INTRODUCTION
Group B streptococcus (GBS, Streptococcus agalactiae) was recognized as a major cause of neonatal sepsis and meningitis in the 1970s [Reference Baker1], but the first Korean case of GBS neonatal meningitis was not described until 1984. Since then, the number of reported cases of neonatal GBS disease has increased steadily [Reference Yoon2].
Neonatal sepsis usually develops within 3 days after birth, and the most frequent causative organism is GBS [Reference Wright Lott3]. Maternal colonization is the primary risk factor; but only 1–2% of babies that become infected develop GBS disease. GBS serotype, neonatal birth weight, and the immune status of the neonate or of the mother during pregnancy modify disease risk [Reference Fischer4]. Asymptomatic colonization with GBS is common worldwide, with estimates from vaginal and rectal sampling ranging from 15–30% depending on the population [Reference Schrag5]. Screening is not standard for care in all Korean hospitals, and the few published reports suggest GBS colonization is considerably lower than elsewhere, ranging from 0·3 to 5·9% [Reference Uh6–Reference Choi8]. However, some of these reports did not use GBS-selective media, and urine specimens were not included.
Penicillin is the intrapartum prophylactic antibiotic of choice for prevention of GBS-induced neonatal sepsis. In pregnant women with penicillin hypersensitivity, erythromycin or clindamycin are recommended; however, since the 1990s, resistance to these two antibiotics has increased significantly [Reference Uh6, Reference Uh9, Reference DiPersio and DiPersio10] exceeding 50% in Korea [Reference Kim7].
The incidence rate of clinically diagnosed neonatal sepsis in Korea of 30·4/1000 live births [Reference Shin11] is significantly higher than the 2/1000 reported for the USA [Reference Sinha12]. However, the reported prevalence of GBS in pregnant women in Korea is low. To help understand this discrepancy, we screened 2624 Korean pregnant women at 35–37 weeks gestation for GBS.
METHODS
Subjects
All consenting pregnant women of 35–37 weeks of gestation seen in Daejeon, at the Eulji University Hospital or the Mote Obstetrics and Gynecology (OBGY) Clinic, or in Seoul, at the Eulji General Hospital or Cheil Women's Hospital between January 2006 and May 2008 who had routine prenatal testing were included. GBS was cultured and identified in the Departments of Laboratory Medicine of the Eulji hospitals in Seoul and Daejeon and, for samples obtained at Cheil Hospital, in the Seoul Clinical Laboratory. The laboratory also carried out antimicrobial resistance testing. The Department of Preventive Medicine of Eulji University performed GBS laboratory work for the Mote OBGY Clinic in Daejeon. The Institutional Review Boards at the Eulji (04-08 and 06-25) and Cheil (SCH-IRB-2005-24) hospitals approved the study protocol. Written informed consent permitting use of the sample materials and medical records for research purposes was obtained from every study participant.
GBS isolates
GBS collection
Physicians collected vaginal mucus or discharge with a swab from the vaginal introitus without inserting a speculum, and placed the swab into Stuart's transport medium. A swab was inserted through the anal sphincter, rotated two or three times, and placed into a separate container of transport medium. Urine samples were self-collected specimens of the first 20 ml of urine. All participating laboratories used the same protocols for GBS incubation and identification. Media and reagents were purchased by the combined research team and distributed to each participating laboratory.
GBS culture
Todd–Hewitt broth supplemented either with gentamicin (8 μl/ml) and nalidixic acid (15 μl/ml), or with colistin (10 μl/ml) and nalidixic acid (15 μl/ml) was used to repress the growth of microorganisms other than GBS. Urine samples were centrifuged, and 1 ml of the sedimented sample was placed into the selective medium. Rectal and vaginal swabs were removed from the transport medium and used to inoculate the selective broth medium. Cultures were shaken three or four times to ensure adequate mixing of the analyte. The lids of the culture tubes were loosely closed and the cultures incubated, along with a negative control, for 18–24 h at 35–37°C in ambient air containing 5% CO2. If the medium in the tubes was still clear after 18–24 h, the cultures were reincubated and inspected again at 48 h. Specimens with evident bacterial growth were subcultured on plates containing sheep blood agar (tryptic soy agar with 5% defibrinated sheep blood; TSAII; KOMED Co., Korea).
GBS identification
GBS was identified using the latex agglutination assay (Streptex; Murex Biotech Ltd, UK) and the catalase test.
