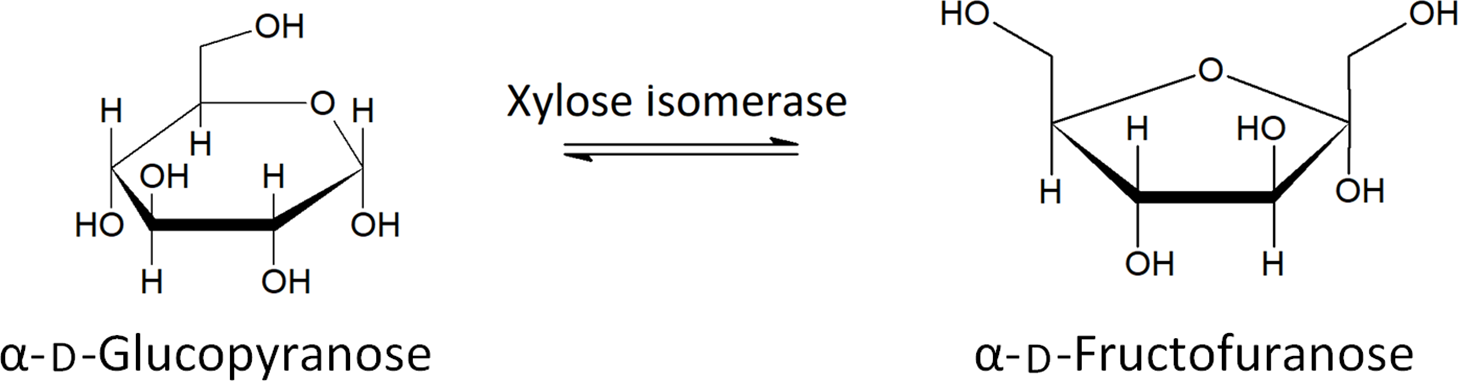It is estimated that irritable bowel syndrome (IBS) affects around 11 % of the global population(Reference Canavan, West and Card1). IBS has symptoms including stomach pains and cramps, bloating, diarrhoea and constipation. Although being a common disease, the exact causes of IBS are typically described as a gut–brain disorder; however, this notion has been challenged in recent years and a number of new potential causes, including anxiety and depression, gut bile malabsorption and inflammation and infection of the nervous system, have been proposed(Reference Holtmann, Ford and Talley2). Fructose malabsorption (FM) has also been suggested as a potential cause of IBS(Reference Di Nicolantonio and Lucan3). FM is believed to affect one in three patients with IBS and is caused by the incomplete absorption of fructose in the small intestine (SI), leading to gastrointestinal (GI) complaints, and has been linked to other diseases, such as early-stage depression(Reference Choi, Kraft and Zimmerman4–Reference Ledochowski, Sperner-Unterweger and Widner6). FM can develop as a result of primary causes, such as congenital deficiency, or by secondary means, including, but not limited to intestinal damage, acute gastroenteritis, medication, coeliac disease, Crohn’s diseases and use of prebiotics. However, due to a lack of knowledge regarding the mechanism of fructose absorption to date, it has been difficult to accurately diagnose and treat FM(Reference Noelting and Di Baise7). In the past few decades, there has been an increase in the amount of fructose consumed, particularly due to high fructose corn syrup (HFCS) and increased consumption of HFCS-rich soft drinks, especially by younger people(Reference Vos, Kimmons and Gillespie8). It has been noted in a survey between 1994 and 1996 that the consumption of artificial sweeteners, including HFCS, results in 1330.5 kJ daily of dietary consumption for US Americans above the age of 2 years; this accounts for 16 % of all caloric intake daily(Reference Bray, Nielsen and Popkin9).
A variety of diseases can result in the malabsorption of sugars, including lactose intolerance, congenital glucose–galactose malabsorption and congenital sucrase-isomaltase deficiency, resulting in the malabsorption of lactose, galactose and maltose, respectively(Reference Ma, Long and Chen10,Reference Burke11) . However, this review will focus on FM and the specific factors affecting the treatment of FM.
Fructose chemistry
Fructose, a ketonic monosaccharide, is able to be directly absorbed into blood from the GI tract; it is one of three dietary monosaccharides that possess this property, alongside glucose and galactose. Fructose is commonly obtained from sugar beets, sugar cane and maize and is the sweetest of all monosaccharides(Reference Moskowitz12). The solubility and sweetness of fructose have been exploited by the food industry in artificial sweeteners, with particular popularity around the use of HFCS, a mixture of glucose and fructose in the monosaccharide form in recent years. Between 1970 and 2004, the share of HFCS as a percentage of total sweetener use has risen from half a percentage point to 42 %(Reference Duffey and Popkin13).
