Depressive disorders are highly prevalent and represent a major health problem with a lifetime prevalence of mood disorders of 20.8%.Reference Ebmeier, Donaghey and Steele1, Reference Kessler, Merikangas and Wang2 Although depression is a serious illness for both men and women there is a large body of evidence showing that depression is twice as common in women as in men.Reference Kessler, Merikangas and Wang2, Reference Fernandez-Pujals, Adams, Thomson, McKechanie, Blackwood and Smith3 The cause for this uneven gender distribution is not fully understood but may partly be explained by biological, genetic, cultural and environmental causes.Reference Fernandez-Pujals, Adams, Thomson, McKechanie, Blackwood and Smith3, Reference Agnafors, Comasco, Bladh, Sydsjo, Dekeyser and Oreland4 There is consistent evidence that depression is associated with other impairments and secondary morbidity that include both somatic and mental disorders.Reference Bromet, Andrade, Hwang, Sampson, Alonso and de Girolamo5, Reference Kessler, Berglund, Demler, Jin, Koretz and Merikangas6 The effect of women's mental health on the child and future generations has been much debated. Questions have been raised concerning the relative importance of genetic and non-genetic factors through twin and two-generation studies.Reference Sullivan, Neale and Kendler7, Reference McAdams, Rijsdijk, Neiderhiser, Narusyte, Shaw and Natsuaki8
In a sequence of articles on a longitudinal family cohort, Weissman et al Reference Weissman, Berry, Warner, Gameroff, Skipper and Talati9, Reference Weissman, Wickramaratne, Nomura, Warner, Verdeli and Pilowsky10 investigated the lifetime rate of psychiatric disorders in three generations. They found high rates of psychopathology in the cohort's third generation which, in the study from 2016, consisted of 351 grandchildren. These rates were especially high if major depressive disorders (MDD) had been diagnosed in both previous generations.Reference Weissman, Berry, Warner, Gameroff, Skipper and Talati9 To our knowledge there are no population-based studies that have gone beyond two generations. The aim of the present study was to follow two generations of women with diagnoses of major depression and to subsequently examine the risk of MDD and use of specialised healthcare among their children, i.e. the third generation.