Antimicrobial resistance
Positive GBS samples were tested for antibiotic resistance by culturing samples of the bacteria on disks containing penicillin G, ampicillin, erythromycin, clindamycin, cefazolin (Sigma Chemical Co., USA), or vancomycin (Daewoong Lilly, Korea) in Muller–Hinton agar (Muller-BAP: KOMED Co., Korea). The size of the inhibitory zone was observed after 18–36 h incubation. Guidelines of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) were used to interpret disk diffusion test. Since the CLSI has not established a resistance standard for cefazolin, the following criteria were used: S⩾28 mm; I=26−27 mm; R⩽25 mm, as previously defined [Reference Foxman and Riley13].
Storage
A 1·8-ml storage tube was filled with 0·75 ml Todd–Hewitt broth, inoculated with two or three pure-cultured GBS colonies, and incubated for 18–24 h at 35–37°C in ambient air containing 5% CO2. Then, 0·75 ml of glycerol was added to the samples, which, after adequate mixing, were frozen at −70°C for storage.
GBS serotyping
GBS serotype was determined using hyperimmune rabbit antisera (Essum, Sweden) against serotypes Ia, Ib, and II–VIII of the capsular polysaccharide of GBS. Capsular serotypes of strains that were non-typable by antigen-antibody precipitation were determined by microarray methods [Reference Borchardt14, Reference Zhang15].
Statistical analysis
All statistical analyses were done using SPSS 14.0 statistical software (SPSS Inc., USA). The GBS prevalence rate by area and specimen, the serotype, and the antimicrobial resistance of the bacteria were tested for statistical significance using the χ2 test.
RESULTS
Colonization rate
A total of 7710 specimens were collected from the urine, vaginal introitus, and anorectal area of 2624 pregnant women at four hospitals in Seoul and Daejeon. From these, 352 GBS isolates were obtained from 211 women (8·0% of the total, Table 1). Prevalence of colonization varied significantly by hospital (P<0·001) with the highest prevalence (10·5%) observed at Cheil Hospital in Seoul (Table 1).
Table 1. Group B streptococcus (GBS) collection characteristics and prevalence (%) in pregnant womenFootnote * (35–37 weeks) of S. Korea (2006–2008)
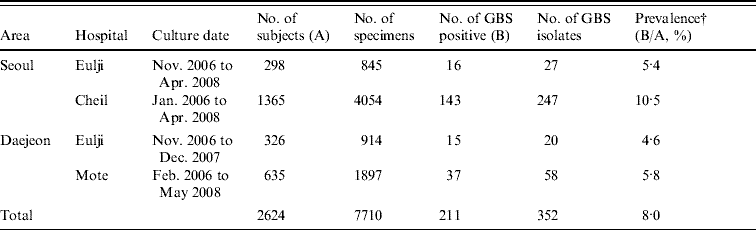
* Pregnant women presenting at each hospital for prenatal care.
† P value obtained by χ2 test was <0·001.
Colonization occurred most frequently in urine (6·2%), significantly (P<0·001) higher than in the vaginal introitus (4·1%) and anorectal specimens (3·4%, Table 2). All three specimen types could not be collected from all participants. Of the 2526/2624 (96%) pregnant women who contributed all three specimens, more than one positive culture was obtained from 204 (8·1%); 36/204 (17·6%) were positive in all three specimens.
Table 2. Prevalence (%) by area, hospital and site of collection. Group B streptococcus isolates collected from pregnant women (35–37 weeks) in S. Korea (2006–2008)
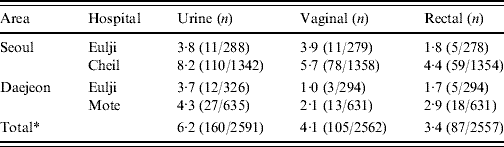
* P value obtained by χ2 test was <0·001.
Serotype distribution
The most common serotypes were III (43·8%), V (20·3%), Ia (12·1%), Ib (9·5%), with 1·6% being non-typable. None of the isolates belonged to serotype IV. The serotype distribution differed by anatomical site (P=0·03, Fig. 1), and study site: serotype V was more common in Seoul than Daejeon (22·9% vs. 13·5%) and serotype III was more frequent in Daejeon (56·9 vs. 40·7%).