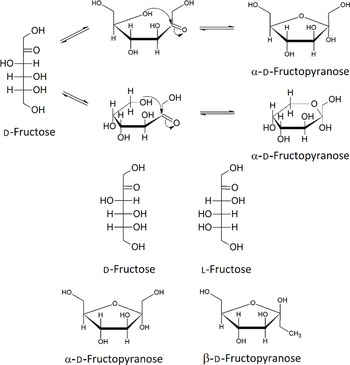
It has been estimated that between 1994 and 1998, the average US American above the age of 2 years consumes 552.3 kJ of HFCS per day. Added sweeteners contribute to 16 % of this daily energetic intake(Reference Ventura, Davis and Goran14–Reference Popkin and Nielsen16). Fructose can be present as a monosaccharide, most abundantly in the furanose form, or as the disaccharide sucrose, in a one to one molecular ratio with glucose. Figure 1 shows the five isomers of fructose; in aqueous solution, fructose exists in the equilibria shown in Fig. 1. The mixture of the five isomers is comprised of 70 % fructopyranose and 22 % fructofuranose; the remaining 8 % consists of the other three forms, including acyclic d-fructose. Fructose can also exist in two anomeric states, α and β, and these are also shown in Fig. 1.

Fig. 1. Structures of the isomers and anomers of fructose.
Causes of fructose malabsorption
FM is caused by the failure to effectively absorb fructose through the enterocytes lining the SI. This results in the accumulation of fructose in the intestinal lumen. The resulting change in osmotic pressure causes the flow of water into the lumen, thus leading to the symptoms associated with IBS. The underlying factors related to the cause of FM will be described in detail in the following section.
Mechanism of fructose absorption
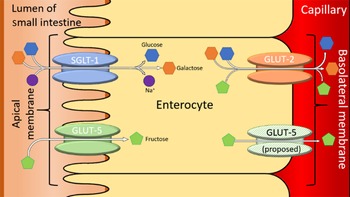
For healthy patients, serum fructose concentration is around 8·1 ± 1·0 μmol/l and for diabetics, serum fructose concentration is around 12·0 ± μmol/l(Reference Kawasaki, Akanuma and Yamanouchi17). The current understanding of FM is underpinned by the transport enzymes present on the borders of the enterocytes lining the SI. The two enzymes in question are GLUT2 and GLUT5. The general understanding of the mechanism of absorption for fructose is that GLUT2 is a high-capacity, low-affinity glucose/galactose transporter that can co-transport fructose in a one-to-one ratio(Reference Leturque, Brot-Laroche and le Gall18). GLUT2 is unable to transport fructose without the presence of glucose, although the mechanism for this is currently unknown. However, it is proposed that GLUT5 is able to selectively transport fructose across the apical membrane of the SI. The low capacity of GLUT5 means that excess fructose leads to the overloading of GLUT5, preventing the complete absorption of fructose(Reference Douard and Ferraris19). The presence of excess fructose in the GI tract leads to increased osmotic load, which, in turn, triggers the symptoms associated with IBS(Reference Choi, Kraft and Zimmerman4). Further research is needed to solidify our understanding of fructose absorptions, so that treatment can be better targeted. One such method could involve targeting the prevention of the fructose accumulation in the GI tract, thus preventing the symptoms resulting from such accumulation. Figure 2 summarises the enzymes involved in the absorption of fructose and other dietary sugars, in the SI. It shows the confirmed and proposed locations of GLUT5 within the enterocyte(Reference White, Jensen and Rajalingam20).

Fig. 2. A simplified diagram of sugar transporters in the enterocytes of the small intestines. Adapted from(Reference Li, Manolescu and Ritzel21–Reference Manolescu, Salas-Burgos and Fischbarg23). SGLT-1 is a sodium-mediated enzyme related to the facilitated co-transport of glucose and galactose and is not linked to the transportation of fructose(Reference Dominguez Rieg and Rieg24).
A total of six GLUT potentially have the capacity to selectivity transport fructose. These are GLUT 5, 7, 8, 9, 11 and 12(Reference White, Jensen and Rajalingam20). However, this review will focus primarily on GLUT5, due to its confirmed selectivity for fructose and its high expression within the SI and duodenum.
