Introduction
The Isles of Scilly are an oceanic archipelago located over 20 nautical miles from the South West coast of the British Isles in the northeast Atlantic Ocean (Figure 1). The archipelago is situated on the European continental shelf, approximately 49°56′N and 6°16′E and is surrounded by shallow waters (<150 m in depth) that are exposed to North Atlantic pelagic currents including the Gulf Stream (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). The archipelago consists of over 300 islands and rocky outcrops, five of which are inhabited, with a population of ~2100 people (Office for National Statistics, 2021).
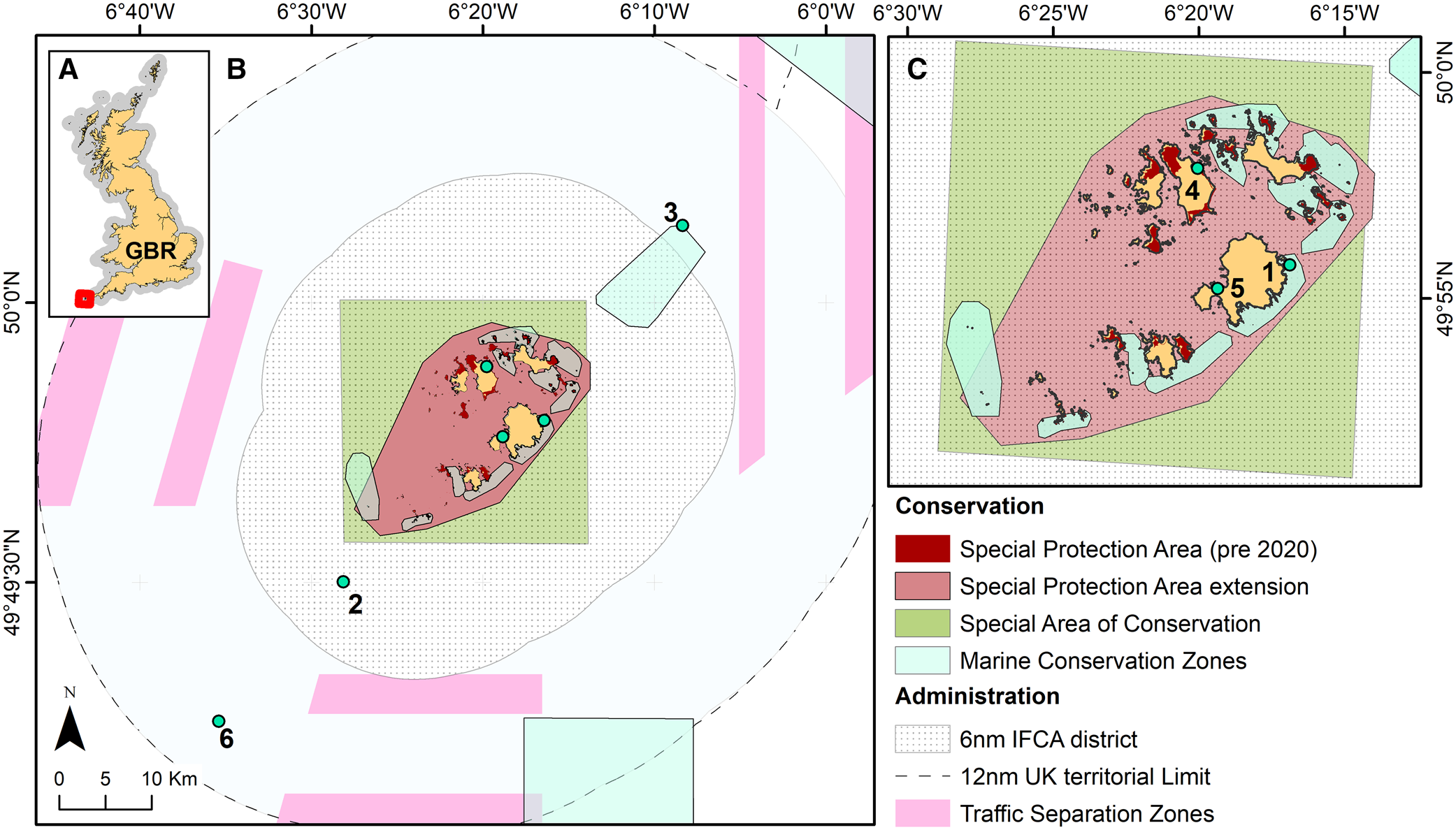
Figure 1. Isles of Scilly study region and key marine management designations and administration boundaries highlighted. (A) Isles of Scilly location relative to the Great Britian, (B) the wider Isles of Scilly region and (C) the inshore zone of the Isles of Scilly. Green points indicate referenced places of interest named in the results 1. Dry Ledge, 2. The Pol Bank, 3. Torrey Canyon and Seven Stones Reef, 4. Old Grimsby Harbour, 5. St Mary's Harbour and 6. Approximate location of the large mud patch considered essential blue skate (Dipturus batis) habitat.
Situated in a biogeographic transition zone between warm and cold temperate waters, the archipelago is considered to be ecologically distinct; supporting warm water species (i.e., sea fans (Eunicella verrucosa) and cup corals (Leptopsammia pruvoti)) at the northerly extent of their distribution and rarely found in the wider British Isles, and cold-water species uncommon on mainland Western Europe (i.e., stalked jellyfish (Calvadosia campanulate) and skate (Dipturus batis)) (Fowler and Laffoley, Reference Fowler and Laffoley1993; Pinnion et al., Reference Pinnion, Mackie, Somerfield and Warwick2007; Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008). This diversity makes the Isles of Scilly an important research and monitoring site for specific species (Faubel and Warwick, Reference Faubel and Warwick2005), habitats (Bull and Kenyon, Reference Bull and Kenyon2021) and at an ecosystem level (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003), particularly in the context of warming waters due to human induced climate change.
In addition to their regionally unique biogeography, the Isles of Scilly are believed to contain ecosystems that are some of the most pristine both nationally and in the wider, highly degraded northeast Atlantic (English Nature, 2000). The small resident population and geographic isolation ensure relatively clean, clear waters that support healthy ecosystems, such as some of the largest seagrass beds (Zostera marina) in the United Kingdom (Jones and Unsworth, Reference Jones and Unsworth2015). The archipelago also forms one of just seven sites in England to have been designated as an ‘assemblage of breeding seabirds’, as they supported the requisite >20,000 breeding seabirds at designation (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008). As a result, the Isles of Scilly have received multiple conservation-based designations over the years and have been highlighted as regionally important for conservation management. For example, they were selected as one of only six All Taxon Biodiversity Inventory reference sites as part of the European Marine Biodiversity Research Sites project aiming to implement long-term and large-scale marine research programmes (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). These six sites were highlighted for their conditions being as close to pristine as one can hope for in European waters. The archipelago's unique flora and fauna also resulted in the designation of the UK's first marine National Parks in 1988 (although this designation was never fully adopted) and it has been a Voluntary Marine Nature Reserve (VMNR) since 1989. All major UK and European marine conservation spatial conservation designations, comprising Special Area of Conservation (SAC) (designated under the ECU Habitats Directive – Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora), Special Protection Area (SPA) (designated under the ECU Birds Directive – Council Directive 79/409/EEC of 2 April 1979 on the conservation of wild birds), and Marine Conservation Zones (MCZs) (Marine Management Organisation, 2009) are found within the inshore zone, designed to protect the range of the marine biodiversity present in the archipelago (Figure 1). These designations, coupled with local byelaws (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020), also result in reduced fishing pressure compared to surrounding regions (Witt and Godley, Reference Witt and Godley2007), with low impact static potting gear and some netting primarily for crustaceans (Isles of Scilly IFCA, 2019).
Despite the Isles of Scilly's ecological value, current state of marine biodiversity knowledge is not well synthesised, particularly at an ecosystem scale. Historical inventories of marine flora and fauna of the Isles of Scilly have been compiled, however, these have either been primarily descriptive (i.e., a series of taxonomic records in natural history journals (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003)) are outdated (i.e., >15 years old (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008), and/or lacking a marine focus. Wider research has similarly been exploratory in nature, focused on taxonomy or single species, rather than directed towards understanding progress towards local conservation and management goals. There is also limited research on the contemporary effects of climate change, fisheries and other extractive activities or vessel noise pollution, despite there being high fluctuations of seasonal recreational and passenger vessel activity (Smyth et al., Reference Smyth, Harvey-Scholes and Wills2021). The current lack of synthesis on existing research may therefore be impeding effective marine management within this biologically unique region, particularly decisions over monitoring resource allocation. To identify potential knowledge gaps we conducted a structured literature review to synthesise the current state of knowledge of the marine environment, human activities and management measures pertaining to the Isles of Scilly. The primary purpose of our review is to help identify future research priorities with maximum impact and applied value for local managers, communities, and biodiversity. Given the current scarcity of data on many marine features, this is critical to support more effective, holistic management of local marine resources and guide targeted data collection that directly addresses relevant questions that are well bounded in time and space (Addison, Reference Addison2011; Wilding et al., Reference Wilding, Gill, Boon, Sheehan, Dauvin, Pezy, O'Beirn, Janas, Rostin and De Mesel2017; Rees et al., Reference Rees, Sheehan, Stewart, Clark, Appleby, Attrill, Jones, Johnson, Bradshaw, Pittman, Oates and Solandt2020).
Material and Methods
Structured literature review
We conducted a structured review of peer-reviewed and grey literature relating to the biotic, abiotic marine environment (mean high water and below) and associated human activities in the Isles of Scilly. All results relevant to the 12 nm inshore zone (delineating the UK's territorial sea) were included, with research beyond this boundary retained on a case-by-case basis. This review followed the PRISMA standardised systematic reviews and meta-analyses statement (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009) which provides a structured approach to conducting a comprehensive and rigorous literature review (see Figure S1 in supporting information).
We searched ‘Web of Science’ (WOS) in January 2023 for all peer reviewed papers including the following combinations of search terms: ‘Isles of Scilly’ AND ‘conservation’, ‘Isles of Scilly’ AND ‘ecology’, ‘Isles of Scilly’ AND ‘fisheries’ and ‘Isles of Scilly’ AND ‘marine’. This search resulted in 148 publications that were subsequently filtered to avoid duplications across search terms. A total of 86 unique studies were identified with each source read in full to determine whether they had relevance to the marine environment or human activities of the Isles of Scilly. This resulted in 59 peer-reviewed publications being retained for further analysis. When reading the resulting publications, any further sources cited that were not yielded in the initial search results (snowball sources) were sourced, read, and incorporated into this study, resulting in an additional eight peer-reviewed publications. Any snowball sources without active links to the full articles were excluded from the review (Supplementary Table 1).
Given that the Isles of Scilly have a range of designations managed by a variety of governmental regulatory bodies, information is often held in statutory reports. Therefore, to supplement the peer-reviewed literature we conducted a complementary search of the grey literature in January 2023. This search involved targeted manual searching for reports and complete datasets on government and non-governmental organisation websites and larger UK Government repositories. These organisations were identified in consultation with local experts that were either data custodians or had significant local knowledge relating to the marine environment of the Isles of Scilly (see Supplementary Table 2 for a list of sources searched). The grey literature search yielded an additional 75 sources that were relevant to the Isles of Scilly marine environment. Reports or datasets that were not available online or were not compiled into a single report or summarised dataset were excluded from the review. For example, the Marine Biological Association (MBA) hosted DASSH data portal (https://www.dassh.ac.uk/) contains >70,000 data records for the approximate Isles of Scilly inshore (<6 nm) zone. It was therefore beyond the scope of this review to process these large data quantities, unless a pre-existing summary of data were available and referenced as part of the DASSH repository metadata (i.e., Seasearch Isles of Scilly Survey reports). Short summary reports (i.e., designation orders for MPAs) were only considered once, with the most recent iteration retained. As with the peer review search, a snowball method was used for any further relevant citations. After screening all potential snowball sources, a further five grey literature publications were incorporated into the results.