Method
Population
This is a population-based, nationwide, retrospective cohort study that included all women who gave birth during 1973–1977 (n = 169 782) or 1978–1982 (n = 154 931) divided into two cohorts. These women will be referred to as ‘grandmothers’ or ‘first-generation women’ throughout this article. Their daughters, who will be referred to as ‘second-generation women’ or ‘mothers’, were also included (n = 244 153 and n = 223 892, respectively) as was the third generation, i.e. the offspring of the second-generation women (n = 381 953 and n = 238 625, respectively). See Fig. 1 for an overview of the inclusion and ‘labelling’ of the study participants. The study population was followed until 2013.
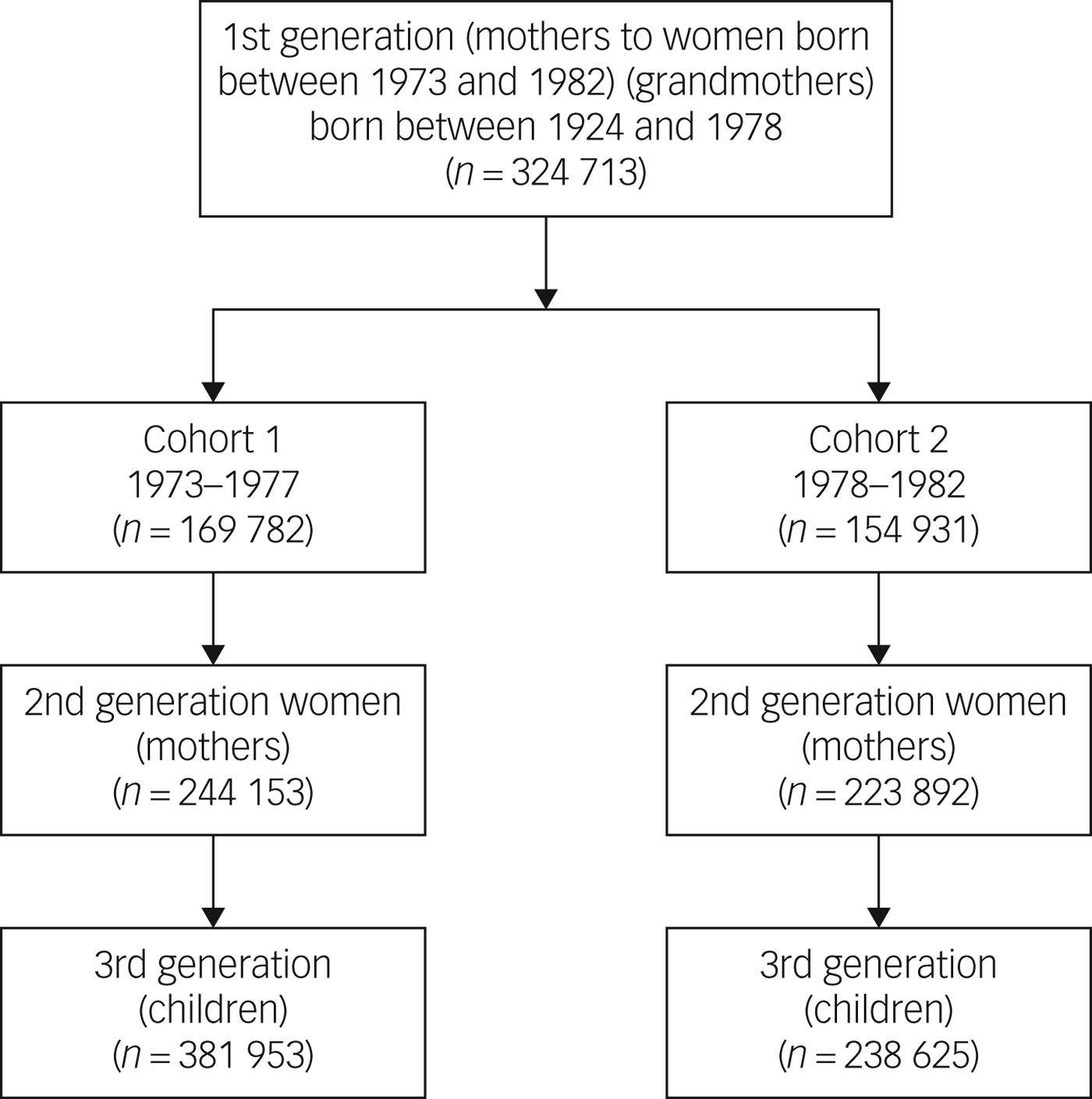
Fig. 1 Flow chart of study participants in the three different generations.
Ethical approval was received from the Regional Ethical Review Board, Linköping, Sweden (no. 03-556, 03-557, 07-M66 08-08-M 233-8 and 2014/112-31). Informed consent is not applicable.
Registers
The information on the first- and second-generation women (grandmothers and mothers) as well as the third-generation children included in this study was retrieved from Swedish population-based registries. All Swedish residents are given unique personal identification numbers, which allow us to individually link the information in the different registers.
Information on the study participants in this study, first- and second-generation women as well as third-generation children, was retrieved from Swedish population registers. Each and every person residing in Sweden is provided a personal identification number allowing linkage of information in different registers. The registers used in this study were:
(a) The Swedish medical birth register (MBR): the MBR is held by the Swedish National Board of Health and Welfare and covers 97–99% of births.11 The register contains medical information pertaining to all births since 1973 and onwards, including prenatal care, delivery and neonatal care.
(b) The total population register (TPR) and the multi-generation register: the TPR is held by Statistics Sweden and was established in 1968.12, 13 The register contains information on variables such as births, deaths, migrations and marital status.
