To the Editor—The consistent delivery of products and services according to established standard is called quality. The concept of quality relates to the degree or grade of excellence of a product or service. Moreover, quality requires effort and participation from everyone in the facility, and to achieve optimal service, time is required to develop, implement, and monitor quality-control processes. The environmental quality monitoring system in a hospital’s central sterile supply department (CSSD) can reduce unnecessary sterilization cost and increase the shelf life of medical devices. The basic functions of a CSSD are cleaning, disinfection, and sterilization of a large number of reusable medical devices in a healthcare institution. The entire decontamination processes (from cleaning to sterilization) can only be safe if the surrounding environment is clean and controlled. 1,2 For proper sterility assurances there are four different environmental quality monitoring systems are discussed: water quality monitoring, air quality monitoring, carbolization and sterility testing.
In the CSSD, purified water is required for cleaning, disinfection, and sterilization purposes. The water used in the CSSD should have low surface tension and should be free from minerals (ie, low total dissolved solids, hardness, and conductivity) and microbes. Mineral-free water is needed to avoid salt deposition on surgical instruments and electrical elements such as heaters (inside the boiler), so that this equipment can run well for a long time. Furthermore, water should be free from microorganisms to avoid the formation of pyrogens after disinfection and sterilization. Therefore, identifying and testing the source of water and the purification system are very important for achieving a high-quality water supply. For water-quality monitoring, periodic chemical tests (ie, chlorine level 0.2–0.5 ppm and pH, ~8) and microbiological tests (eg, for Pseudomonas and coliform bacteria) are required. Reference Bhalchandra, Chandy and Ramanan3
The CSSD is mainly divided into 3 zones: a dirty zone, a packaging zone, and a sterile zone. Purified and moisture-free air is essential for preparing sterile materials and preserving them for a long time. According to the international standard, the temperature of a CSSD (including sterile storage) should be 20–22 °C and the relative humidity should be 30%–60%. Air should always move from the sterile zone to the dirty zone to avoid cross contamination. The clean air must be particle free, and the air-filter pore size should range between 0.05 µm and a maximum of ≤10 µm. Furthermore, tests for differential air pressure (minimum, 2.5 Pascals), air velocity (minimum, 2,500 cfm), air exchange rate (≥10 per hour), and air microbiology (measuring bacterial and fungal colony) are required to minimize air impurities in the sterile zone. 4
The process of cleaning articles with antiseptic solution is called carbolization. This process includes daily cleaning of floor, walls and ceiling, tables and trolleys, and other equipment in the CSSD. Stainless steel equipment is cleaned using a neutral enzymatic solution because chloride solution may induce rust, but other areas and equipment are cleaned using a hypochloride solution (ie, 150 ppm chlorine concentration). Cleaning should be carried out twice daily to ensure a clean and dust-free environment. A daily audit should be required to assess cleaning performance. 5
Sterility testing is one of the outputs of environmental quality monitoring that examines how clean the CSSD is. It is widely considered that “worldwide sterility maintenance is event related rather than time related.” But in some exceptions, such as poor design, incorrect process flow, faulty equipment or technique, or poor environmental conditions, sterility can be maintained on a time-related basis. In time-related sterility assurance, 2 times are maintained related to product shelf life: manufacturing date and expiry date. After sterilization, the sample is kept in sterile storage until the date of expiry, then the sample is sent to the microbiology department for culture. To confirm shelf life (based on work practices, sterile barrier system, storage, and environmental conditions), sterility testing is the only method that can ensure that a product has retained its sterility in a certain environmental condition. 6,7
The total costs of environmental quality monitoring systems are high. Here, we have adopted a cost calculation method from a cancer center in eastern India where the total costs of environmental quality monitoring systems are based on standard operational procedures (SOPs) and that have been standardized by the hospital infection control committee. The frequency of monitoring may vary from 1 day to 1 month, but the standards are quite similar to international standards. A comprehensive cost analysis is provided as a reference in Table 1. If test results are unacceptable according to the standard, then continuous assessment is required until satisfactory results are achieved, and this process increases costs as well.
Table 1. Cost of Consumables Used for Central Sterile Supply Department (CSSD) Environmental Quality Monitoring Purposes
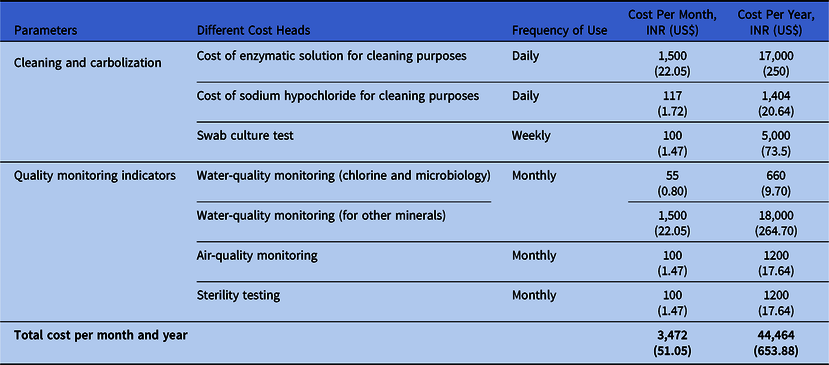
Note. INR, Indian rupees.
In conclusion, the objective of a hospital CSSD is to provide the right items to the user at the right time, in the right place, and under the right conditions. The CSSD plays an important role in infection control practices where a well-designed and well-equipped department combine with an efficient staff to achieve high-quality work. However, providing a sterile item in the right condition to the user’s area requires not only good work practices (skills or technique) but also good environmental conditions. The challenge is to ensure that environmental monitoring systems are used routinely (according to the standard) for proper sterility assurance and that human intervention and faulty documentation do not hinder the process. Reference Basu, Bhattacharya, Mahajan, Ramanan and Chandy8
Acknowledgment
None.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2020.41