Literature review and categorisation
The finalised list of grey and peer reviewed sources (including snowballing) yielded 150 sources for analysis, with information subsequently extracted on the characteristics of each report/study. Characteristics included: authors, year of the publication, research topic, key findings or results and whether the research was directly relating to the Isles of Scilly or part of a broader study with only partial reference to the archipelago and its marine environment (see Supplementary Table 3 for finalised list of sources retained for analysis). Based on the research topic, all sources were categorised into broad and focused research themes to explore temporal trends in the literature. At a broad level, sources were categorized based on their primary focus being: (1) marine habitats; (2) species research (either single species or multi-species); (3) human activities and impacts; and (4) management and monitoring. Many studies, particularly relating to habitats and species that are a designated feature of local MPAs were applicable to both monitoring and habitats or species categories. Sources were therefore only classed as ‘management and monitoring’ if they explicitly stated the focus of the research was to directly inform an MPA condition assessment. Focused themes within each broad category were generated iteratively. For example, custom functional groupings were used for focused species themes (i.e., Benthic infauna and epifaunal communities') based on the number of related sources found. In addition to theme categorisation, all sources had key findings extracted and synthesised.
Results
For the 150 sources retained for analysis (66 peer reviewed, 84 grey literature), temporal trends revealed increasing annual publication rates and diversification in research theme for the Isles of Scilly. A mean 0.8 studies per year were published between 1956 (first source) and 2000, increasing to 5.0 per year from 2000 to 2022 (Figure 2A). At a broader-scale, peer reviewed studies relating to species historically dominated the literature prior to 2000, however, since 2000 research covers a broader range of topics relating to habitats, management and monitoring, and the characterisation of human activities and impacts (Figure 2A). Of the 150 sources, 129 were localised studies, with 21 studies considered to be at a broader spatial scale with significant, but only partial reference to the Isles of Scilly. All retained sources are available on a public Zenodo repository (https://zenodo.org/records/10461881, DOI: 10.5281/zenodo.10461881).
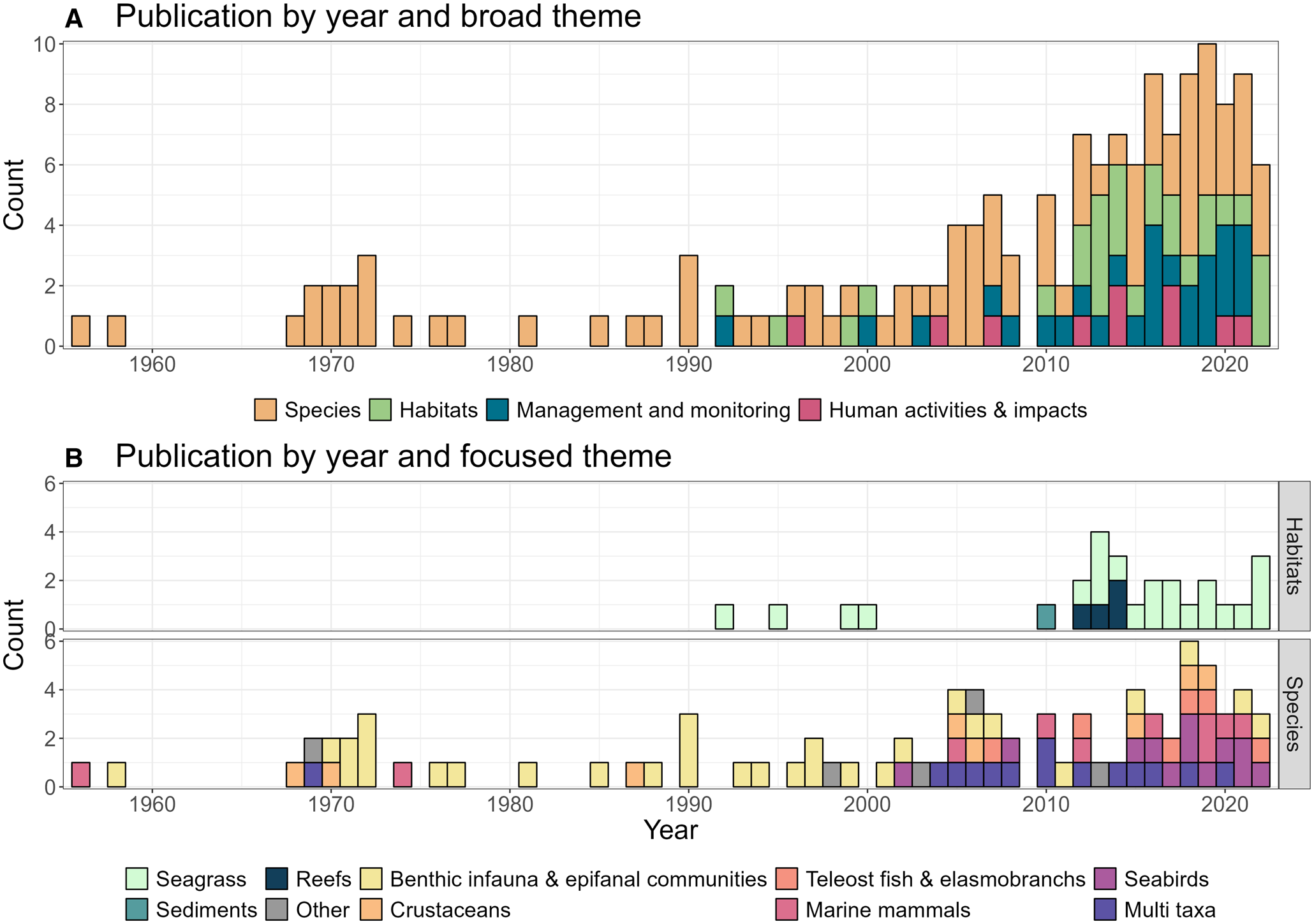
Figure 2. Trends in all source themes over time with: (A) the broad research theme of literature by year published; and (B) the focused theme groups into broadscale habitats and descriptive taxonomic groups.
Prior to 2000, species specific research was primarily focused on benthic infauna and epifaunal communities (Figure 2B). From 2000 onwards there was an increasing diversification towards other taxa, notably seabirds and marine mammals (Figure 2B). Research focusing on specific habitats and their associated communities has been almost exclusively driven by seagrass research (22 of 27 habitat-based sources) with limited publications relating to other sediment and rocky reef habitats (Figure 2A).
At a finer scale, the majority of literature dedicated to species research or management were published as peer reviewed literature (53 of 86), whilst habitat focused literature were primarily published as grey reports (21 of 27) with all habitat focused peer reviewed publications focused on seagrass. For species focused literature, 49 of 86 publications were relating to invertebrate research, driven by a series of articles in the Journal of Marine Natural History to develop a biodiversity inventory for primarily benthic infauna and epifaunal communities of the Isles of Scilly. Marine vertebrate research was found to have increased post 2005 and large-bodied vertebrate studies now the dominant subject for species literature, primarily due to seabird and marine mammal research (combined representing 22 of 35 vertebrate publications). In the following sections we present an overview of the key findings relating to the health of the biota of the Isles of Scilly.
Habitats
Seagrass beds and sediments
The seagrass beds in the Isles of Scilly which are comprised of Z. marina are one of the largest and most studied in English waters (Figure 3A). Despite their considerable present-day extent, anecdotal reports suggest local seagrass sites were heavily impacted (Harvey, Reference Harvey1969) by an outbreak of disease that spread across the North Atlantic in the 1930's (Turk and Seaward, Reference Turk and Seaward1997). Regular local surveys were initiated in 1984 by the Nature Conservancy Council, before commencement of a voluntary monitoring programme in 1992 in response to the presence of wasting disease (Fowler, Reference Fowler1992; Cook, Reference Cook2000, Reference Cook2011). Annual monitoring surveys led by a voluntary diving group (more recently in association with the NGO, Project Seagrass) of five sites (Broad Ledges Tresco, Higher Town Bay, Little Arthur, Old Grimsby Harbour and West Broad Ledges) have been in place since 1996 (Bull and Kenyon, Reference Bull and Kenyon2015, Reference Bull and Kenyon2016, Reference Bull and Kenyon2017, Reference Bull and Kenyon2018, Reference Bull and Kenyon2019, Reference Bull and Kenyon2020, Reference Bull and Kenyon2021) with the beds now a designated sub-feature of the SAC – referred to as ‘sandbanks which are slightly covered by sea water all the time’ (English Nature, 2000).

Figure 3. Image grabs of the four main habitat types found within the Isles of Scilly inshore zone taken from baited remote underwater video surveys in 2023 with: (A) Zostera marina seagrass beds, (B) infralittoral reefs dominated by marine macro algae, (C) subtidal sediments and (D) circalittoral reefs.
Results of the long-term voluntary dive surveys have shown changes in wasting disease, epiphyte cover and shoot density over time, but with no clear trends emerging (Bull and Kenyon, Reference Bull and Kenyon2021). Where declines were identified, they were not attributed to either restricted light availability or nutrient status and it is likely that another, unknown factor is the cause (Howard-Williams, Reference Howard-Williams2022a). Further Natural England commissioned surveys revealed a net seagrass extent loss of 49.3 ha in five years (184.1 ha in 2010 to 134.8 ha in 2016) although surveys relied on different data collection methods and therefore may have overestimated the extent of loss (Jackson et al., Reference Jackson, Higgs, Allsop, Cawthray, Evans and Langmead2011; Maher and Goodchild, Reference Maher and Goodchild2017). Long-term monitoring has, however, revealed the seagrass beds to be naturally resilient over time (Bull and Kenyon, Reference Bull and Kenyon2015). Patch occupancy (defined as the probability of occurrence in unit sample) has shown more concerning trends, with a total decline of 19.5% since monitoring commenced in 1994 (Bull and Kenyon, Reference Bull and Kenyon2021). Whilst the reason for site level variation in occupancy across the archipelago is unclear (Bull and Kenyon, Reference Bull and Kenyon2021), the greatest declines (65%) have been observed in the Old Grimsby Harbour (Figure 1) (Bull and Kenyon, Reference Bull and Kenyon2021), with low allelic diversity levels also being recorded at this site (Alotaibi et al., Reference Alotaibi, Kenyon, Cook, Börger and Bull2019).
Despite localised declines, additional studies found seagrass sampled in the Isles of Scilly to be in the healthiest ecological state compared with 10 other sites sampled across the southern British Isles (Jones and Unsworth, Reference Jones and Unsworth2015). Seagrass across the islands had the longest and widest leaves, highest shoot biomass and tallest seagrass in the study, which was attributed to high local light levels supporting productive seagrass habitat (Jones and Unsworth, Reference Jones and Unsworth2015). The seagrass beds are also believed to display the highest abundance of ‘fairy circles’ (large circular patches of seagrass growth with bare centres) anywhere in the world, although exact reasons for this remains unclear (Warwick, Reference Warwick2022). Long-term (1996 to 2012) flowering shoot data (linked to increased reproductive success and contributing to colonisation of bare sediment) revealed a positive effect of increasing sea surface temperature (SST) on eelgrass flower density at certain Isles of Scilly sites (Potouroglou et al., Reference Potouroglou, Kenyon, Gall, Cook and Bull2014). However, further long-term datasets (1997 to 2010) on the impacts of wasting disease prevalence revealed an increased negative effect of disease occurring at higher temperatures (Bull et al., Reference Bull, Kenyon and Cook2012). Given the archipelago is situated mid-latitude within the geographic range of Z. marina, the impacts on local seagrass beds under future increases in SST associated with human induced climate change remains unclear.