(c) The education register and the population and housing census: since 1985, Statistics Sweden has continuously collected information on the educational level of the population in the Education Register.14–16
(d) The national patient register (NPR): the NPR was originally established in 1964. Its main focus is on psychiatric diagnoses. From 1987 all in-patient visits have been included, and in 2001 out-patient visits were added to the register.Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall17, 18
These registers have all been previously evaluated.11, 16–Reference D'Amico, Agozzino, Biagino, Simonetti and Marinelli21 The Inpatient Register was most recently evaluated by Ludvigsson et al in 2011, where it was concluded that the register is of good quality with a high validation rate.Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim and Reuterwall17 Similarly, the education register, the MBR, the TPR and the cause of death register have been evaluated and deemed to be of high quality.
Diagnoses
In order to identify individuals with MDD in the three generations, psychiatric in- and out-patient care diagnoses were collected from the NPR. The depression diagnoses are based on the Swedish version of ICD from the World Health Organization (WHO).22 Between 1969 and 1987 ICD-8 was in use. In 1997, the healthcare system changed so that ICD-9 to ICD-10 were used. During 1997, ICD-9 and ICD-10 were used interchangeably. MDD diagnoses in ICD-8 were identified as codes 296.00–296.09, 296.20–296.29, 298.00–298.09, in ICD-9 as codes 296B, 296X, 298A, 300E, 311-and in ICD-10 codes F32–33. WHO conversion tables were used to translate ICD-10 codes into ICD-9 codes and then ICD-8 codes.23 Adjustments in the translation between ICD versions were performed by three of the authors, two medical doctors (A.J. and J.V.) and a behavioural scientist (G.S.) in order to only include MDD diagnoses.
Definitions
The variable ‘MDD diagnosis’ was divided into two categories according to ICD-10, diagnosis present and diagnosis not present. For the grandmothers (first-generation women), the included sociodemographic variables were: parity (no previous children/previous children), highest attained level of education (elementary, high school and graduate/postgraduate), region of origin (Nordic (Sweden, Norway, Finland, Denmark, and Iceland)/non-Nordic (all other countries)), marital status (married, unmarried and divorced/widowed), age at childbirth (<20 years, 20–26 years, 27–33 years and >33 years). The same set of sociodemographic factors were collected for the mothers (second-generation women) except for region of origin since the MBR is restricted to births occurring in Sweden.
For the third-generation children, data on the total number of out- and in-patient visits for any cause of morbidity were also collected. A cut-off of incidences of 0–1 visits/≥2 visits for in-patient data and 0–10 visits/≥11 visits for out-patient data was determined arbitrarily. These served as proxies for general morbidity among the third-generation children.
Statistical analysis
In order to examine the risk of receiving an MDD diagnosis among the second-generation women (mothers) and third-generation children, Pearson's χ2 analyses were performed to examine bivariate differences. Data was also analysed using unadjusted as well as adjusted logistic regression analyses in order to estimate the odds ratio (OR) and the corresponding 95% confidence interval of being diagnosed with MDD. The dependent variable was the presence of MDD diagnoses, as well as number of in- and out-patient visits among the third-generation children. The independent variables included in the analyses were educational level, region of origin, marital status, age when gave birth and indicator of an MDD diagnosis in the previous generation(s). All analyses were stratified by the mothers' (second-generation women) year of birth, where cohort 1 pertained to women born between 1973 and 1977, and cohort 2 to women born between 1978 and 1982. This stratification was as a result of the fact that the follow-up of the grandchildren (third-generation children) of those in the older cohort was more complete (cohort 1 were nearing the end of their reproductive era, and their children were, on average, somewhat older allowing for a more complete collection of data) than for those in the younger cohort. A P < 0.05 (two-sided) was considered statistically significant. All analyses were performed using SPSS, version 22.0.
Results
A total of 17 413 of 324 713 (5.4%) grandmothers (first generation) had been diagnosed with an MDD; 8724 of 169 782 (5.1%) in cohort 1 and 8689 of 154 931 (5.6%) in cohort 2. Among their daughters, the second-generation women, 32 221 of 468 045 (6.9%); 15 880 of 244 153 (6.5%) in cohort 1 and 16 341 of 223 892 (7.3%) in cohort 2 had been diagnosed with an MDD. Finally, among the ‘third-generation children’, 2033 of 381 953 (0.5%) in cohort 1, and 273 of 238 625 (0.1%) in cohort 2 had been diagnosed with MDD. Of these of 381 953 ‘third-generation children’, a total of 65 838 had had more than ten out-patient visits (median 4, range 1–261) and 53 649 had had two or more in-patient visits (median 4, range 1–504), as presented in Table 1.