Expression of GLUT5
Expression of GLUT5 is coded by the solute carrier family 2, facilitated GLUT member 5 (SLC2A5) gene and although expression is highest in the SI and duodenum, the RNA resulting from SLC2A5 expression is found in other tissue as well(Reference Blakemore, Aledo and James25). Despite glucose being the primary energy source in the brain, RNA production from SLC2A5 has resulted in GLUT5 expression being found in the blood–brain barrier of rats, as well as the microglia and fetal cerebellar Purkinje cells of humans(Reference Shu, Isenberg and Cormier26–Reference Nualart, Godoy and Reinicke28).GLUT5 is also expressed in the cerebellum of mice and the hippocampus of rats. These cells may be capable of using fructose as a source of energy; however, this is currently unconfirmed. It should be noted the expression of GLUT 5 in the brain is significantly lower than in the SI(Reference Dyer, Wood and Palejwala29).
Diabetes has a major effect on GLUT5 expression in the SI. It has been found that in type 2 diabetic patients, there is a three- to four-fold increase in the expression of GLUT 5 proteins and mRNA in duodenal and small intestinal cells(Reference Dyer, Wood and Palejwala29). Reversing blood hyperglycaemia results in the reversal of the elevated levels of GLUT 5 expression(Reference Dyer, Wood and Palejwala29). A positive link between blood hypertension, linked to diabetes, and up-regulation of GLUT5 has also been investigated(Reference Kawasaki, Akanuma and Yamanouchi17). However, more research into this finding is required.
The effect diabetes has on serum fructose concentration is currently unclear. Serum fructose concentration and urinary fructose increased significantly in Japanese diabetic patients indicating FM(Reference Pitkänen30). However, a similar study in Finland, comparing healthy volunteers with type 1 and 2 diabetics, indicated similar concentrations of serum fructose(Reference Buddington and Diamond31,Reference Ferraris32) . These contradictory results complicate what the precise effect of diabetes is on serum fructose concentration. However, it is apparent that fructose may have a key role in the development of metabolic disorders that cause the adverse effects resulting from diabetes.
FM may have a role to play in causing infantile colic(Reference Duro, Rising and Cedillo33). It has been noted that in the prenatal and suckling periods of rats, rabbits and humans, GLUT5 levels in the intestine are very low(Reference Nobigrot, Chasalow and Lifshitz34). Additionally, a study into breath hydrogen of colic-affected patients indicated an increase in breath hydrogen in children, less than 1 year of age, but not in those 2 years or older(Reference Chan, Chan and Chung35). These results indicate that GLUT5 is not initially expressed in infants and the resulting FM may result in the colic that many infants suffer from.
Interestingly, elevated levels of GLUT5 mRNA and protein expression have been found in some cancer cell lines. Despite GLUT5’s poor expression in typical mammary epithelial cells, high amounts of GLUT5 mRNA and protein expression have been found in the breast carcinoma cell lines MCF-7 and MDA-MB-231, resulting in high fructose transport rates(Reference Godoy, Ulloa and Rodríguez36), which are models of early and late stage cancer, respectively. GLUT5 knockdown studies have shown inhibition of the growth of both MCF-7 and MDA-MB-231, highlighting the transporter’s importance to the cancer cell lines(Reference Levi, Cheng and Gheysens37).
Large-scale screening of the GLUT family, using western blotting, in both cancerous and normal human tissues showed GLUT5 is significantly overexpressed in 27 % of cancerous tissues tested, including tumours of the brain, breast, colon, liver, lung, testes and uterus, with GLUT5 showing moderate to high amounts of staining in all but one cancer tissues tested(Reference Godoy, Ulloa and Rodríguez36,Reference Levi, Cheng and Gheysens37) .
Most of the tumours cells presenting an overexpression of GLUT5 additionally possess an elevated rate of fructose uptake and the extensive overexpression of GLUT2 and GLUT5, and indicate that fructose may be preferred as the energy source for the growth and propagation of some tumour cells(Reference Peralta, Cottone and Doveri38). More research is needed to determine whether this characteristic can be exploited for anti-cancer activity. It could be possible that locally inducing FM in cancer cells, may result in apoptosis of such cells. The impact of the GLUT5’s presence in some cancerous tissue needs to be fully delineated, to confirm whether GLUT5 can be exploited as a target for novel chemotherapy methods.