The Isles of Scilly also holds other significant sediment habitats, notably tide swept sandbanks and channels between the islands (Figure 3C) that are unique in the UK for their extent and diversity (English Nature, 2000). Sediment habitats on the Isles of Scilly support diverse communities of fauna, with over 50 species of mollusc recorded from a single sandflat (notable species included the tellin – Angulus tenuis, rayed artemis -Dosinia exoleta, the heart urchin – Echinocardium cordatum, and razor shell -Ensis siliqua), many of which are at the extreme north of their range (English Nature, 2000). To date research relating to these habitats has focused on the associated invertebrate communities, in part to underpin the monitoring of the SAC (Warwick and Light, Reference Warwick and Light2002; Warwick and Somerfield, Reference Warwick and Somerfield2015), and there exists relatively limited data on wider habitat condition or other sediment associated taxa.
As of 2020 a new four year ‘LIFE Recreation ReMEDIES’ project, led by Natural England, has engaged in active conservation management of the seagrass (‘sub-tidal sandbanks (eelgrass bed communities)’) feature of the SAC (Bull and Kenyon, Reference Bull and Kenyon2021). The project aims to increase public engagement and awareness of the seagrass resource found on the Isles of Scilly, with both locals and visitors alike, facilitating greater protection of the seagrass through mitigation of recreational boating impacts, and mapping the ecosystem services of the beds (Howard-Williams, Reference Howard-Williams2022b). Initial outputs of this project have also identified the seagrass beds in proximity to Little Arthur as having the greatest ecosystems services benefits including blue carbon and nitrogen sequestration potential (Howard-Williams, Reference Howard-Williams2022a).
Intertidal and infralittoral reefs
Infralittoral rocky reefs (Joint Nature Conservation Committee, 1996a) of the Isles of Scilly (Figure 3B) support a high diversity of marine algae species. The archipelago is considered a nationally important site for algal diversity (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008) with a comparable number of species recorded along the mainland coast of Cornwall (~400 species of red algae), as well as nationally rare species (i.e., Cryptonemia lomation) and listed species of principal importance as defined by Natural England under their legal obligations set out in Section 41 of the Natural Environment and Rural Communities Act 2006 (formerly Biodiversity Action Plan (BAP) species) (Cruoria cruoriiformis and Dermocorynus montagnei). This diversity of algae can be attributed to the archipelago's latitude straddling warm and cool temperature zones, to limited freshwater runoff and consistent west to east currents. This results in the archipelago experiencing stable levels of salinity and low turbidity (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). Clear water and high light levels have also enabled algae growth as deep as 30 m for species such as golden kelp Laminaria ochroleuca (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003).
The large granite boulder shores across the islands are also important for nationally rare ‘Intertidal under-boulder communities’, a Section 41 listed habitat of principal importance, which are a designated sub-feature of the intertidal reef feature of the Isles of Scilly SAC. Under-boulder habitat occurs from mid to the extreme lower shore and includes boulders >256 mm diameter. The accompanying fissures and crevices form a series of complex microhabitats that provide refuge to important communities of species such as sea mats, sponges, and coralline seaweed which encrust the under surfaces of boulders. Surveys of these habitats have also identified nationally rare species such as red speckled anemone Anthopleura ballii, the bulbous encrusting bryozoan Turbicellepora magnicostata (which has only been recorded on the Isles of Scilly in the UK) and gold star coral Balanophyllia regia (Selley et al., Reference Selley, Bailey and McNair2014). Monitoring of this priority habitat provided a benchmark for future surveys to measure against and found sites to be in a ‘favourable condition’ with a ‘biodiverse range of sponges, algae, bryzoans and anthozoans typical of the biotope’ present (Selley et al., Reference Selley, Bailey and McNair2014). No subsequent monitoring has been conducted however.
Circalittoral reefs
The Isles of Scilly contain a large area of circalittoral reef habitat, the majority of which is protected under the Isles of Scilly SAC (Eggleton and Meadows, Reference Eggleton and Meadows2013). Circalittoral reefs are dominated by animal communities (i.e., sponges and corals) (Figure 3D) as opposed to the shallower, algae dominated communities found on infralittoral reefs (Joint Nature Conservation Committee, 1996b). Across the islands these reefs support a range of cold-water coral and sponge species, including a high level of nationally rare species such as Axinella infundibuliformis, Desmacidon fruticosum with the yellow sponge, Axinella damicornis, recorded by citizen science divers at Dry Ledge (Figure 1) (Seasearch, 2004)’. The BAP habitat ‘Fragile Sponge and Anthozoan Communities on Rocky Habitats’ is common on Scillonian circalittoral reefs and are typically dominated by large, slow growing branching sponge and sea fan species such as the pink sea fan (Eunicella verrucosa) (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008). The Isles of Scilly has >200 mapped wrecks (UK Hydrographic Office, 2023) of varying ages, five of which are legally protected (Historic England, 2015), and so it is likely that these structures provide important habitat for a range of species. However, whilst several sites have been studied using multi-beam echo sounder (Camidge et al., Reference Camidge, Goskar and James2019) there has been limited research into the composition of ecological communities that they host or their role in supporting fish and invertebrate populations.
Species
The Isles of Scilly have long been considered an ecologically important site for a disproportionally large number of marine species despite the archipelago's relatively small spatial extent (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). Existing inventories of marine species have shown high number of conservation priority species to be present (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008), with the Islands considered a hotspot of marine megafauna species richness relative to the wider English Channel (McClellan et al., Reference McClellan, Brereton, Dell'Amico, Johns, Cucknell, Patrick, Penrose, Ridoux, Solandt, Stephan, Votier, Williams and Godley2014).
Invertebrates (excluding commercial species)
Considerable research has been conducted on marine invertebrates notably epifaunal and benthic infauna communities at various sites across the Isles of Scilly. Kendall et al. (Reference Kendall, Widdicombe, Davey, Somerfield, Austen and Warwick1996) found comparable levels of macrofauna between mainland and Scillonian sites, contradicting historical theories of relative species impoverishment on the Isles of Scilly (Crisp and Southward, Reference Crisp and Southward1958; Harvey, Reference Harvey1969). Further studies on the diversity and abundance of mollusc species showed the Isles of Scilly contain a diversity of species comparable to Devon and Cornwall (Turk and Seaward, Reference Turk and Seaward1997). Historical surveys conducted between 1984 and 1991 have also revealed the Isles of Scilly to host a high diversity of epifaunal species, attributed to the Isles' geographic position between warm and cool temperate zones (Fowler and Laffoley, Reference Fowler and Laffoley1993). Species recorded during these surveys include the pink sea fan (Eunicella verrucosa), red and pink sea fingers (Alcyonium glomeratum, Parervthropodium coralliodes) and large numbers of the sunset star coral (L. pruvoti); (Fowler and Laffoley, Reference Fowler and Laffoley1993), which are considered indicators of ecosystem health due to their high sensitivity to substrate loss, physical disturbance, salinity changes and other stressors (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008).
An extensive taxonomic inventory of marine flora and fauna of the Isles of Scilly have been published through a series of articles in the Journal of Natural History (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). Publications in this series include inventories of Diatoms, Macroalgae, Foraminifera (Atkinson, Reference Atkinson1970), Cnidaria and Ctenophora (Robins, Reference Robins1969), Digenea (Newell, Reference Newell1986), free-living Nematoda (Warwick and Coles, Reference Warwick and Coles1977), Gastrotricha (Hummon and Warwick, Reference Hummon and Warwick1990), Cephalochordata (Rowe, Reference Rowe1972), Polychaeta (Harris, Reference Harris1972), Tardigrada (King et al., Reference King, Fordy and Morgan1981), Acari (Pugh, Reference Pugh1988), Crustacea (Eucarida (Thurston, Reference Thurston1970), Ostracoda (Neale, Reference Neale1970), Copepoda: Harpacticoida (Wells, Reference Wells1970), Mysidacea (Makings, Reference Makings1987), Pycnogonida (King, Reference King1972), Mollusca, Bryozoa and Entoprocta (Hayward, Reference Hayward1976, Reference Hayward1971), Echinodermata (Rowe, Reference Rowe1971), Enteropneusta, Ascidiacea, Thaliacea, Larvacea and Cephalochordata (Rowe, Reference Rowe1972). Many of these studies identified multiple new species to science and the UK (Wells, Reference Wells1970; Warwick, Reference Warwick1977; Warwick and Coles, Reference Warwick and Coles1977; Michael Gee, Reference Michael Gee2005, Reference Michael Gee2006; Faubel and Warwick, Reference Faubel and Warwick2005; Bamber, Reference Bamber2011) and provide an invaluable baseline against which future range shifts may be measured; particularly relating to local extinctions and colonisation associated with warming waters.
Further local studies focusing on marine invertebrate communities include understanding drivers of diversity of epifaunal communities (Gee and Warwick, Reference Gee and Warwick1994), biodiversity assessments of wider fauna using death assemblages of shelled molluscs (Warwick and Light, Reference Warwick and Light2002), community composition dynamics (Somerfield et al., Reference Somerfield, Dashfield and Warwick2007) and multivariate methods for analysing variation in ecological communities (Somerfield et al., Reference Somerfield, Clarke and Gorley2021, Reference Somerfield, Dashfield and Warwick2018). Benthic infauna received similar attention in the literature, notably research on the drivers of assemblage structure (Clarke and Warwick, Reference Clarke and Warwick1999; Warwick et al., Reference Warwick, Dashfield and Somerfield2006; Somerfield et al., Reference Somerfield, Dashfield and Warwick2007) and to support monitoring of the SAC (Warwick and Somerfield, Reference Warwick and Somerfield2015). Other records of note include hundreds of sea hare (Aplysia punctata) in St Mary's Harbour (Figure 1) and surrounding sand flats (Hiscock et al., Reference Hiscock, Earll and White2020), and the nationally rare lagoon snail (Paludinella globularis), which is common in the Isles of Scilly (Light and Killen, Reference Light and Killen2001).
Commercially exploited crustaceans
High densities of brown (edible) crab (Cancer pagurus), European lobster (Homarus gammarus) and the European spiny lobster, or crawfish (Palinurus elephas) are found throughout the Isles of Scilly and form the majority of landings from local fisheries (Figure 4) (Holt and Kelly-Flectcher, Reference Holt and Kelly-Flectcher2015). In 2013 the Isles of Scilly Inshore Fisheries and Conservation Authority (IFCA) initiated a tagging study to gather data on the biology and ecology of the European lobster and European spiny lobster (i.e., population characteristics, individual movements, growth and recapture rate) to inform local fisheries management measures and ascertain sustainability of current levels of fisheries mortality (Holt and Kelly-Flectcher, Reference Holt and Kelly-Flectcher2015). Results found European lobsters to be exploited within sustainable levels, Spiny lobster, however, were characterised by population declines, which was considered a result of increased netting effort and hand removal by divers (Holt and Kelly-Flectcher, Reference Holt and Kelly-Flectcher2015). Spiny lobster have experienced significant populations declines throughout the Northeast Atlantic, with a peak in English landings of >100 tons in 1969 falling to 12 tons in 2014 (Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018). The Isles of Scilly, however, appears to be a regionally important location for the species, with models of larval dispersal suggesting its population is an important source of larvae for the south and west of Ireland (Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018). In turn, dispersal models suggest limited self-seeding of larvae within the Isles of Scilly, with recruitment likely dependent on distant populations outside the South West UK (Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018; Ellis et al., Reference Ellis, MacLeod, Jenkins, Rato, Jézéquel, Pavičić, Díaz and Stevens2023).