Table 1 Descriptive information on major depression diagnosis and hospital visits for the three generations included in the study

a. All mothers were born between 1973 and 1982.
b. All children were born between 1987 and 2012.
c. All grandmothers were born between 1924 and 1978.
In Table 2, sociodemographic background data are presented for the first-generation women (grandmothers), and all women (second generation), whether or not they had had any children during the study period. Both cohorts of grandmothers (first generation) diagnosed with an MDD had a lower level of education and were more likely to have been divorced or widowed compared with those without an MDD diagnosis. The first-generation women diagnosed with an MDD were also younger when they gave birth. Moreover, the second-generation women diagnosed with an MDD had a lower level of education, were more likely to be either widowed or divorced, and were less likely to have given birth to at least one child, although those who had had a child were of a younger age compared with second-generation women not diagnosed with an MDD.
Table 2 Sociodemographic characteristics of the study participants born between 1973 and 1982 and who had and had not become mothers
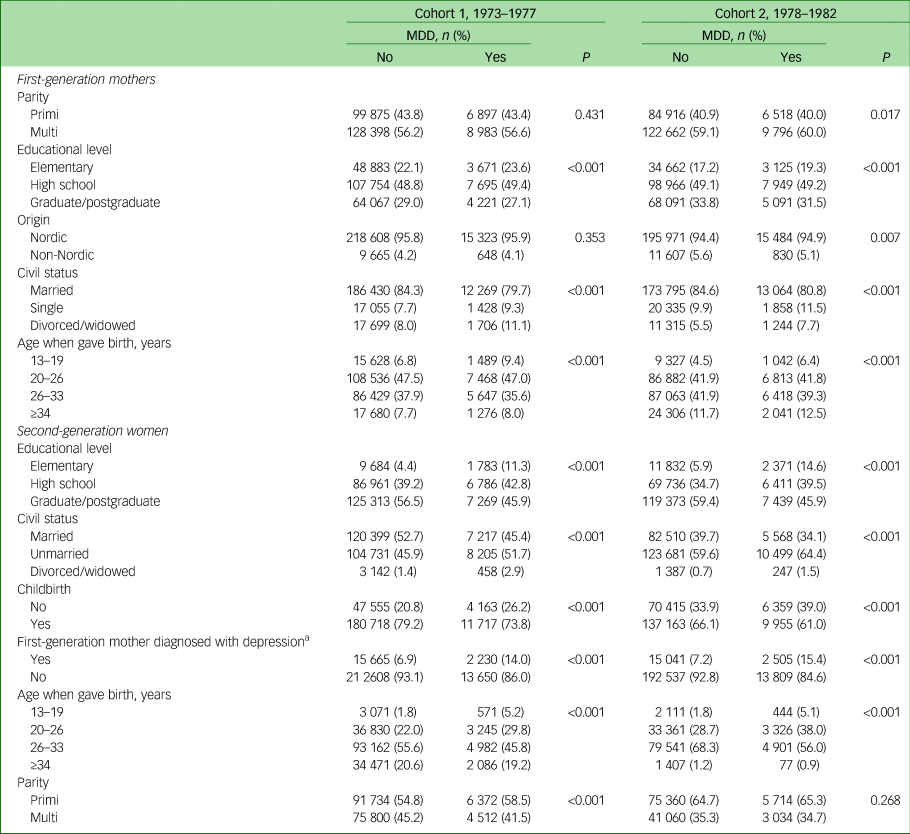
MDD, major depressive disorder.
a. This number does not add up to the number presented in Table 1 since each first-generation woman may have had more than one child.
In addition, women (second generation) diagnosed with an MDD had mothers (first-generation women) who to a greater extent had been diagnosed with an MDD, but they had also had more frequent visits to the hospital because of their MDD compared with women (second generation) whose mothers (first-generation women) were not diagnosed with an MDD.