Small intestinal bacterial overgrowth
It has been proposed that some symptoms of IBS may not be as a result of FM, but rather as a result of fructose fermentation by bacteria in the SI and the resulting bacterial respiration(Reference Lin39). These symptoms include bloating and elevated hydrogen breath test (HBT) levels. Hydrogen gas excretion is significantly increased in 84 % of IBS patients after lactulose ingestion (BTLact) and an increase of 75 % after the use of local antibiotics. There is also a strong correlation between the nature of gas excreted and bowel movement patterns(Reference Ghoshal, Ghoshal and Das40,Reference Rumessen41) .
The BTLact test is a tool used to assess the prevalence of small intestinal bacterial overgrowth (SIBO) within a patient. When compared with analysing jejune aspiration, BTLact is simpler and more tolerable for patients, providing information quicker to the clinician, since a jejune aspiration testing requires an endoscopic retrieval of jejunal flora and subsequent microbiological culture(Reference Rumessen41).
BTLact uses the ingestion of lactulose, which cannot be absorbed through the SI, resulting in the lactulose being metabolised by bacterial flora, producing gases, including methane and hydrogen.
The sensitivity of the BTLact test in SIBO diagnosis is reported to be 86 %, with 44 % specificity, indicating that some symptoms of IBS may not be due to SIBO exclusively(Reference Attaluri, Paulson and Jackson44–Reference Stone-Dorshow and Levitt46). This has directed some clinicians to consider that BTLact is not the best method for diagnosing SIBO and they prefer more indirect methods, such as serum vitamin B12 and folate levels instead. It must also be noted that BTLact uses lactulose; this is significant because the flora of the SI is varied and complex (and may respond differently to other sugars, including fructose and fructans). Currently, there is no standardised test for the absorption of fructans and research in the area is limited(Reference Kleessen, Hartmann and Blaut47,Reference Rumessen and Gudmand-Høyer48) .
Fructans are naturally derived carbohydrate storage polymers found in plants. They are fructose polymers with terminal glucose molecules. The degree of depolymerisation (DP), defined as the number of monomers that make up an oligomer or polymer, thereby indicating the length of the oligomer or polymer, can be as low as one and can reach several hundreds(Reference Cummings and Macfarlane49). A couple of examples of common fructans are sucrose (DP = one) and insulin (DP = thirty-five). Fructans are typically found in cereals, onions, asparagus, scorzonera and Jerusalem artichokes, as well as some non-edible plants, for example, chicory(Reference Ghoshal, Ghoshal and Das40). Fructans are commonly used as artificial sweeteners(Reference Ghoshal, Ghoshal and Das40). It has been shown that dietary fructans can alter the intestinal mucosal environment, releasing mucins and mucosa-associated bifidobacterial in gnotobiotic rats(Reference Rumessen41). Fermentation patterns of fructans tend to remain fairly consistent regardless of DP; however, there is a significant positive correlation between DP and transit time(Reference Jung, Seo and Cho51). However, the same study showed that undesired abdominal symptoms only occurred in single doses of fructans greater than 20 g where given to the participants of a trial(Reference Wang and Gibson52).
These studies indicate that some symptoms of IBS perhaps are better attributed to SIBO, rather than FM alone. However, it must be noted that the two conditions are not mutually exclusive; rather, it is important to note that a patient with IBS may have SIBO and FM, or only one of the two conditions. Since it is apparent that the symptoms of IBS cannot be exclusively attributed to one of these two causes, the nature of their interactions may cause difficulty when attempting to determine the appropriate therapeutic actions to pursue. This is because certain treatments that are affective for symptoms resulting in SIBO may be ineffective for symptoms resulting from FM and vice versa(Reference Rivière, Selak and Lantin53).
It has also been proposed that the uses of prebiotics have a beneficial effect on intestinal health(Reference Markowiak and Śliżewska54). However, this may result in SIBO and the production of unwanted side-effects in patients affected by IBS. Prebiotics can selectively stimulate the growth and activity of gut bifidobacteria and lactobacilli(Reference Cherbut55,Reference Barrett and Glbson56) . Prebiotics are non-digestible, short-chain carbohydrates with a DP between two and approximately sixty. However, this evidence related to non-digestible prebiotics is mostly circumstantial. Oligofructose that has been incubated in vivo with either human saliva or rat pancreatic homogenate has been shown to be ‘hardly digested’, rather than completely non-digestible(Reference Jung, Seo and Cho51). This means that oligofructose may contribute to the accumulation of fructose in the GI tract. Prebiotics are relatively simple, water soluble, molecules; therefore, fermentation by GI flora is highly likely.