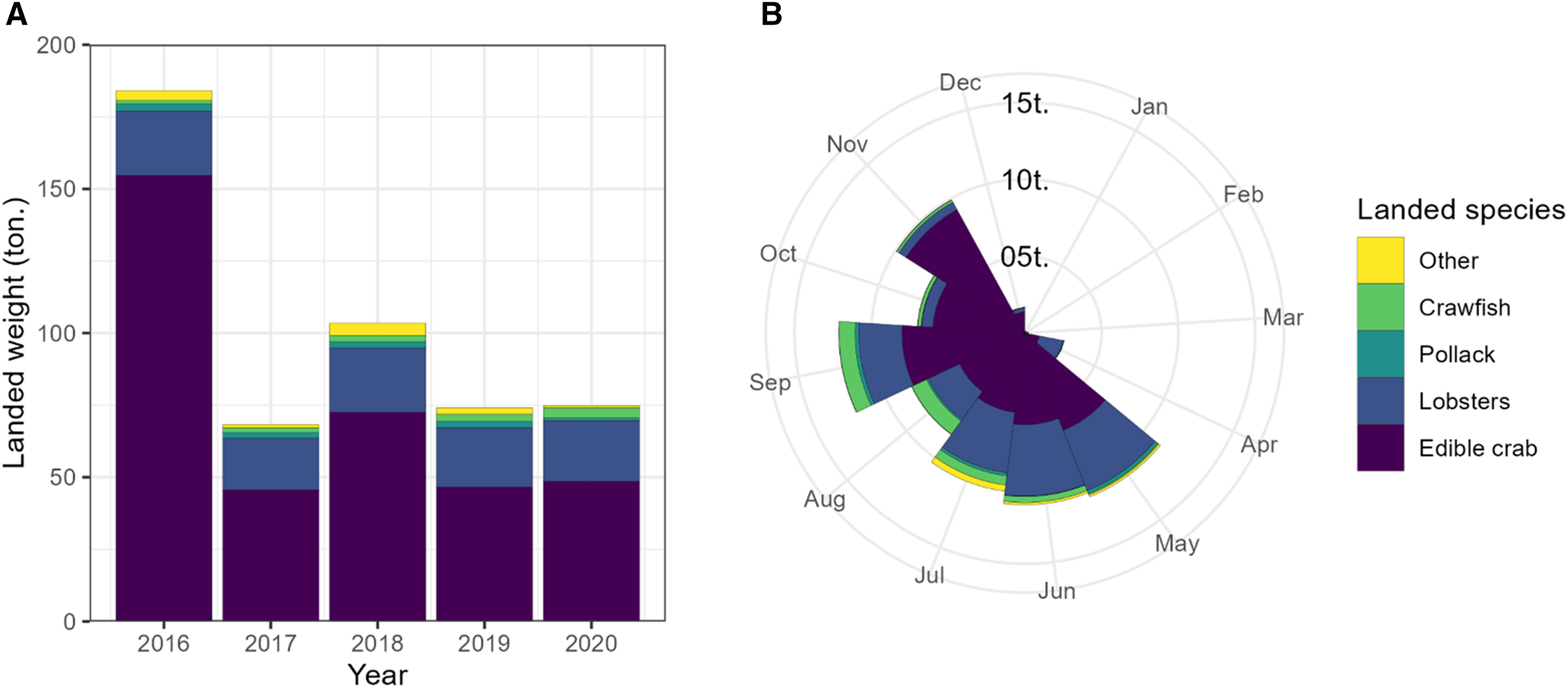
Figure 4. Annual landings to Isles of Scilly ports from MMO ‘Landings to Port’ dataset with: (A) the annual total weight by top four landed species with all other species aggregated; and (B) 2020 landings by month to show the seasonality of the fishery.
Teleost fish and elasmobranchs
A total of 32 BAP species of marine or euryhaline fish and elasmobranchs have been identified locally, with the archipelago remaining an important site for species such as the European eel (Anguilla Anguilla), lesser sand eel (Ammodytes tobianus) and Atlantic cod (Gadus Morhua) whilst some previously recorded species are now assumed locally extinct (i.e., angel shark, Squatina squatina) (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008). Local ecological knowledge has also been used to provide additional detail on the species found within the archipelago (Herdson, Reference Herdson2010). This includes occasional rare records of nationally important conservation priority species including European sturgeon (Acipenser sturio) and seahorse species (Hippocampus guttulatus and Hippocampus hippocampus). Trawl surveys in 2016 and 2017 conducted by the Centre for Environment, Fisheries and Aquaculture Science (CEFAS) have confirmed the waters surrounding the Isles of Scilly to be nationally important for blue skate, with subsequent demographic modelling estimating the adult breeding population to be between 15,000 and 25,000 individuals regionally (Bendall et al., Reference Bendall, Jones, Nicholson, Hetherington and Burt2017; Hetherington et al., Reference Hetherington, Nicholson, Nelson, Skirrow, Barreau and Spence2018; Delaval et al., Reference Delaval, Bendall, Hetherington, Skaug, Frost, Jones and Noble2023). Critical habitat for this species is a mud patch located west of Bishop's Rock lighthouse (Figure 1) (Herdson, Reference Herdson2010), with individuals displaying high site fidelity to the area (Delaval et al., Reference Delaval, Bendall, Hetherington, Skaug, Frost, Jones and Noble2023). Sightings and recreational catches of porbeagle sharks (Lamna nasus), a BAP listed species, occur most years, typically on outer reefs (Seasearch, 2008; Earll and Hiscock, Reference Earll and Hiscock2017; Earll et al., Reference Earll, Hiscock and White2018; Hiscock and Earll, Reference Hiscock and Earll2021), with large numbers of blue shark (Prionace glauca) recorded around the Isles from mid-June until mid-September (Earll and Hiscock, Reference Earll and Hiscock2017; Earll et al., Reference Earll, Hiscock and White2018). Notable hotspots include Pol Bank (Figure 1), a submerged reef south west of the archipelago (Herdson, Reference Herdson2010). Similarly, recreational angling catches indicate a potential hotspot for bluntnose sixgill (Hexanchus griseus) sharks in deep waters south west of the archipelago (Hiscock and Earll, Reference Hiscock and Earll2022). Other pelagic species including basking (Cetorhinus maximus) and common thresher (Alopias vulpinus) sharks are occasionally sighted in proximity to the archipelago (Leeney et al., Reference Leeney, Witt, Broderick, Buchanan, Jarvis, Richardson and Godley2012; Hiscock and Earll, Reference Hiscock and Earll2022).
Atlantic bluefin tuna sightings are increasingly common in the Isles of Scilly and South West UK more generally (Hiscock and Earll, Reference Hiscock and Earll2021). Of UK sightings recorded between 2014 and 2018 and analysed by Horton et al. (Reference Horton, Block, Davies, Hawkes, Jones, Jones, Leeves, Maoiléidigh, Righton, van der Kooij, Wall and Witt2021), 86% were observed around the Isles of Scilly and the southern English coast, with a 77% increase in sightings during this period. Ocean sunfish (Mola mola) are recorded in reasonable, if inconsistent numbers around the Isles (Earll and Hiscock, Reference Earll and Hiscock2017), with 2017 seeing a peak in sightings despite low numbers recorded across mainland Cornwall (Earll et al., Reference Earll, Hiscock and White2018). In waters between the 6 and 12 nm zones, the Pelagic ecosystem survey in the Western Channel and Celtic Sea (PELTIC) has shown recent declines in Atlantic mackerel (Scomber scombrus) in the Isles and surrounding waters since 2019, in contrast to increases of anchovy (Engraulis encrasicolus) (Hiscock et al., Reference Hiscock, Earll and White2020; Hiscock and Earll, Reference Hiscock and Earll2021). The PELTIC survey also regularly recorded large catches of boarfish (Capros aper) in deeper waters surrounding the archipelago (Earll et al., Reference Earll, Hiscock and White2018). In recent years, rare and unusual records have been collated annually and include the blackfish (Centrolophus niger), scale-rayed wrasse (Acantholabrus palloni), Portuguese or red blenny (Parablennius ruber) and giant goby (Gobius cobitis) (Goodwin and Picton, Reference Goodwin and Picton2007; Hiscock and Earll, Reference Hiscock and Earll2020; Hiscock et al., Reference Hiscock, Earll and White2020).
Marine mammals
Records from strandings and sightings data reveal 14 species of marine mammals in the waters of the archipelago (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008; Leeney et al., Reference Leeney, Witt, Broderick, Buchanan, Jarvis, Richardson and Godley2012; Earll and Hiscock, Reference Earll and Hiscock2017; Hiscock et al., Reference Hiscock, Earll and White2020; Hiscock and Earll, Reference Hiscock and Earll2021, Reference Hiscock and Earll2022). These range from species that are sighted annually (i.e., harbour porpoise (Phocoena phocoena)), to rarer species with lags of serval years between sightings (Risso's dolphin (Grampus griseus)) or single records (sperm whale (Physeter macrocephalus)) (Lewis et al., Reference Lewis, Parslow, Gall and McCartney2008; Leeney et al., Reference Leeney, Witt, Broderick, Buchanan, Jarvis, Richardson and Godley2012). The Isles of Scilly are a regionally important haul-out site for the South west population of grey seals (Halichoerus grypus), with counts ranging from 565 seals in October to 399 seals by the end of November (Sayer and Witt, Reference Sayer and Witt2019). Annual surveys suggest the population is stable, with the Western Rocks (282 individuals ± 48) and Eastern Isles (108 ± 49), that are subject to interannual fluctuations in numbers, considered the two most important haul out sites (Leeney et al., Reference Leeney, Broderick, Mills, Sayer, Witt and Godley2010, Reference Leeney, Witt, Broderick, Buchanan, Jarvis, Richardson and Godley2012; Sayer et al., Reference Sayer, Hockley and Witt2012; Sayer and Witt, Reference Sayer and Witt2019). Whilst there is a resident population year-round, analysis of photo identification catalogues and satellite tracking data have provided direct evidence of connectivity with populations on the UK mainland (Sayer et al., Reference Sayer, Allen, Hawkes, Hockley, Jarvis and Witt2019), and the Molène archipelago in France (Vincent et al., Reference Vincent, Fedak, McConnell, Meynier, Saint-Jean and Ridoux2005) with corroborating historical surveys (Summers, Reference Summers1974). Strandings and post-mortems of grey seals are rare locally, with the most recent cases including a seal shot in 2015 and a fatal septicaemia infection recorded in 2018 (Crosby et al., Reference Crosby, Hawtrey-Collier, Niki and Williams2015; Clear et al., Reference Clear, Hawtrey-Collier and Williams2018).