The same findings were present when we limited the study population to only include second-generation women who had become mothers during the study period (Table 3). Moreover, mothers (second-generation women) diagnosed with MDD more often had children who had had multiple visits to the hospital, both as in- and out-patients, compared with mothers (second generation) without MDD diagnoses. Children of mothers (second-generation women) diagnosed with an MDD were also diagnosed with an MDD to a greater extent than those with mothers (second-generation women) without an MDD diagnosis.
Table 3 Sociodemographic characteristics of the study participants, limited to women born between 1973 and 1982 who had become mothers
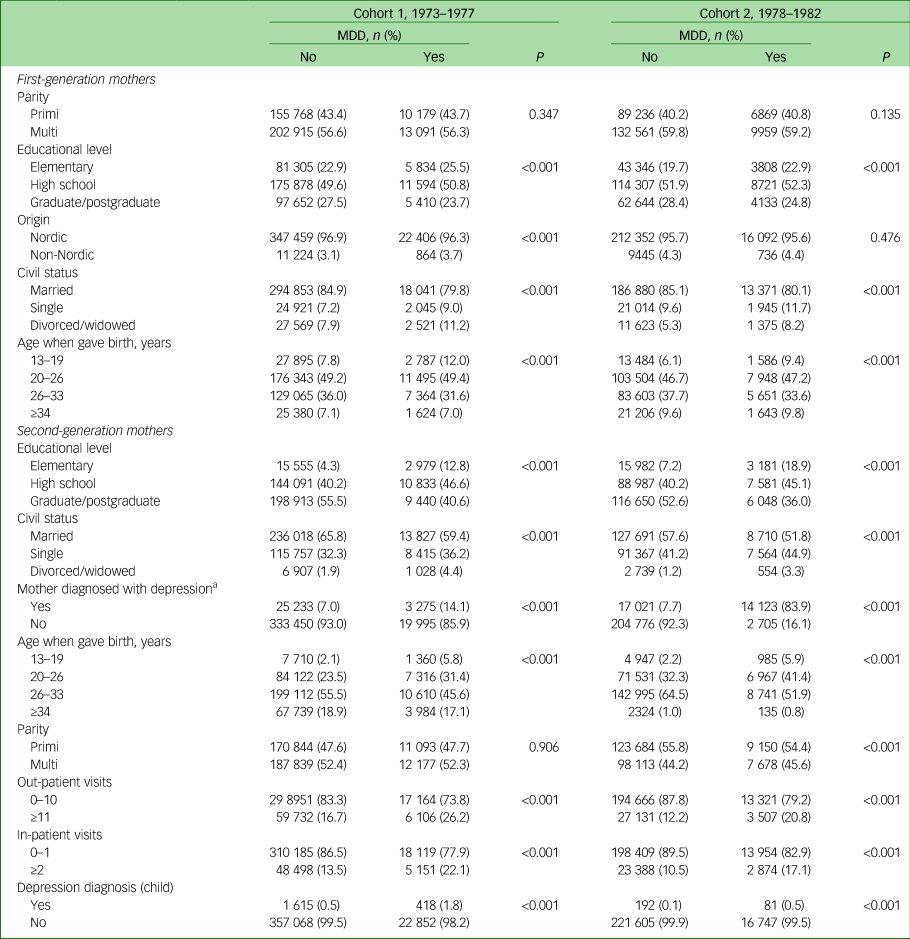
MDD, major depressive disorder.
a. This number does not add up to the number presented in Table 1 since each first-generation woman may have had more than one child.
The overall mean age of the third-generation children was 7.40 (s.d. = 4.66) in cohort 1 and 4.36 (s.d. = 3.48) in cohort 2, whereas the mean age of the children who had received an MDD diagnosis was 16.06 (s.d. = 2.80) in cohort 1 and 13.39 (s.d. = 2.36) in cohort 2.