Human studies have shown a consistent failure to recover inulin and oligofructose in faecal matter, indicating their complete metabolisation by intestinal flora(Reference Poeker, Geirnaert and Berchtold57). In vitro studies have demonstrated the ability of prebiotics to support intestinal bacteria growth and result in the production of a variety of fermentation-derived end products(Reference Parnell and Reimer58). Two early studies showed the in vitro utilisation of oligofructose, derived from sucrose, by bifidobacteria. However, oligofructose lacks selectivity towards bifidobacterial species and a variety of enteric bacteria were able to grow on a wide range of prebiotics, in particular species of Bacteroides(Reference Ventura, Davis and Goran14,Reference Trelis, Taroncher-Ferrer and Gozalbo59) . Wang and Gibson later showed that both inulin and oligofructose could selectively promote the growth of bifidobacteria(Reference Walker, Dumke and Goran60). It has been shown that 42 % of patients with FM also have a high prevalence of intestinal parasites, with Giardia intestinalis being present in twenty-six and a half percentage of cases of FM, highlighting a significant association between FM and the presence of intestinal parasites(Reference Jones, Burt and Dowling61).
The main products of the metabolism of prebiotics are SCFA, hydrogen gas, carbon dioxide and bacterial cell mass(Reference Szilagyi, Malolepszy and Yesovitch62). However, there has been little research into the relationship of the type of carbohydrate and its fermentability. This research could provide profound insight into the effect prebiotics have upon the symptoms of IBS resulting from SIBO.
Trends in the diagnosis of fructose malabsorption
A number of different factors have been linked to an increased likelihood of developing FM. It is important to consider these factors, as FM is often misdiagnosed, due to the similarity in symptoms with other forms of carbohydrate malabsorption.
A key difference in FM cases when compared with lactose intolerance and other cases of carbohydrate malabsorption is the relationship between FM and age. A study of 1093 patients showed that the probability of testing positive for FM decreased by a factor of 0·82 per year of age, for patients under the age of 15 years; this trend is not found in cases of lactose intolerance(Reference Macha, VanWagoner and Tolia63). This may be linked with the late expression of fructose-selective GLUT5 enzymes(Reference Nobigrot, Chasalow and Lifshitz34).
There may also be a relationship between sex and FM. A study in 2007 showed that women complained more frequently about symptoms associated with FM (P = 0·04) as well as presenting a greater number of cases than men (P = 0·0527)(Reference Halmos, Power and Shepherd64). However, the present study needs expansion to a larger sample size (greater than n 29) and more research is needed to determine whether there is a causal link between sex and FM.
Unfortunately, there has been no research into comparing the pervasiveness of FM between different ethnicities on a national level. Therefore, it is currently difficult to determine any possible correlation between ethnicity and the likelihood of FM.
Diet is a key factor in the prevalence of FM. A study of 3476 patients with FM showed that 52 % of patients consumed a fructose-rich diet, including high consumption of soft drinks, fruit juices, candy and fructose-rich fruits, such as apples, pears, peaches and oranges. The effects of diet on FM will be discussed in more detail later on in this review(Reference Nanayakkara, Skidmore and O’Brien65). According to the same study, FM is most commonly accompanied by gastroesophageal reflux disease, followed by lactose intolerance(Reference Nanayakkara, Skidmore and O’Brien65). It should be noted again that the symptoms of lactose intolerance are similar to those of FM, which may lead to potential misdiagnosis of FM.
Effect of diet relating to fructose malabsorption
It is important to consider the trends in fructose consumption and the changes in diet related to sugar that may contribute towards FM and diseases caused, at least in part, by FM.
Logically, it could be expected that high fruit consumption is a primary cause of FM because of its sugar content. However, based upon the hypothesis that the critical factor is the amount of excess free fructose (EFF), many fruits may not fit this criterion. According to a study by Barrett and Gibson on sugar content in fruits, only apples, pears, mangoes and Asian pears have more than a gram of EFF per average serving, potentially leading to FM after consumption. All other fruits contain fructose, with either glucose in excess or a very slight excess in fructose, making them an unlikely factor for FM(Reference Vos, Kimmons and Gillespie8). Another key component here is the consumption of soft beverages, which often use HFCS as an artificial sweetener, which is high in fructose. Studies by Ventura and Walker have shown that the vast majority of soft drinks in North America contain EFF(Reference Ventura, Davis and Goran14,Reference Szilagyi, Malolepszy and Yesovitch62) . The study by Ventura assessed that eighteen soft drinks that used HFCS had an average of 1·5 g of EFF per 100 ml; this results in an average of 37 % more fructose than glucose(Reference Ventura, Davis and Goran14). Two notable exceptions are Mexican Coca-Cola and Pepsi throwback, which both use cane sugar, rather than HFCS. Walker’s study showed cane sugar has a lower fructose quantity than HFCS; the two beverages using cane sugar recorded an average of 0·25 g of EFF per 100 ml, resulting in 5·6 % more fructose than glucose for the two drinks(Reference Ventura, Davis and Goran14,Reference Szilagyi, Malolepszy and Yesovitch62) . Although there are other harmful health effects of soft drink consumption that may be reduced by the introduction of HFCS, its introduction in recent times has likely caused an increased risk of FM, particularly in communities where soft drink consumption is high.
