1); 12 of these carried IMP-14, and one was VIM-2 positive. Another isolate with a bla IMP gene was negative for class 1 integron genes. The SpeI-digested genomic DNA patterns of 14 isolates were classified into three types; A, B and C according to the criteria of Tenover et al. [Reference Tenover7] (Fig. 2). Pattern A was dominant and consisted of five closely related restriction patterns for the 12 bla IMP-producing isolates. The VIM-2 strain was unique (pattern C), and had a susceptibility profile identical with most strains of pattern A.
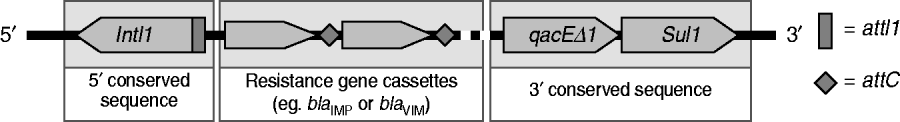
Fig 1. Schematic representation of class 1 integron.

Fig 2. Pulsed-field gel electrophoresis (PFGE) pattern of some of the P. aeruginosa isolates carrying MBL genes after digestion with SpeI. Lane M, lambda ladder (Promega, USA); lanes 1-14, isolates 20 042, 20 689, 20 055, 20 717, 60 334, 60 546, 20 062, 60 154, 20 288, 60 172, 20 453, 20 697, 20 055, and 20 832, respectively.
Corresponding with data from the National Antimicrobial Resistance Surveillance Center of Thailand, clinical isolates in the present study showed high rates of resistance to most anti-pseudomonal agents especially quinolones, β-lactam/inhibitor combinations, cephalosporins and carbapenems, but no resistance to CL was found underlining its potential as a reserve antibiotic for multidrug-resistant strains of P. aeruginosa.
As found in a previous study [Reference Gu8], integron-positive strains were significantly associated with resistance to aminoglycosides, quinolones, carbapenems and cephalosporins (P⩽0·001). Interestingly, one strain carried the bla IMP gene but not the integrase (intI-1) gene and this raises the possibility of insertion of the bla IMP gene into another class of integrons [Reference Shibata2]. Moreover, two strains which were phenotypically positive for MBLs but negative for bla IMP and other MBL-encoding genes (e.g. VIM-, GIM- and SPM-type), clearly produced MBL enzymes [Reference Lee9], one of which was identified as VIM-2 type. By contrast, two strains harboured the genes for bla IMP and class 1 integrons but were negative by the MBL E-test. This was previously observed by Collis & Hall [Reference Collis and Hall10] who suggested that the expression of the cassette had been affected by the position of the antibiotic resistance gene cassette which was inserted in the region of the integron.
We have shown the emergence of bla IMP-14 and bla VIM-2 genes in MBL-producing P. aeruginosa strains. The IMP-14 MBL gene was first described in a P. aeruginosa clinical isolate from Thailand in 2004 (GenBank accession no. GQ302617, unpublished data), and the VIM-2-type MBL gene has been reported from many countries including Japan, South Korea, Portugal, Spain, and USA [Reference Walsh11].
PFGE showed that all isolates in pattern A were from urine samples of patients from two wards suggesting that this was the predominant clone circulating in the hospital. Although seemingly related by DNA profile the remaining four strains with the IMP-14 gene originated from various specimens and wards. Thus MBL-producing strains exhibited both clonality and diversity, which underlines that their prevalence cannot be explained solely by clonal expansion of a particular strain type.
In Thailand, the prevalence of MBL (bla IMP and bla VIM)-producing P. aeruginosa strains associated with class 1 integrons has not yet been investigated in a large-scale study. Our preliminary survey showed that 17·3% of P. aeruginosa strains isolated over 2 months tested positive for MBL-producing genes. These data underline the importance of the emergence of MBLs associated with class 1 integrons and highlight the need for the development of infection control strategies in order to combat the spread of multidrug-resistant strains in hospitals by more efficient and appropriate prescribing of selected reserve drugs such as the carbapenems.
ACKNOWLEDGEMENTS
This study was financially supported by a Graduate thesis grant, Graduate School, Chulalongkorn University, and Chulalongkorn University Centenary Academic Development Project. Cherman Piyakul received a graduate tuition fee scholarship and teaching assistant fellowship from the Faculty of Allied Health Sciences, Chulalongkorn University. The authors thank Mr Somsak Rahul for providing P. aeruginosa clinical isolates.
DECLARATION OF INTEREST
None.