In studying the transmission of depression, data were analysed through unadjusted and adjusted logistic regression. The unadjusted logistic regression revealed that if the grandmother (first-generation women) had been diagnosed with MDD the odds ratio of the mothers (second-generation women) being given an MDD diagnosis was more than twice as high (1973–1977: OR = 2.13, 95% CI 2.02–2.25; 1978–1982 OR = 2.35, 95% CI 2.23–2.47 for cohort 1 and cohort 2, respectively) (Table 4). Moreover, third-generation children whose mothers (second-generation women) and/or grandmothers (first-generation women) had been diagnosed with an MDD had a higher odds ratio of having two or more out-patient visits, and a higher odds ratio of having more than ten out-patient visits and for being diagnosed with MDD in both cohorts when compared with third-generation children where none of the women in the previous two generations had been diagnosed with an MDD. Also, children in the third generation exhibited a substantially increased odds ratio for being diagnosed with an MDD if both first- and second-generation women had been diagnosed with an MDD (OR = 5.07, 95% CI 4.06–6.34; OR = 7.20, 95% CI 4.41–11.77 in cohort 1 and cohort 2, respectively) compared with third-generation children where none of the women in the first or second generations had been diagnosed with an MDD. Although adjusting for sociodemographic factors reduces the odds ratios somewhat, the increased likelihood still remains statistically significant (Table 5).
Table 4 Unadjusted odds ratios and corresponding 95% confidence intervals on the intergenerational effect of depressive disorder in three generationsa
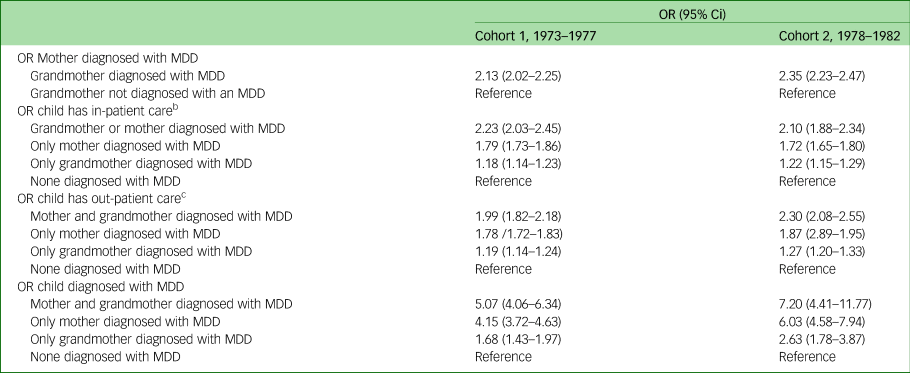
MDD, major depressive disorder.
a. All women were born between 1973 and 1982. All children were born between 1987 and 2012. All mothers were born between 1924 and 1978.
b. In-patient care categorised into 0–1 visits and ≥2 visits.
c. Out-patient care categorised into 0–10 visits and ≥11 visits.
Table 5 Adjusted odds ratios and corresponding 95% confidence intervals on the intergenerational effect of depressive disorder in three generationsa
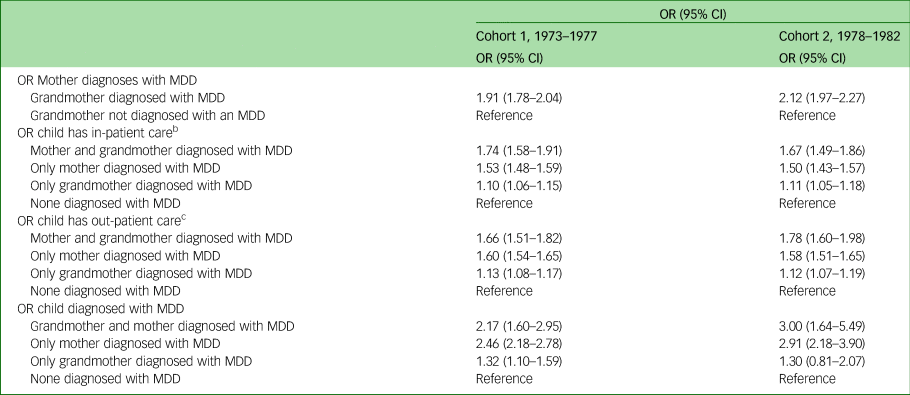
a. All women were born between 1973 and 1982. All children were born between 1987 and 2012. All mothers were born between 1924 and 1978.
b. In-patient care categorised into 0–1 visits and ≥2 visits.
c. Out-patient care categorised into 0–10 visits and ≥11 visits.