In terms of cetaceans, aerial surveys have revealed harbour porpoise, and common dolphin (Delphinus delphis) to be regularly sighted within inshore waters, and minke whales (Balaenoptera acutorostrata) and bottlenose dolphins (Tursiops truncatus) observed in larger numbers at the eastern edge of the 6 nm limit (Leeney et al., Reference Leeney, Witt, Broderick, Buchanan, Jarvis, Richardson and Godley2012; Buttifant, Reference Buttifant2021). There have been increasing sightings of humpback whales (Megaptera novaeangliae) close to the islands since 2018, primarily during winter months, and fin whales (Balaenoptera physalus) are now being regularly recorded, with the Isles of Scilly Bird Group recording 21 sightings in 2021 (Hiscock and Earll, Reference Hiscock and Earll2021). Ongoing citizen science surveys by the NGO ORCA suggest substantial interannual variation in numbers of common dolphin sightings for the period 2006 to 2017 (Robbins et al., Reference Robbins, Babey and Embling2020). As with pinnipeds, reports of cetacean strandings on the islands are relatively rare, with recent records including a single case of a bottlenose dolphin stranding (Crosby et al., Reference Crosby, Hawtrey-Collier and Clear2016), a common dolphin with potential evidence of entanglement (Clear et al., Reference Clear, Hawtrey-Collier and Williams2018) and the stranding of a juvenile minke whale (Hiscock and Earll, Reference Hiscock and Earll2021). In terms of rare and unusual records the first ever UK record of a bowhead whale (Balaena mysticetus) was observed off St Martin's in 2015 (Hiscock and Earll, Reference Hiscock and Earll2016), and a visiting walrus (Odobenus rosmarus) occupied St Mary's Harbour in summer 2021 (Hiscock and Earll, Reference Hiscock and Earll2022).
Seabirds
The islands were designated a SPA in 2001 in recognition of the international importance of its seabird assemblages (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008). At the time of its designation approximately 20,000 seabirds of 13 different species were recorded breeding across the islands (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008), resulting in the Isles being one of only seven sites in England to qualify as an ‘assemblage of breeding seabirds’. Whilst subsequent surveys have confirmed that the islands continue to support a greater diversity of seabirds than any other site in England, (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008), they also reveal that between 1983 and 2015 there has been a 31.3% decrease in total seabird population size, and the loss of breeding populations of the roseate (Sterna dougallii) and Sandwich tern (Thalasseus sandvicensis) (Heaney and St Pierre, Reference Heaney and St Pierre2017). Declines were most evident for the common tern (Sterna hirundo, −85%) and black-legged kittiwakes (Rissa tridactyla, −72%), with similar concern that without intervention kittiwakes could also be lost as a breeding species (Heaney and St Pierre, Reference Heaney and St Pierre2017). There have, however, been some notable successes with historic declines in the burrow nesting European storm petrel (Hydrobates pelagicus) and Manx shearwater (Puffinus puffinus) partially reversed by the successful eradication of rats from the islands of St Agnes and Gugh in 2016, with these populations monitored annually on the islands of St Agnes, Gugh and Annet (Heaney et al., Reference Heaney, Ratcliffe, Brown, Robinson and Lock2002; RSPB, 2016; Earll and Hiscock, Reference Earll and Hiscock2017; Heaney and St Pierre, Reference Heaney and St Pierre2017; Heaney, Reference Heaney2021).
With a local breeding population of ~1300 pairs (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008), the Isles of Scilly are an important site for the European shag (Phalacrocorax aristotelis). GPS tracking and aerial surveys revealed multiple colonies were dependent on a spatially limited area of shallow, inshore waters at the centre of the archipelago for foraging (Evans et al., Reference Evans, Dall, Bolton, Owen and Votier2016; Webb and Irwin, Reference Webb and Irwin2021), that are highly susceptible to disturbance from vessel traffic and pollution events. The results of these studies were subsequently used to support the expansion of the SPA (Natural England, 2018, 2019a), adopted in 2020 (Figure 1).
Other species
The offshore location of the archipelago results in the presence of pelagic species that are less common around the mainland UK. The Continuous Plankton Recorder study revealed evidence of sharp (1 km) transition boundaries between distinct phytoplankton communities, showing highly heterogeneous pelagic environments surrounding the archipelago (Cunningham et al., Reference Cunningham, McKee, Craig, Tarran and Widdicombe2003). The ecosystem services provided by these phytoplankton communities are considerable (Mangi et al., Reference Mangi, Davis, Payne, Austen, Simmonds, Beaumont and Smyth2011). The contribution to gas and climate regulation from photosynthetic production in the Isles of Scilly was estimated at 136,495 tC y−1, with phytoplankton productivity far outweighing the contribution of seagrass and kelp habitats (annual production in tC y−1 18,776.6 and 2519.8 respectively) (Mangi et al., Reference Mangi, Davis, Payne, Austen, Simmonds, Beaumont and Smyth2011). No studies relating to the temporal trends of phytoplankton communities surrounding the Isles of Scilly were found during this review. Data collect from the Western English Channel between 1992 and 2007 suggest considerable shifts between the communities may be occurring however (Widdicombe et al., Reference Widdicombe, Eloire, Harbour, Harris and Somerfield2010).
Mass strandings of pelagic jellyfish and similar species are also relatively common, with rare mass strandings of salps on St Mary's (Clear et al., Reference Clear, Hawtrey-Collier and Williams2018; Hiscock and Earll, Reference Hiscock and Earll2021), Portuguese Man O’ War (Physalia physalis) and mauve stingers (Pelagia noctiluca) in 2017 (Earll et al., Reference Earll, Hiscock and White2018). Pelagic algal blooms, typically occurring offshore, are also able to encroach into the islands, a recent example being the 2020 coccolithophore bloom (Hiscock and Earll, Reference Hiscock and Earll2021). Sightings and strandings of loggerhead (Caretta caretta), leatherback (Dermochelys coriacea) and Kemp's ridley (Lepidochelys kempii) turtles occur near annually, however, no clear species specific trends were evident (Witt et al., Reference Witt, Penrose and Godley2007; Earll and Hiscock, Reference Earll and Hiscock2017; Crosby and Hawtrey-Collier, Reference Crosby and Hawtrey-Collier2020; Hiscock and Earll, Reference Hiscock and Earll2021).
Human activities and impacts
Activities
Fisheries
The Isles of Scilly supports a small inshore fishery targeting crustaceans with static pots and nets, with only 16 active vessels out of a total 25 present on the islands (Isles of Scilly IFCA, 2019). A limited (<1 tonne in 2020) take of European pollack (Pollachius pollachius) also occurs, with no other finfish species of note in landings (Figure 4). Due to the small size of vessels and high exposure to winter storms the fishery is seasonal and typically operates between March and November (Isles of Scilly IFCA, 2019) (Figure 4). European lobster are the most economically important species targeted (33.35 tonnes of landings worth £366,880 in 2018) whilst brown crab are the most landed species in total live weight (83.7 tonnes worth £167,000 in 2018) (Isles of Scilly IFCA, 2019). Landings of European lobster have seen a 97% increase between 2015 (16.9 tonnes) and 2018 (33.3 tonnes), with brown crabs peaking in 2016 (154 tonnes) but remaining high from a 2012 baseline of just 22 tonnes (Isles of Scilly IFCA, 2019).
Recreational potting effort is currently managed by the Recreational Fixed Gear Permit byelaw (2020) that limits recreational pot numbers to 6 per person. There are currently between 150 and 200 recreational pots worked in the inshore waters each summer (Isles of Scilly IFCA, 2019), although distribution of this recreational effort in time and space is unknown. No mariculture operations exist in the Isles of Scilly to date, with the exposed nature of the islands making them less suitable than mainland sheltered bays for either finfish or shellfish production (Exeter et al., Reference Exeter, Kerry, Pikesley, Turner and Witt2021).
Recreation
A wide variety of marine recreational activities have occurred for decades within the inshore zone, notably yachting, motorised water sports, SCUBA diving, snorkelling, and wildlife watching (English Nature, 1996). There is limited data on the current distribution and intensity of recreational activities for the Isles of Scilly. The Marine Management Organisation's modelled recreational activity data layers, developed to inform the South West Marine Spatial Plan (Marine Management Organisation, Reference Marine Management Organisation2014), were considered too coarse to make accurate estimates of the recreational footprint or quantify potential impacts on local conservation features, such as the SAC grey seal population or SPA protected seabirds.
Energy and aggregates
Currently no wind or wave energy operations occur in the Isles of Scilly. Data from wave buoys west of the islands were included in a regional feasibility study (Smith et al., Reference Smith, Fairley, Robertson, Abusara and Masters2017), and the high levels of device development and testing in the wider Celtic Sea suggests this may be an area of future development. Historical (end date unknown) aggregate extraction has occurred at Bar Point, St Mary's (English Nature, 1996) but no modern activities exist.
Impacts
Pollution
Despite having a small resident human population (2100 in the 2021 Census), sediment samples from across South West UK have identified the Isles of Scilly as a hotspot for microplastic pollution (Nel et al., Reference Nel, Sambrook Smith, Harmer, Sykes, Schneidewind, Lynch and Krause2020). Pollution levels were greater than sites sampled at the regional population hubs of the port town of Falmouth and the port city of Plymouth (Nel et al., Reference Nel, Sambrook Smith, Harmer, Sykes, Schneidewind, Lynch and Krause2020). Causes of local high plastic pollution loads may include poor waste disposal practices, however, it is highly likely that distant sources (i.e., Americas) are responsible due to the archipelago's exposure to prevailing currents of the Gulf Stream which circumnavigate the islands (Nel et al., Reference Nel, Sambrook Smith, Harmer, Sykes, Schneidewind, Lynch and Krause2020). Novel sources of pollution are also potentially prevalent around the Isles of Scilly due to the high density of shipwrecks, including the Torrey Canyon wreck in 1967 on the Seven Stones reef (Figure 1), considered to be one of the worst maritime ecological disasters in North West Europe (Wells, Reference Wells2017). Smith (Reference Smith2004) detailed a significant pollution event due to stranding of the cargo vessel MV CITA on rocks near Porth Hellick, with both oil and large quantities of polythene being lost at sea. Pollution events from shipping vessels (e.g., oil spills) were also perceived to be the greatest future threat to habitats and species of the Isles of Scilly during interviews with local communities (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020).
The Isles of Scilly SAC and SPA Site Improvement Plan highlighted discharges of sewage and trade effluent directly into the SAC as a key cause of local pollution and nutrient enrichment, with potential for harmful phytoplankton blooms and overgrowth of green algae, reduced water quality, increasing turbidity, decreasing algal communities, or siltation and smothering on reefs (Natural England, 2014). It is estimated 59,761 m3 of raw sewage containing 108,521 kg BOD is discharged into inshore waters per annum with the marine biota around the Isles of Scilly performing a bioremediation service valued at £227,894 per year (equivalent cost of treating the waste to tertiary level) (Mangi et al., Reference Mangi, Davis, Payne, Austen, Simmonds, Beaumont and Smyth2011). Discharges from boats are also an understudied but potentially significant source of pollution (Butt, Reference Butt2007) given this region records the second highest annual vessel density (considered to 12 nm) in the South West (Natural England, 2010; Exeter et al., Reference Exeter, Kerry, Pikesley, Turner and Witt2021). Despite these findings, a separate study which compiled multiple existing datasets relating to the state of the marine environment, showed the Isles of Scilly to have the best bathing water status, the clearest water (suspended mater) and the second lowest modelled urban runoff in the entire South West UK study area (Exeter et al., Reference Exeter, Kerry, Pikesley, Turner and Witt2021). These results were attributed to the small resident population and limited industrial or agricultural pollution sources (Exeter et al., Reference Exeter, Kerry, Pikesley, Turner and Witt2021). Vessel source pollution results were ranked second worst for the region however, and were more aligned with the wider literature on pollution levels in the Isles of Scilly highlighted here.