It is estimated that, on average, 10 % of an US American’s daily energy intake is derived from fructose(Reference Vos, Kimmons and Gillespie8). A study by Vos et al. assessed that fructose consumption per capita in Americans (excluding that which occurs naturally in fruits and vegetables) increased from less than 0·5 g/d in 1970 to more than 40 g/d in 1997(Reference Vos, Kimmons and Gillespie8). This may be due to the increase in consumption of HFCS, with consumption increasing by 26 %, from 64 g/d in 1970 to 81 g/d in 1997. HFCS contains fructose (55 % by weight; 56·7 % of total energy content) and glucose (42 % by weight; 43·3 % of total energy content) in their monosaccharide forms, thus highlighting a potential increase in fructose consumption from HFCS(Reference Bray, Nielsen and Popkin15).
Another study by Vos et al., in 2008, provided important statistics on fructose consumption. Fruit and fruit juices provide the largest amount of fructose for children aged 2–5 years and adults over the age of 50, whereas for people aged between 12 and 30 years, sugar-sweetened drinks account for nearly half of all fructose consumption, perhaps due to HFCS(Reference Vos, Kimmons and Gillespie8). The study found that processed food, regardless of food category, is responsible for a significant contribution towards fructose consumption. Seventy-four percentage of fructose consumed originated from foods excluding whole foods and vegetables(Reference Vos, Kimmons and Gillespie8). It is important to highlight that these data are from the USA; surveys for other countries are limited and conclusions drawn from other territories may differ significantly, especially due to the local availability of HFCS.
A study of national HFCS production and diabetes prevalence was conducted by Goran et al in 2013(Reference Martínez-Azcona, Moreno-Álvarez and Seoane-Pillado66). It was found that there is a 20 % increase in the prevalence of type 2 diabetes in HFCS-producing countries, with a P-value = 0·013. Although the values in the paper are skewed by the significantly higher than average prevalence of type 2 diabetes in the USA (the present study examined type 2 diabetes), it could be argued that due to the estimates of HFCS production, that FM prevalence may follow a similar trend.
Diagnosis of fructose malabsorption
Diagnosing FM is made difficult due to the fact that symptoms caused by FM can be caused by a number of other conditions, including other sugar intolerances. The current test for FM is the HBT(Reference Peterson and Fred67). Excess sugar in the GI tract is fermented by intestinal bacteria, resulting in the production of hydrogen which can be measured during exhalation. The chemical reaction for this is shown in Fig. 3. The patient is given pure fructose to consume and after a period of fasting (usually between 8 and 12 h), the patient’s breath is collected and analysed for the concentration of gases in the breath sample. Elevated levels of hydrogen may indicate the patient suffers from FM. Typically, a positive test is recorded in the concentration of hydrogen and methane that are greater than 20 ppm above baseline values, recorded prior to the test, after 60 min(Reference Lee, Barrie and Levinson68).

Fig. 3. Chemical reaction for the fermentation of fructose by bacteria in the GI tract(Reference Helwig, Koch and Koppka69).