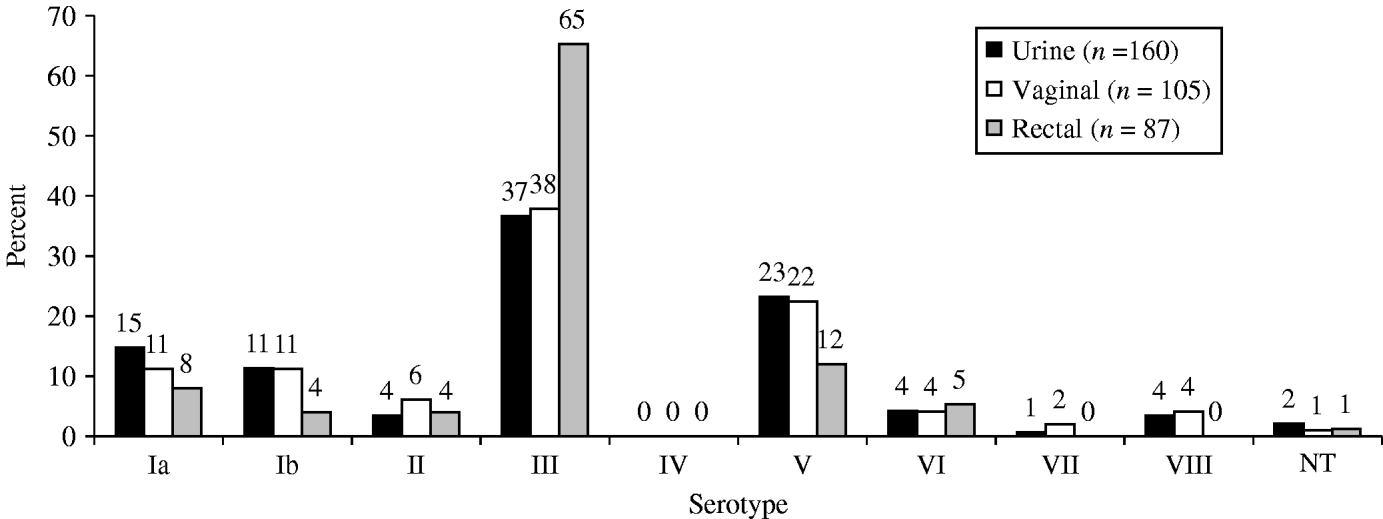
Fig. 1. Serotype distribution by site of collection. Group B streptococcus isolates collected from pregnant women (35–37 weeks) in S. Korea (2006–2008).
Antibiotic resistance
None of the GBS isolates were resistant to penicillin G, ampicillin or vancomycin, and there was no significant difference in resistance between Seoul and Daejeon. Cefazolin resistance was observed in 13 (3·7%) of the isolates. Clindamycin resistance was detected in 54·0% of the isolates and 25·6% of the isolates showed erythromycin resistance. All isolates resistant to erythromycin were also resistant to clindamycin, except in two cases (Table 3).
Table 3. Antimicrobial resistance (%) by site collected. Group B streptococcus isolates collected from pregnant women (35–37 weeks) in S. Korea (2006–2008)

R, resistance; I, intermediate.
* Two isolates were missed.
† All but two isolates resistant to erythromycin were also resistant to clindamycin.
Resistance was associated with serotype: more than three quarters of serotype V isolates were resistant to clindamycin or erythromycin or both. All isolates susceptible to erythromycin and resistant to clindamycin were of serotype III (Table 4).
Table 4. Antimicrobial resistance (%) by serotype. Group B streptococcus isolates collected from pregnant women (35–37 weeks) in S. Korea (2006–2008)Footnote *
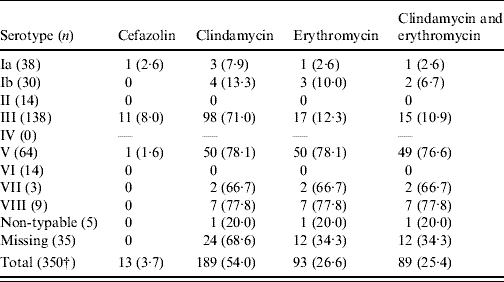
* All isolates were susceptible to ampicillin, penicillin G and vancomycin.
† Two isolates were missed.