Invasive and introduced species
The prevailing Westerly currents of the Gulf Stream may limit the ability of invasive species that have colonised the South and West UK coastlines from reaching the Isles of Scilly. For example, the Pacific oyster (Magallana gigas) is now well established in the South West UK (Hiscock and Earll, Reference Hiscock and Earll2022), but has yet to be recorded on the islands, with limited larval connectivity from the mainland to the archipelago (Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018) a possible cause. The presence of many Mediterranean invertebrate species however, suggests connectivity between Northern France and the Isles of Scilly (Turk and Seaward, Reference Turk and Seaward1997), so colonisations are probable in the future. Brown rats have proven a significant historical threat to the Island's burrow nesting seabirds (RSPB, 2016). The successful eradication on St Agnes and Gugh islands, at the time the largest community led rat removal project for seabirds globally, has led directly to improved breeding successes for several species (Heaney and St Pierre, Reference Heaney and St Pierre2017). Sargassum muticum, an invasive Pacific brown seaweed (commonly known as wireweed and with the potential to outcompete native seagrass species), has been observed at low levels during seagrass surveys in the Isles of Scilly (Bull and Kenyon, Reference Bull and Kenyon2020), although no formal quantification of abundance or extent was recorded making comparisons with other sites difficult.
Fisheries exploitation
As early as 1822 reports of decreases in fish abundance have been recorded around the islands (Harvey, Reference Harvey1969). Beyond the 6 nm fisheries limit the variety of gear types deployed diversifies, with analysis of Vessel Monitoring Systems (VMS) data revealing that an area just south of the 6 nm fisheries limit to be some of the most intensively fished in English waters, with bottom towed gears common (Witt and Godley, Reference Witt and Godley2007).
The current focus on static potting for crustaceans within the 6 nm fisheries zone (Figure 1B) is considered low impact and compatible with most MPA objectives and ecosystem functions (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020), however, damage to long-lived, slow growing sessile taxa is a potential area of concern (Gall et al., Reference Gall, Rodwell, Clark, Robbins, Attrill, Holmes and Sheehan2020). Netting for European spiny lobster and monkfish (Lophius budegassa) is less established, but rapidly expanding (Isles of Scilly IFCA, 2019) with an assessment of bycatch an area in need of further research.
Disturbance, wildlife conflict and novel threats
An estimated 100,000 people visit the islands each year and wildlife disturbance has been highlighted as a key area of conflict (Smyth et al., Reference Smyth, Harvey-Scholes and Wills2021). However, the spatial footprint of marine tourist activities and the potential impact for vulnerable species (i.e., grey seals, and seabird nesting colonies) has yet to be quantified. In 2022 a global outbreak of avian flu reached the Isles of Scilly with some reported mortalities (pers. Comm Isles of Scilly Wildlife Trust). The population impacts of this disease are not yet clear, however, it is likely to be significant in coming years and the Isles of Scilly Wildlife Trust's annual bird counts will be essential in quantifying this impact (Heaney, Reference Heaney2021).
Marine management
Marine protected areas
Three main types of marine protected area are present in the archipelago: the Isles of Scilly SAC, MCZs and the SPA. Local Sites of Special Scientific Interest (SSSIs) lack marine components below mean high water (with one exception being the intertidal ‘St Martin's Sedimentary Shore SSSI) so were not included in the analysis. SSSIs do, however, cover important bird habitat and provided the legal underpinning for the original SPA and SAC designations.
The Isles of Scilly SPA and SAC were designated under the European Birds and Habitats Directives, respectively (English Nature, 1997). The SPA was established in 2001 and expanded in 2020 to cover a total of 133 km2 (Figure 1). SPA designated features are: European storm petrel (H. pelagicus), Lesser black-backed gull (Larus fuscus), Great black-backed gull (Larus marinus), European shag (Phalacrocorax aristotelis) and for a seabird assemblage supporting over 20,000 breeding seabirds at designation (Heaney et al., Reference Heaney, Lock, Pierre and Brown2008). The SAC was established in 2000 and covers approximately 268 km2. Designated under the European Habitats Directive, the site covers Annex I habitats: Subtidal sandbanks which are slightly covered by sea water all the time, Intertidal mudflats, and sandflats not covered by seawater at low tide, Reefs and species: grey seals and shore dock (Rumex rupestris) (Natural England, 2010). These broad habitat classifications contain numerous sub-features including seagrass bed communities, kelp forest communities and rocky shore communities.
A total of 11 MCZs covering 58 km2 were established in 2013 following the 5-year stakeholder led ‘Finding Sanctuary’ collaborative decision-making process for South West English waters (Lieberknecht et al., Reference Lieberknecht, Hooper, Mullier, Murphy, Neilly, Carr, Haines, Lewin and Hughes2011). These sites were established to protect a number of designated features, with additional features added in 2019 (Natural England, 2019b). Ten of these sites all fall within the SAC boundary (Figure 1), are small in size (mean 3.1 km2) and are designated for similar features; primarily intertidal habitats, European Spiny lobster and stalked jellyfish species. The Bristows to the Stones MCZ is the largest (~27 km2) and most spatially distinct of the inshore sites, its north eastern edge falling outside the 6 nm fisheries limit. This site has been the most thoroughly surveyed prior to designation, with multibeam acoustic and ground-truthing video capture used to inform accurate broadscale habitat maps and help identify the extent of the extensive fragile sponge and anthozoan communities (Defra, 2014). This site is distinct from other inshore MCZs both geographically, and due to the features it is designated to protect. ‘High energy circalittoral rock’, ‘Fragile sponge and anthozoan communities on subtidal rocky habitats’ and ‘Pink sea-fan (Eunicella verrucosa)’, are all features of the site which are not designated for any other inshore Isles of Scilly MCZ, however, these features are covered under features of the SAC designation (Natural England, 2019a). At the edge of the 12 nm zone the offshore MCZ site; South of the Isles of Scilly is designated for the features ‘Subtidal coarse sediment’ and ‘Subtidal sand’ (Stubbles et al., Reference Stubbles, Harper, McLeod, Goold, Chaniotis, Burney and Moffat2019). These features were assigned a ‘recover’ condition during the MCZ designation process due to ‘continued high exposure of the seabed to the pressures associated with benthic trawling’ determined from analysis of VMS data between 2014 and 2016 (Stubbles et al., Reference Stubbles, Harper, McLeod, Goold, Chaniotis, Burney and Moffat2019). No evidence of any management being introduced to underpin this recovery was found as part of this review. The Marine Management Organisation, however, have begun drafting assessments and advice for the site as of 2023 (Marine Management Organisation, Reference Marine Management Organisation2023).
The MCZ network in the Isles of Scilly is considered a rare UK example of bottom-up, locally supported marine spatial management (Pieraccini and Cardwell, Reference Pieraccini and Cardwell2016). Local support for the MCZ network was attributed to increased stakeholder confidence in MCZ locations after extensive scientific dive surveys, coupled with the bottom–up nature of the design process resulting in high levels of consensus between stakeholders (Pieraccini and Cardwell, Reference Pieraccini and Cardwell2016). The MCZ designations were, in effect, a future-proofing tool, confirming the sustainability of existing practices (Pieraccini and Cardwell, Reference Pieraccini and Cardwell2016), with most designated features assigned a ‘Maintain in favourable condition’ rather than ‘Recover to favourable condition’ (Figure 5).
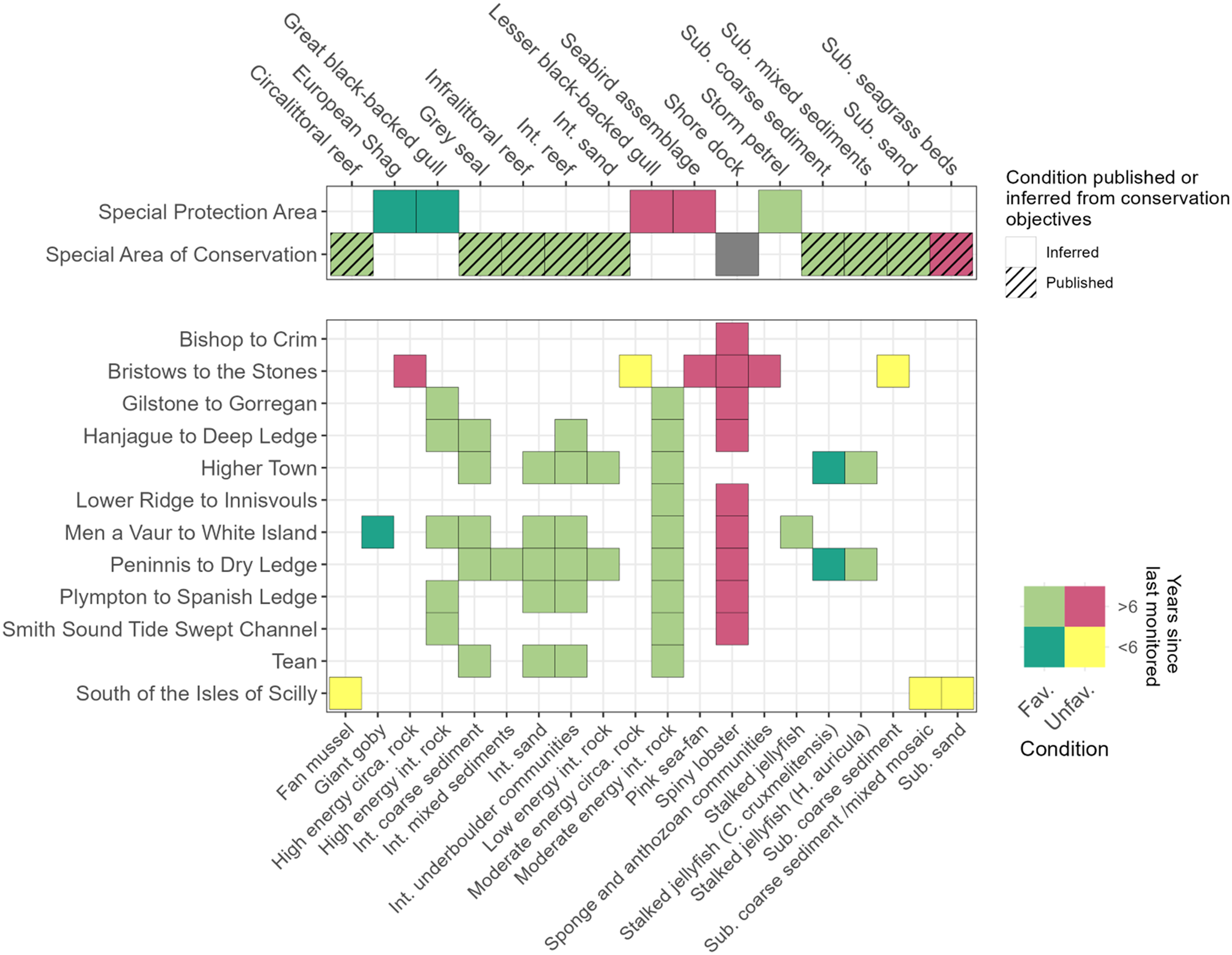
Figure 5. Monitoring and condition matrix of features of Isles of Scilly MPA sites. Showing the published or inferred condition of features (either ‘Favourable’ or ‘Unfavourable’) and the time lag since the last assessment (grouped into </>6 year windows) as of 01/01/2023. Monitoring strictly refers to literature used to inform feature condition assessments. Additional studies that have not been applied as formal assessments or statutory reporting (i.e., recent seagrass surveys) are not included here. As only certain features had condition assessments published (no shading), condition values were often inferred (shaded boxes) from the features conservation objectives. If a site was designated <6 years ago then it was considered to be within <6 time window regardless of the presence of a post-designation condition assessment. The 6 year time window was considered appropriate based on historical condition reporting requirements between the UK and European Union for European designated sites. No published surveys or condition were found during the review for the SAC ‘Shore dock’ feature that could be used to infer condition. The underpinning SSSI condition assessment lists the feature as ‘Favourable’ (Site feature condition (naturalengland.org.uk)), however unpublished Natural England reports (Bennallick, Reference Bennallick2018) have found the species to have almost completely disappeared from the islands.