However, there are a number of issues with the HBT. Lee’s group estimated that between 8 and 12 % of all patients tested for lactose malabsorption will result in false negatives from the HBT, if tested for hydrogen alone, since many patients will produce methane, rather than hydrogen(Reference Chang70). A key problem with the HBT is the lack of specificity of the test; elevated breath hydrogen can be as a result of a multitude of reasons, including FM, but also potentially SIBO and malabsorption of other carbohydrates, which will need alternative treatments. Furthermore, subjective assessment of diet is needed to fully assess the GI complaints and misdiagnosis is possible. In addition, a number of HBT result in false positives, leading to inaccurate data in the epidemiology of FM. In order to accurately diagnose FM, a fructose-selective analysis of the GI tract is required. Helwig et al. described the HBT as possessing no predictive value for the outcome of fructose-free diets, indicating doubt in the HBT’s ability as a predictive test for FM. However, Helwig did also describe a positive correlation between the concentration of hydrogen measured in the HBT and the prevalence of FM symptoms, indicating some validity to the use of HBT(Reference Schmulson and Drossman71). FM cannot be directly diagnosed via the Rome criteria, used for GI complaints. The Rome criteria, used to diagnosed IBS, are in its forth iteration and require a patient to have recurrent abdominal pain at least once a week for 3 months, accompanied by at least two of the following for a positive diagnosis of IBS: a change in stool frequency, a change in stool form or discomfort during defecation(Reference Tuck, Ross and Gibson72,Reference Komericki, Akkilic-Materna and Komericki-Strimitzer73) .
Treatment of fructose malabsorption
The primary method of treating GI complaints, such as IBS, is through dietary change. A common diet change is the low-fermentable oligo-, di-, monosaccharide and polyol (FODMAP) diet. This is a restrictive diet aimed at limiting the group of carbohydrates that are poorly absorbed in the SI and subsequently fermented by intestinal bacteria. Table 1 shows a list of foods that may be prescribed as part of a low FODMAP diet. It should be noted that Tuck et al. showed that the simple addition of glucose to fructose had no effect on HBT results of patients with symptoms of FM, when compared with fructose consumption alone(Reference Manichanh, Eck and Varela74).
Table 1. Table of high- and low-fermentable oligo-, di-, mono-saccharides and polyol (FODMAP) foods

A low FODMAP diet has been shown to have a beneficial effect in randomised, single-blind, crossover studies. In the present study spearheaded by Halmos et al., patients (n 30 patients with IBS) consumed either a low FOFMAP diet or the average diet of an Australian person(Reference Martínez-Azcona, Moreno-Álvarez and Seoane-Pillado66). Their results showed that 70 % of subjects experienced an improvement in symptoms on the low FODMAP diet. The present study is hindered by a low number of subjects and the subjective nature of the assessment of symptoms improvements.
Although dietary intervention is well-known and relatively straightforward to implement, it is not without complications. The FODMAP diet is somewhat complex and with an absence of FODMAP information on food packaging; support from specialist dietitians is required for sufficient adherence, since clinical studies show that not every patient sees improvements to symptoms on a low FODMAP diet, patient compliance is an issue(Reference Chumpitazi, Cope and Hollister75).
In addition, there is little knowledge concerning the long-term health effects of being on a low FODMAP diet. One such issue is the alteration to the ecology of GI bacteria due to the change in diet. Studies by both the Sloan and Chumpitazi groups have found a decrease in bacteria in the SI following adherence to a low FODMAP diet, which has the potential to negatively alter the effect the bacteria have as part of the immune system(Reference Halmos and Gibson76,Reference Satherley, Howard and Higgs77) . More research is needed to determine the exact changes to the bacterial ecology as a result of a low FODMAP diet, because the microbiology of the intestines plays a key role in health and the immune system.
Another concern is the increased prevalence of eating disorders caused by the need for strict monitoring of food intake. It has been proposed by Halmos and Gibson that patients adhering to strict diet controls are at an increased risk of the eating disorder orthorexia nervosa(Reference Blow, Collyer and Goldberg78). This disorder is linked to symptoms including an obsessive focus on food choice, planning, purchase, preparation and consumption; food as a source of health rather than pleasure; the belief that particular foods can prevent or cure disease and alter well-being. There is a lack of research into such eating disorders and more study is needed into the management of such conditions, given the prevalence of such disorders could be between 5 and 44 % for patients on strict diets(Reference Lindfors, Unge and Arvidsson79). There are also concerns regarding potential nutritional deficiencies associated with being on a low FODMAP diet; more research is needed to ascertain the exact level of deficiency and to what extent such deficiencies cause adverse health effects(Reference Komericki, Akkilic-Materna and Strimitzer80).
An alternative to strict dietary controls is found in gut-directed hypnotherapy. A recent randomised clinical trial showed that gut-directed hypnotherapy led to similar efficacy to treating the symptoms of IBS as a low FODMAP diet, without the increased prevalence of eating disorders(Reference Silano and Barat Baviera81). However, there is currently limited understanding of the brain–gut axis and the mechanism of how gut-based hypnotherapy improves symptoms and lack of availability of hypnotherapists with suitable training means that such treatment may be inaccessible to most patients(Reference Komericki, Akkilic-Materna and Strimitzer80).