DISCUSSION
GBS colonization is reportedly low in Asia, and especially Korea (0·3–5·9%) [Reference Uh6–Reference Choi8]. Therefore, even though the incidence of neonatal sepsis in Korea is much higher than in other countries [Reference Shin11] there are no guidelines for GBS prevention. Using media selective for GBS, we found a colonization rate of 8% (4·6–10·5% depending on the hospital), predominance of the more virulent serotypes (serotypes III and V), and very high levels of antimicrobial resistance (54·0% for clindamycin, 25·6% for erythromycin, 3·7% for cefazolin). The higher prevalence of colonization observed in our study compared to previous reports probably reflects the large sample size (2624 pregnant women in four hospitals), use of selective media, and inclusion of urine samples with vaginal and anorectal swabs for GBS culture. Although the colonization prevalence is still much lower than in USA and Europe, the high rates of resistance suggest that GBS is an emerging problem in Korea, and that screening measures and chemoprophylaxis guidelines regarding GBS infections, especially in pregnant women should be established.
The GBS prevalence rate of 8·0% detected in our study is similar to that in neighbouring countries: 8·2% in Japan, 11·2% in Taiwan and 12% in India and Pakistan [Reference Stoll and Schuchat16–Reference Matsubara18]. However it is lower than the 26% found in the USA and 10–36% in Europe [Reference Trijbels-Smeulders19]. In a study from The Netherlands prevalence differed by country of origin: 13% in Asia, 21% in Europe, and 29% in Africa [Reference Valkenburg-van den Berg20].
The distribution of GBS serotypes found in our study is similar to that reported for USA and Canada, with Ia, III and V predominating [Reference Davies21, Reference Edwards and Baker22], and to Taiwan, although in Japan serotypes VI and VIII are most common [Reference Lachenauer23]. This is in contrast to the serotype distribution reported for Korean isolates collected from general hospital laboratories in the 1990s, when serotypes Ib and III were the most prevalent, and serotype V accounted for only 11% [Reference Uh9, Reference Lee24].
We found a higher rate of resistance to clindamycin and a lower rate of resistance to erythromycin than reported for isolates collected from general hospital laboratories between 1990–2002 in Korea (54·0 vs. 35·0% for clindamycin, and 25·6 vs. 30·0% for erythromycin, respectively) [Reference Uh9]. The resistance rate reported in the current study is still much higher than found in pregnant women in the USA and Canada [Reference DiPersio and DiPersio10, Reference Fernandez25, Reference Morales26]. We found no previous reports of resistance to first-generation cephalosporins in Korea [Reference Lee24, Reference Uh27]. The prevalence found here, of 3·7% to cefazolin is similar to that found in the USA (2–4%) [Reference Berkowitz28, Reference Chohan29] and Japan (5·1%) [Reference Morikawa30]. Similar to previous reports from the USA, GBS serotypes III and V were more likely to be resistant to clindamycin and erythromycin [Reference Manning31]. However, a previous report from Korea did not observe an association between serotype III and high rates of erythromycin and clindamycin resistance [Reference Uh9].
The frequency of maternal GBS colonization and the incidence of early-onset GBS infection in newborns varies by country, influencing the choice of GBS prevention strategy. In the USA, a screening based strategy is used to prevent as many GBS neonatal infections as possible [Reference Schrag5]. However, in many European countries, a risk-factor-based strategy is used, as it avoids the increased risk to the infant of allergic reactions later in life, the overgrowth of penicillin-resistant pathogens, and maternal anaphylaxis caused by penicillin allergy [Reference Valkenburg-van den Berg20, Reference Pettersson32]. In Korea, no guidelines exist for GBS prevention and no data for the risk of GBS colonization; given the high rates of sepsis, and increasing GBS colonization, it is time for Korea to implement a screening-based strategy to prevent GBS neonatal infections.
ACKNOWLEDGMENTS
We thank SCL (Seoul Clinical Laboratories & Seoul Medical Science Institute), and the following physicians for providing some of the isolates used in this study: Ji-Hee Yu (Motae Women's Hospital, Daejeon), Jung Yeol Han, Hyun-Mi Ryu, Hyun-Kyong Ahn, Jin-Hun Jung, Jun-Sik Choi and Min-Hyung Kim (Cheil General Hospital & Women's Healthcare Center, Kwandong University, Seoul); Jun-Suk Park, In-Tack Hwang, and Jung-Hun Rho (Eulji University Hospital, Daejeon); and Seo-Yoo Hong and Won-Il Park (Eulji Hospital, Seoul). This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD), (KRF-2005-015-E00106).
DECLARATION OF INTEREST
None.