To further elucidate the relationship of the increased odds ratio for a third-generation child having received an MDD diagnosis and having a mother (second-generation woman) and/or grandmother (first-generation women) diagnosed with an MDD, data was stratified by age of diagnosis of the child. This analysis revealed that in both cohorts there appeared to be a dose–response effect, in both of the age groups, for the risk of the third-generation child being diagnosed with an MDD. The lowest risk was present when only the grandmother (first generation) had been diagnosed with an MDD (cohort 1: OR = 1.12, 95% CI 1.07–1.18 and OR = 1.15, 95% CI 1.07–1.24, <13 and ≥13 years old, respectively; cohort 2: OR = 1.19, 95% CI 1.12–1.26 and OR= 1.22, 95% CI 0.98–1.52, <13 and ≥13 years old, respectively) and the highest risk was present if both mother (second generation) and grandmother (first generation) had been diagnosed with an MDD (cohort 1: OR = 1.90, 95% CI 1.68–2.14 and OR = 1.96, 95% CI 1.68–2.28, <13 and ≥13 years old, respectively; cohort 2: OR = 2.00, 95% CI 1.78–2.24 and OR = 1.85, 95% CI 1.29–2.63, <13 and ≥13 years old, respectively) (Table 6).
Table 6 Unadjusted odds ratios and corresponding 95% confidence intervals on the intergenerational effect of depression in three generations, stratified by age of the child (third generation)

MDD, major depressive disorder.
Discussion
Main findings
This large three-generation, nationwide population-based retrospective cohort study shows a strong intergenerational impact on the transmission of MDD. Mothers (second generation) are more than twice as likely to be diagnosed with MDD if their mothers (first generation) have received such a diagnosis. Although the presence of MDD in the first generation increases the risk of MDD for the children of the third generation, the risk is even higher if MDD is present in the second generation. Furthermore, the risk is highest in the third-generation children who have a mother (second generation) as well as a grandmother (first generation) with MDD history. These children also have an increased overall consumption of specialised healthcare indicating a higher degree of ill health. Hence, it appears that there is a dose–response effect in the transmission of depression but also that the strength of transmission decreases with an increasing number of generations.
Comparison with findings from other studies and interpretation of our findings
In the present study, 5.4% of the grandmothers (first generation) and 6.5% of the mothers (second generation) had, at some point in their lives, received an MDD diagnosis. Some community-based studiesReference Rorsman, Grasbeck, Hagnell, Lanke, Ohman and Ojesjo24, Reference Moffitt, Harrington, Caspi, Kim-Cohen, Goldberg and Gregory25 have found lifetime MDD prevalence rates as high as 45% in women, whereas other studies have found these rates to be about 14%.Reference Bromet, Andrade, Hwang, Sampson, Alonso and de Girolamo5 In their study, Bromet et al Reference Bromet, Andrade, Hwang, Sampson, Alonso and de Girolamo5 commented on the large variations in rates between studies and between countries, noting that rates are higher in high, compared to low, income countries. According to Ebmeier et al,Reference Ebmeier, Donaghey and Steele1 one-sixth of individuals will experience an MDD episode during their lives of which only 25–50% will seek healthcare. Thus, the relatively low rates found in the present study are probably explained by the fact that these individuals have actually sought healthcare, and are further reduced because the NPR only covers specialised, and not general, care. Even though the oldest mothers (second generation) were only 40 years of age at the end of the study period, the cumulative prevalence rates were higher in these women compared with the lifetime prevalence rates of the women in the first generation. This is probably because of an increased acceptance and decreased stigmatisation of mental disorders and thus an increased tendency in these women to seek psychiatric care.