Xylose isomerase has been proposed as a potential treatment of FM in recent years(Reference Singh, Singh and Pandey82). Xylose isomerase is used to convert fructose to glucose in industrial settings and has been shown to produce no allergic response in humans(Reference Singh, Singh and Pandey82) . The ability of xylose isomerase to convert between glucose and fructose, shown in Fig. 4, has led to the proposal of its use as a treatment for FM. A double-blind, placebo-controlled study showed a significant decrease in breath hydrogen upon oral administration of xylose isomerase, after ingestion of fructose, as well as significant improvement regarding nausea and abdominal pain, two symptoms related to FM and IBS(Reference Singh, Singh and Pandey82). More research is needed to assess the long-term health effects and to determine which patients are best suited to treatment with xylose isomerase.
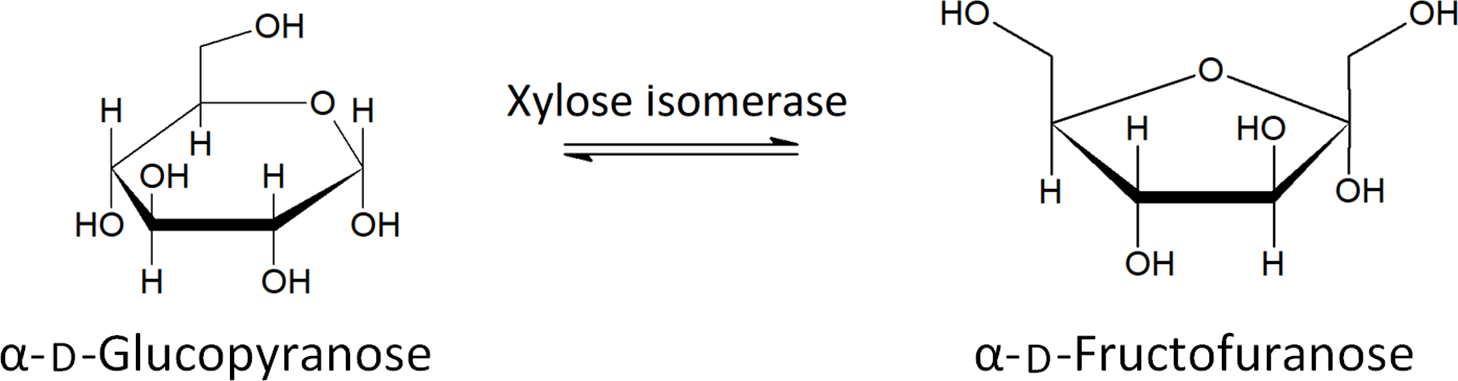
Fig. 4. The conversion of glucose to fructose by xylose isomerase(Reference Silano and Barat Baviera81) .
Conclusions and future outlook
With the increased global consumption of fructose in recent decades comes the increasing prevalence of IBS and other GI complaints caused by FM. However, with limited understanding into the mechanism of fructose absorption and the currently inability to accurately diagnose FM, treatment of such disorders is somewhat difficult.
Current treatment regimens for FM are fraught with limited understanding of the long-term health effects of following such routines. Alternative approaches to treatment, such as the use of medicinal intervention, need to be investigated. It is also important that the relationship between SIBO and FM is investigated thoroughly, so that the symptoms of IBS can be accurately addressed as the result of accurate diagnosis of the causes of IBS. However, this is hindered significantly by the inter-relationship between these two factors and the similarities of the symptoms that SIBO and FM cause. Dietary intervention can prevent the causes of IBS, by removing the nutrients in questions from the GI tract; however, precise determination of the food groups responsible for symptoms can be difficult and as is the case with any diet, patient compliance is often the main factor in the success of these diets.
Our understanding of FM and its role to play in various diseases are still in its infancy. However, it is apparent that FM may have significant role to play in a variety of diseases, not just IBS.
Acknowledgements
The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilised in the production of this manuscript.
Conceptualisation: S. P. W., A. L. G. and A. E.; formal analysis: S. P. W. and M. B.; investigation: S. P. W. and M. B.; methodology: S. P. W. and M. B.; project administration: S. P. W., A. L. G. and A. E.; supervision: S. P. W.; writing – original draft: M. B. and S. P. W.; writing – review and editing: S. P.W., A. L. G. and A. E.
We have no conflicts of interest in the publication of this review.