Monitoring
The Nature Conservancy Council (subsequently English Nature and now Natural England) first identified the Isles of Scilly as an important monitoring site for rare and unusual UK species of conservation importance in 1983/4 (Fowler and Pilley, Reference Fowler and Pilley1992; Fowler and Laffoley, Reference Fowler and Laffoley1993). A monitoring programme was initiated focusing on Z. marina beds, under-boulder communities and rock pools (Fowler and Pilley, Reference Fowler and Pilley1992).
Since designation, features of the Isles of Scilly SAC and SPA have required monitoring for condition assessment on a 6-year cycle. The frequency of condition monitoring assessments for SAC features varies greatly, with grey seal pup counts (Sayer et al., Reference Sayer, Hockley and Witt2012; Sayer and Witt, Reference Sayer and Witt2019) an example of a well monitored feature (Figure 5). The seagrass sub-feature has had repeated annual voluntary monitoring, but the last published condition assessment was in 2017 (Cook, Reference Cook2011; Natural England, 2017) (Figure 5). Regular monitoring of the infaunal communities of the St Martin's Flats intertidal sandflats (Figure 5 abbreviated to ‘Int. sand’) also used to occur regularly, but has not occurred since 2009 (Natural England, 2010; Warwick and Somerfield, Reference Warwick and Somerfield2015). Monitoring of the infauna communities of the SAC features ‘sandbanks which are slightly covered by sea water all the time’ were conducted between 1997 and 2013 (Johnson et al., Reference Johnson, Burrows, Crabtree and Warner2022) with no published studies since. Based on these surveys favourable condition assessments were assigned, however, it has been noted that these surveys lacked standardised protocols between sites and years making results difficult to interpret (Johnson et al., Reference Johnson, Burrows, Crabtree and Warner2022).
Less accessible features of the SAC were generally found to be in a favourable condition (Figure 5) but lacking recent in-situ monitoring surveys likely due to depth and the highly exposed nature of the islands. Circalittoral, infralittoral and intertidal rock features are all reassigned ‘Favourable’ condition status as of 2021 (https://designatedsites.naturalengland.org.uk/), however, these assessments assumed no decline in condition from baseline surveys with no in-situ monitoring being conducted within the last 6 years. Baseline condition assessments of Annex I reefs were determined using the abundance and condition of pink sea fans (Eunicella verrucosa) and Ross coral (Pentapora fascialis) colonies from towed video arrays as a proxy (Eggleton and Meadows, Reference Eggleton and Meadows2013). These surveys found pink sea fans condition to range from excellent to poor throughout the Isles of Scilly, with Ross corals more commonly in good condition (Eggleton and Meadows, Reference Eggleton and Meadows2013). Dive surveys were also conducted in 2011 to assess the condition of the four sub-features: ‘Kelp forest communities’, ‘Vertical Rock’ and ‘Subtidal rock and boulder communities’ and ‘Subtidal faunal turf’ (Irving and Northern, 2012). These dive surveys were partially repeated in 2013 to further develop the monitoring methodologies and make temporal comparisons for any replicate sites (Axelsson et al., Reference Axelsson, Dewey and Wilson2014). As with the towed video arrays, condition was determined by the abundance and condition of static features, notably kelp species, erect sponges, cup corals, anthozoan communities and pink sea fans. Independent monitoring conducted by the University of Plymouth combining towed video and benthic grab survey data with literature reviews and interviews to develop a natural capital asset and risk register found variation in the status of habitats assessed (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). Intertidal habitats were determined to be in ‘good’ condition, whereas infralittoral rock was considered to be in ‘acceptable’ condition, and circalittoral rock and sublittoral macrophyte dominated sediment (seagrass beds) to be ‘of concern’ (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). The majority of degraded circalittoral reef condition was attributed to historical fishing pressures, with <1% of current degradation attributed to current fishing practices (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020).
All-species seabird population counts have been conducted in 1999/2000, 2006 and 2015 to meet the SPA condition monitoring requirements (Heaney and St Pierre, Reference Heaney and St Pierre2017). Between the SPA baseline survey in 1999/2000 and 2015 a 14.3% decrease in seabird breeding pairs counts (31.3% total seabird population decrease) was recorded (Heaney and St Pierre, Reference Heaney and St Pierre2017). As features of the SPA, changes in the population of European storm petrel (−7%), Lesser black-backed gull (−24%), great black-backed gull (+14%), European shag (−22%) between 2006 and 2015 surveys are of particular importance (Heaney and St Pierre, Reference Heaney and St Pierre2017). A further all-species seabird population counts was conducted in 2023 (delayed by covid), with results expected soon.
No published literature on the monitoring of MCZs sites were found during the literature search (Figure 5). Whilst the majority of MCZ features were in favourable condition at designation, the European spiny lobster was designated as requiring recovery at all nine relevant sites (Figure 5). Developing a fisheries independent monitoring programme for the species to inform MCZ condition assessments should therefore be prioritised.
Byelaws and non-spatial fisheries management
Isles of Scilly IFCA byelaws active as of 2021/22 (Table 1) included minimum landing sizes and retention bans on berried females, and towed fishing gear bans including dredges and nets (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). Many of these were found to have reasonable or high support from local communities in interviews conducted by the University of Plymouth (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). The IFCA has also conducted annual monitoring of the key crustacean stocks using fisheries dependent surveys, providing a regular source of data on stock status and population dynamics of the key commercial species exploited (Isles of Scilly IFCA, 2019).
Table 1. Fisheries byelaws specific to Isles of Scilly Inshore Fisheries and Conservation Authority applicable to 6 nautical miles as of 2022

Discussion
The Isles of Scilly is a biologically distinct archipelago, supporting an exceptionally high diversity of temperate marine species and is often considered to be in a far less degraded state than the surrounding waters of the Northeast Atlantic (Warwick et al., Reference Warwick, Emblow, Feral, Hummel, Van Avesaath and Heip2003). Despite the diversity of both coastal and pelagic habitats and species, marine research in the Isles of Scilly has been historically dominated by biological inventories, notably of small invertebrate taxa. This trend may partially be an artefact of literature availability, with these studies more often published in peer reviewed journals compared to habitat assessments that were commonly found in grey literature (government research reports) and therefore less likely to be housed in a future-proofed repository. For example, multiple historical reports relating to seagrass monitoring identified had no active links on any government repository or online search engine (Supplementary Table 1).
Trends in research themes have recently diversified to include marine vertebrate groups (notably commercial crustaceans, seabird species, and marine mammals) and seagrass beds. There remains, however, limited research relating to marine teleost fish and elasmobranch communities, or the current condition of non-seagrass habitats. For instance, no in-situ surveys of designated reefs were found to have been conducted since 2014 (although independent opportunistic surveys did occur in 2019 on previously unmapped veneer reefs [Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020]), making their true condition hard to determine. Sources relating to inshore marine fish species were found to be reliant on ad hoc records, with no indicators or proxies available for the health of fish populations, to aid identification of essential fish habitat or to understand their role in supporting seabird populations. Addressing the knowledges gaps highlighted here will be key to designing effective marine management interventions in the Isles of Scilly and reflect the UK Government's shifting focus to an ecosystem approach to marine management as laid out in the Benyon review into UK protected areas (Benyon, Reference Benyon2019). Key research gaps and priority areas highlighted from this review which are discussed in more detail below are:
• Quantifying anthropogenic impacts within the archipelago, notably the effects of warming waters on marine communities (with a particular focus on forage fish and seabird populations) and seasonal trends in potential vessel disturbance.
• Developing repeatable survey protocols that can underpin long-term ecosystem-based management and monitoring across all designations – with a particular focus on the spiny lobster feature within relevant MCZ boundaries, and reef and non-seagrass sediment features of the SAC.
• Addressing the lack of published literature on the elasmobranch and marine teleost fish communities, notably population trends and spatial mapping of essential nursery and foraging habitat.
Contemporary threats to the marine environment of the Isles of Scilly
Limited research relating to anthropogenic threats to the Isles of Scilly marine environment exists. Only one publication specific to the Isles of Scilly on marine pollution (plastics) was documented, with other studies (n = 2) reliant on UK wide analyses that provide only coarse information at a local scale. This compares to other point source marine pollution events globally that have received considerably more attention in the literature. Examples include impacts on the ability of marine habitats to maintain ecosystem services (DeLaune and Wright, Reference DeLaune and Wright2011) to declines in macroinvertebrate crustaceans (Zengel et al., Reference Zengel, Pennings, Silliman, Montague, Weaver, Deis, Krasnec, Rutherford and Nixon2016) and implications for the local fisheries (McCrea-Strub et al., Reference McCrea-Strub, Kleisner, Sumaila, Swartz, Watson, Zeller and Pauly2011). Conversely, man-made marine structures have been shown to act positively as artificial reefs and fish aggregation sites (Fujii, Reference Fujii2015; Todd et al., Reference Todd, Lavallin and Macreadie2018; Sánchez-Caballero et al., Reference Sánchez-Caballero, Borges-Souza and Abelson2021). Research into the long-term ecological impacts of pollution associated with local wrecks, and their potential to act as fish aggregation sites should therefore be encouraged.
A lack of climate change research undertaken around the Isles of Scilly was also found during this review, with projects such as the MBA run Marclim survey (Natural England, 2006) not extended to the Isles of Scilly to date. Increasing sea temperatures may have an acute impact on the archipelago's unique biogeography, particularly given the high number of nationally rare species at the southerly or northerly extremes of their range. Given the extensive historical inventory of marine fauna and flora collated in the literature, the Isles of Scilly presents a unique opportunity to track the impacts of warming seas on Northeast Atlantic communities, if repeat surveys were prioritised.
The Isles of Scilly experience large seasonal spikes in tourism (Smyth et al., Reference Smyth, Harvey-Scholes and Wills2021), with high densities of service, cruise, recreation, and transport vessel activities during summer months. Given the limited geographic area of the inshore zone, significant vessel activity is believed to overlap with key habitats such as seagrass beds and sand flats, as well as conservation priority species including grey seals and European shags. Potential cumulative impacts associated with this vessel activity have yet to be quantified locally. Physical disturbance and noise pollution from vessel represent an under-researched and under-managed threat to marine wildlife in UK waters in general (Merchant et al., Reference Merchant, Brookes, Faulkner, Bicknell, Godley and Witt2016). Their impacts on marine ecosystems have been shown to be considerable at other locations however, with anchor abrasion within seagrass beds (La Manna et al., Reference La Manna, Donno, Sarà and Ceccherelli2015) and disturbance of seabirds (Velando and Munilla, Reference Velando and Munilla2011; Wong et al., Reference Wong, Gjerdrum, Gilchrist and Mallory2018) a potential concern. Proposed ‘non-disturbance areas’ (i.e., ‘Smith Sound non-disturbance area’ including features as such ‘Tide-swept channels’) were recommended by Scillonian fishermen during the Finding Sanctuary MCZ process, but ultimately they were not included when the sites were designated in 2013 (Lieberknecht et al., Reference Lieberknecht, Hooper, Mullier, Murphy, Neilly, Carr, Haines, Lewin and Hughes2011). These zones would have introduced higher levels of restriction of vessel activities than elsewhere within MCZs and could be considered an area for further research into their potential as a spatial tool for managing vessel impacts on marine communities.