The rates of MDD diagnoses among the children (third generation) were 0.5% in the older cohort 1 and 0.1% in cohort 2. The reason for these low prevalence rates was probably that the children of the third generation were still very young. The mean age in cohort 1 was about 7 years, and in cohort 2 it was 4 years. Costello et al Reference Costello, Mustillo, Erkanli, Keeler and Angold26 estimated that the cumulative prevalence rate of any depression disorder would be 9.5% before 16 years of age. Meanwhile, Domènech-Llaberia et al Reference Domènech-Llaberia, Vinas, Pla, Jane, Mitjavila and Corbella27 found in their community-based study on 1427 children aged 3–6 years that 1.12% met MDD criteria. The differences between these results and those of the present study are of course, once again, that the children in the present study had sought and been diagnosed in specialist psychiatric care.
The present study also showed that grandmothers (first generation) and mothers (second generation) with an increased incidence of MDD had a lower educational level and gave birth at a younger age, which might demonstrate a greater socioeconomic burden. This might either be an effect of the depressive morbidity itself or a contributing factor. It is also possible that mothers with a lower socioeconomic status are more prone to depression.
Our finding that maternal MDD is associated with an elevated risk for MDD diagnoses among the children is consistent with the results of earlier studies on parental depression.Reference McAdams, Rijsdijk, Neiderhiser, Narusyte, Shaw and Natsuaki8–Reference Weissman, Wickramaratne, Nomura, Warner, Verdeli and Pilowsky10 In addition, the results of the present study are partially in accordance with those seen in the three-generation interview study on the familial aggregation of psychiatric disorders conducted by Weissman et al.Reference Weissman, Berry, Warner, Gameroff, Skipper and Talati9 Both studies have found the risk of MDD to be highest in third-generation children if both previous generations have been afflicted by depression. Taken together, the results indicate the importance of screening for family history beyond the parental generation.
Transmission of MDD risk across generations is likely to be the result of a combination of genetic, biological and psychosocial factors. In a three-generation study by Hammen et al,Reference Hammen, Shih and Brennan28 information on MDD diagnoses in the first generation of women was retrieved through accounts from the second-generation women. They found that transmission of depression across generations was mediated by interpersonal stress, family discord and parenting perceived negatively by the younger generations. In addition, previous studies have shown that maternal depression can have negative effects on the development and mental health of children.Reference Goodman, Rouse, Connell, Broth, Hall and Heyward29, Reference Stein, Pearson, Goodman, Rapa, Rahman and McCallum30 In a 10-year follow-up study on offspring and parents with depression and panic disorders, Hirsfeld-Becker and colleaguesReference Hirshfeld-Becker, Micco, Henin, Petty, Faraone and Mazursky31 found that parental depression predicted mood disorders, more impaired functioning and psychiatric hospital admission for the children.
Strengths and limitations
The national registers on which this study is based are validated and reliable, with a high level of coverage and thus allow for studies on large populations while adjusting for important confounding factors. Another strength of register-based studies is that the risk of recall bias is circumvented. However, a major limitation of this study is the lack of information on diagnoses from general practice, where, of course, many of those with less severe MDD are treated, and the incomplete information on out-patient psychiatric care diagnoses prior to 2001. In addition, only women are included in the MBR; hence, the results of the present study are only generalisable to MDD in women that require psychiatric care. Another limitation of this study is the lack of information on paternal mental health and comorbidity, which might have served as confounding factors. Finally, large populations also confer the risk of finding significant differences that do not have clinical relevance. In addition, the children of the third generation were still very young; hence, further studies are needed to ascertain that the results from the present study are still valid as the third-generation children age. Since this is only the second study on transmission of MDD across three generations, and the first study based on psychiatric diagnoses, more studies are needed to verify the findings of this study.
Implications
In conclusion, this study indicates that there is a strong generational impact in the transmission of MDD. The risk of MDD and an increased overall consumption of specialised psychiatric care is especially high in individuals with MDD in both previous maternal generations. There is evidence that screening for family history should extend further than the parental generation.
Acknowledgements
We thank Professor Lawrence Lundgren for language corrections.










eLetters
No eLetters have been published for this article.