In comparison, local fisheries appear to be well managed with targeted data collection in recent years to minimise any potential impacts (Holt and Kelly-Flectcher, Reference Holt and Kelly-Flectcher2015; Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018; Isles of Scilly IFCA, 2019). The low impact nature of the gears used within the region, the strong buy-in from local fishers for conservation byelaws and adoption of annual data collection on exploited stocks suggest the Isles of Scilly to be a rare example of a sustainable fishery with a bottom-up approach to management (Pieraccini and Cardwell, Reference Pieraccini and Cardwell2016). The recent increase in European spiny lobster landings (Isles of Scilly IFCA, 2019) and subsequent diversification of gear to tangle nets does, however, pose a novel source of potential population decline, non-target bycatch and benthic abrasion that requires future monitoring.
Monitoring
The Isles of Scilly MCZ network currently lacks structured monitoring, especially compared to some SAC features and SPA sites (Figure 5). No literature on direct monitoring of MCZ designated features were found during this review. The spiny lobster is a feature of particular concern due to historical population declines across its range and its current designation status requiring recovery (Whomersley et al., Reference Whomersley, Van der Molen, Holt, Trundle, Clark and Fletcher2018), although recent landings data suggest localised population recovery for the species may already be underway (Figure 5). A further MCZ research priority is the Bristows to the Stones site. Despite the higher variety and uniqueness of features compared to other MCZs, Bristows to the Stones does not currently benefit from the same level of management as the other 10 sites, with no bottom towed gear restrictions in effect (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020) however new management byelaws to address this are expected in the near future (pers. comms Isles of Scilly IFCA 2023). This is despite the MCZ being highlighted as one of 11 nationally in the highest risk category of damage or deterioration to non-sensitive and sensitive features (JNCC, 2012).
Literature relating to habitats found within the Isles of Scilly is dominated by seagrass surveys. Despite considerable monitoring effort, subtidal seagrass beds are one of the few designated features across the Isles of Scilly MPA network to be in an unfavourable and declining condition. Less accessible features of the SAC have proven harder to develop spatially comprehensive, repeatable monitoring methodologies, due to their depth and the highly exposed nature of the islands. Circalittoral, infralittoral, and intertidal rock features are all listed as being in ‘Favourable’ condition with assessments completed in 2021, however, there is no evidence of these features being subject to onsite monitoring within the last 6 years (Figure 5). Subtidal coarse sediment (A5.1), Subtidal mixed sediments (A5.4) and Subtidal sand (A5.2) were assigned a favourable condition based on decade old grab surveys (Johnson et al., Reference Johnson, Burrows, Crabtree and Warner2022) and Subtidal seagrass beds (A5.53) ‘Unfavourable Declining’. Condition assessments of Annex I reefs were conducted using the presence and quality of pink sea fans and Ross coral colonies from towed video arrays as a proxy (Eggleton and Meadows, Reference Eggleton and Meadows2013). However, dive surveys have shown the pink sea fan is relatively uncommon locally and often small due to the high exposure and strong currents (Seasearch, 2004), making their role as a proxy for wider reef condition unclear. Dive surveys to assess a further four reef sub-features in 2011 (partially repeated in 2013 to develop the methodologies and make temporal comparisons for replicate sites): ‘Kelp Forest, communities’, ‘Vertical Rock’ and ‘Subtidal rock and boulder communities’ and ‘Subtidal faunal turf’ also concluded a ‘favourable’ condition assessment (Irving and Northern, 2012; Axelsson et al., Reference Axelsson, Dewey and Wilson2014). No repeat surveys have been published to track long-term temporal trends and these assessments are now over a decade old. Independent monitoring conducted by the University of Plymouth in 2019 to develop a natural capital asset and risk register for the region is therefore the only recent source of primary data for any updated condition assessments (Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). The lack of repeat assessments, limited spatial coverage of existing surveys and a lack of consensus between independent and statutory surveys suggest improvements to the monitoring of deeper water habitats in particular should be prioritised in future.
Identification of low-cost survey techniques that can generate meaningful indicators of ecosystem health for these features should therefore be prioritised in future monitoring efforts in the Isles of Scilly (Noble-James et al., Reference Noble-James, Bullimore, McBreen, O'Connor, Highfield, McCabe, Archer-Rand, Downie, Hawes and Mitchell2023). Baited remote underwater video systems (BRUVS) are an example of non-invasive, low cost and accessible sampling methodology that have been applied to study the health of marine ecosystems both in the UK (Unsworth et al., Reference Unsworth, Peters, McCloskey and Hinder2014; Bean et al., Reference Bean, Greenwood, Beckett, Biermann, Bignell, Brant, Copp, Devlin, Dye, Feist, Fernand, Foden, Hyder, Jenkins, van der Kooij, Kröger, Kupschus, Leech, Leonard, Lynam, Lyons, Maes, Nicolaus, Malcolm, McIlwaine, Merchant, Paltriguera, Pearce, Pitois, Stebbing, Townhill, Ware, Williams and Righton2017; Davies et al., Reference Davies, Holmes, Bicknell, Attrill and Sheehan2022) and in temperate systems globally (Whitmarsh et al., Reference Whitmarsh, Fairweather and Huveneers2017). Environmental DNA (eDNA has also been highlighted recently as having potential to complement traditional methods for MPA monitoring (Noble-James et al., Reference Noble-James, Bullimore, McBreen, O'Connor, Highfield, McCabe, Archer-Rand, Downie, Hawes and Mitchell2023). However, eDNA data are not considered comparable with the precision of traditional methods currently, particularly relating to abundance and biomass measurements (Noble-James et al., Reference Noble-James, Bullimore, McBreen, O'Connor, Highfield, McCabe, Archer-Rand, Downie, Hawes and Mitchell2023). These methods are capable of remotely and safely sampling a range marine species and habitats, and therefore have the potential to complement existing methodologies (i.e., dive surveys) particularly in less accessible habitats and MPAs (i.e., Bristow's to the Stones). Methods targeting more mobile and cryptic species would also complement existing data from dive and towed video studies that have primarily been used to assess the distribution and condition of sensitive habitats and sessile species (i.e., sponge and coral communities) (Eggleton and Meadows, Reference Eggleton and Meadows2013; Ashley et al., Reference Ashley, Rees, Mullier, Reed, Cartwright, Holmes and Sheehan2020). In addition to the flexibility and access provided by employing remote survey tools, any MPA monitoring strategies should strive to adopt established best practices, such as the ‘good monitoring framework’ (Lindenmayer and Likens, Reference Lindenmayer and Likens2010; Addison, Reference Addison2011). Representative sampling design, choice of appropriate biological indicators, appropriate data presentation and statistical analysis and regular reporting of results (Addison, Reference Addison2011) are all principles that would future proof monitoring for the region (Rees et al., Reference Rees, Sheehan, Stewart, Clark, Appleby, Attrill, Jones, Johnson, Bradshaw, Pittman, Oates and Solandt2020).
Knowledge gaps relating to marine fish communities and food webs
Published research relating to marine fish species and communities is notably scarce in the Isles of Scilly. All publications on spatial or temporal trends related to surveys beyond the 12 nm inshore zone (Bendall et al., Reference Bendall, Jones, Nicholson, Hetherington and Burt2017; Hetherington et al., Reference Hetherington, Nicholson, Nelson, Skirrow, Barreau and Spence2018) or were part of wider surveys with only coarse local resolution (Horton et al., Reference Horton, Block, Davies, Hawkes, Jones, Jones, Leeves, Maoiléidigh, Righton, van der Kooij, Wall and Witt2021). This data paucity can be attributed to the local fishery's targeting of crustaceans with static pots, with traditional fisheries dependent data from trawl surveys, a common source of data on the mainland UK, not available locally (Bean et al., Reference Bean, Greenwood, Beckett, Biermann, Bignell, Brant, Copp, Devlin, Dye, Feist, Fernand, Foden, Hyder, Jenkins, van der Kooij, Kröger, Kupschus, Leech, Leonard, Lynam, Lyons, Maes, Nicolaus, Malcolm, McIlwaine, Merchant, Paltriguera, Pearce, Pitois, Stebbing, Townhill, Ware, Williams and Righton2017; Liu et al., Reference Liu, Collins, Baillie, Rainbird, Brittain, Griffiths, Sims, Mariani and Genner2022). Considerable data relating to marine teleost fish also likely exists from ad hoc recording schemes (Hiscock and Earll, Reference Hiscock and Earll2020, Reference Hiscock and Earll2022) and targeted surveys (i.e., Seasearch) (Seasearch, 2007, 2008). However, as a systematic a review of the published literature, these records were not considered here. Future analyses of these data repositories could therefore provide important benchmarks of species presence and indicators of inter-annual trends. The application of new survey methodologies (outlined above) for mobile fish, elasmobranch and crustacean populations at other UK sites have also proven an effective source of data from which to measure the impacts of management actions (Blampied et al., Reference Blampied, Rees, Attrill, Binney and Sheehan2022; Davies et al., Reference Davies, Holmes, Bicknell, Attrill and Sheehan2022; Liu et al., Reference Liu, Collins, Baillie, Rainbird, Brittain, Griffiths, Sims, Mariani and Genner2022). Development of similar surveys in the Isles of Scilly would allow comparison against historical and anecdotal records (i.e., Herdson, Reference Herdson2010), and provide management groups a benchmark from which to measure the future impacts of warming waters, fisheries pressures and other novel threats. Remote methods, such as BRUVS, could also address the spatial data gaps in the local MPA network, as they have been shown effective for surveying highly remote locations (i.e., Birt et al., Reference Birt, Cure, Wilson, Newman, Harvey, Meekan, Speed, Heyward, Goetze and Gilmour2021) such as the western circalittoral reefs and offshore sites including Bristows to the Stones MCZ, that are currently underrepresented in monitoring data and generally inaccessible for dive surveys. Given the continuing population declines in certain seabird species across the archipelago (Heaney, Reference Heaney2021), understanding the health and spatial distribution of small forage fish, a key food source for many species, should also be highlighted for further research. Finally, whilst the blue carbon and other natural capital benefits of local seagrass beds are currently being mapped (Howard-Williams, Reference Howard-Williams2022b), their role as fish nursey sites remains unclear in UK waters compared with other locations (Blandon and zu Ermgassen, Reference Blandon and zu Ermgassen2014; Díaz-Gil et al., Reference Díaz-Gil, Smee, Cotgrove, Follana-Berná, Hinz, Marti-Puig, Grau, Palmer and Catalán2017). Their nursery role should therefore be explored further, especially as anecdotal records suggest shifts in the communities compared to historical recreational fisheries records, notably for flat fish species (Herdson, Reference Herdson2010). Developing our understanding of the marine fish communities of the Isles of Scilly would both complement established surveys of other taxa and provide a more holistic understanding of the state of marine ecosystems in the Isles of Scilly.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424000699.
Data
All retained sources used to inform this review are available on a public Zenodo repository (https://zenodo.org/records/10461881).
Acknowledgements
All authors wish to thank the reviewer for their time and effort, and greatly appreciate their constructive comments that contributed clarity to the findings.
Author Contributions
K. M., A. C. B. and O. E. conceived the study; O. E. conducted the literature review and synthesised findings; O. E. wrote the first draft; and all authors contributed to the revision of the manuscript and gave approval for publication.
Financial Support
O.E. was supported by funding from the University of Exeter through a Natural Environment Research Council GW4+ DTP PhD Case studentship award (Grant Ref number: NE/S007504/1). For the purpose of open access, the author has applied a ‘Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.
Competing interest
The authors declare no conflict of interest.
Ethical Standards
This review followed the PRISMA standardised systematic reviews and meta-analyses statement (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009) which provides a structured approach to conducting a comprehensive and rigorous literature review (see Figure S1 in supporting information).








