Introduction
Multiple sclerosis (MS) is the most common disabling neurological condition affecting young adults.Reference Leary, Porter and Thompson 1 Prevalence estimates in Canada range from 177 to 267 cases per 100,000.Reference Kingwell, Zhu and Marrie 2 , Reference Marrie, Fisk and Stadnyk 3 The female-to-male ratio is approximately 3:1.Reference Kingwell, Zhu and Marrie 2 The mean age at symptom onset is 32 years, although diagnosis is typically delayed for 3-5 years. 4
The clinical signs and symptoms of MS are the result of autoimmune dysregulation, in which activated T and B cells cross the blood-brain barrier into the central nervous system (CNS) and release inflammatory mediators that result in demyelination and axonal loss.Reference Trapp, Peterson, Ransohoff, Rudick, Mork and Bo 5 Known risk factors for the development of MS include the HLA-DRB*1501 allele, which is most common in individuals of northern European descent; lower sun exposure and/or lower serum vitamin D levels; cigarette smoking; and exposure to the Epstein-Barr virus (EBV) or other herpes viruses during adolescence.Reference McKay, Jahanfar, Duggan, Tkachuk and Tremlett 6
The clinical hallmarks of relapsing-remitting MS (RRMS) are relapses, defined as onset or exacerbations of neurological signs/symptoms persisting for at least 24 hours, not attributable to elevated body temperature (due to fever, infection, heat exposure), and not better explained by an alternative diagnosis. Typically, clinical symptoms related to relapses evolve over days to weeks, followed by varying degrees of recovery. About 85% of patients present with RRMS, in which neurological exacerbations alternate with periods of remission; recovery may be complete or partial. In untreated patients with RRMS, the frequency of relapses diminishes over time, but a majority of patients will eventually develop secondary-progressive MS (SPMS),Reference Trojano, Paolicelli, Bellacosa and Cataldo 7 in which there is ongoing neurodegeneration with few or no relapses. In contemporary cohorts, there is evidence that earlier diagnosis and initiation and maintenance of disease-modifying therapies (DMTs) may reduce disability in the long term and the proportion of RRMS patients reaching SPMS,Reference Ford, Goodman and Johnson 8 , Reference Kappos, Kuhle and Multanen 9 but this is controversial because of the inherent biases of observational cohorts. About 10%-15% of patients present with primary-progressive MS (PPMS), where there is progressive neurological worsening from onset.
Over the past two decades, the treatment paradigm for people living with RRMS has changed markedly, partly owing to ever-increasing DMT options, but also owing to improved understanding of the pathophysiology of MS. Currently, 11 DMTs are available in Canada to target different aspects of the dysfunctional immune response seen in RRMS (Table 1). In Canada, treatment initiation is largely restricted to neurologists specialized in MS care at one of the country’s 32 MS clinics (see Appendix 1).Reference Marriott, Mamdani, Saposnik, Gomes, Manno and O’Connor 10 Long-term treatment is generally recommended throughout the relapsing-remitting phase of the disease, along with periodic safety monitoring.
Table 1 Disease-modifying therapies approved for use in relapsing-remitting multiple sclerosis (MS)
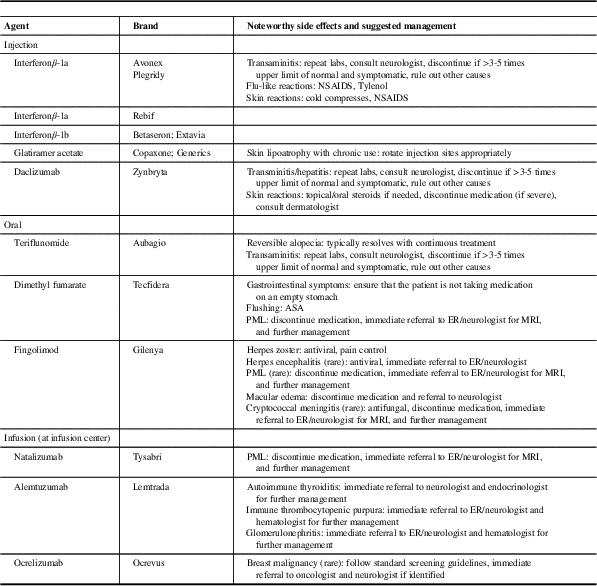
ASA=acetyl-salicylic acid; ER=emergency room; NSAIDS=non-steroidal anti-inflammatory drugs; PML=progressive multifocal leukoencephalopathy.
Primary care physicians (PCP) can play a central role in the ongoing management of MS. Although no shared-care model that includes PCPs has yet been developed for MS, given the increasing complexity of chronic management of MS patients, it is apparent that such a model would greatly benefit patients. The following is a proposal outlining how PCPs can more fully participate in the care path to meet the complex, multidisciplinary healthcare needs of their MS patients, particularly in the evolving modern era of MS treatment.
Early Detection
Multiple sclerosis can affect many areas of the CNS and can present clinically in myriad ways. Common symptoms related to an MS relapse can include a history of sensory disturbances (e.g., asymmetrical numbness or dysesthesia/hyperesthesia), neurologic pain (e.g., burning sensation, trigeminal neuralgia), motor symptoms (weakness, spasticity), visual symptoms (e.g., monocular visual impairment with pain, loss of color vision, disc pallor, diplopia), fatigue, heat sensitivity, imbalance, and poor coordination. Other common symptoms include bladder urgency or retention, bowel dysfunction, sexual dysfunction, and neurocognitive symptoms (depression, anxiety, cognitive impairment).
The differential diagnosis can be challenging as a number of other conditions can mimic MS. A diagnosis of MS is less likely if there is a sudden, rapid onset of symptoms (<1 hour to maximal deficit); later onset (after age 50 years); transient, migrating symptoms; peripheral neuropathy; fever; diffuse, whole-body pain; arthralgias; skin rash; or prominent cortical features (e.g., seizures). When there are atypical clinical features present, in the appropriate clinical context, some conditions that may need to be taken into consideration include infections (e.g., human immunodeficiency virus, Lyme disease, syphilis), systemic inflammatory diseases (e.g., Behçet syndrome, sarcoidosis, Sjögren syndrome, systemic lupus erythematosus), vascular disease (e.g., migraine, vasculitis), nutritional deficiencies (e.g., folate, vitamin B12, vitamin E), psychiatric conditions (e.g., anxiety, conversion disorder), genetic conditions (mitochondrial disorders, spastic paraparesis),Reference Saguil, Kane and Farnell 11 and other inflammatory diseases of the CNS (neuromyelitis optica and solitary sclerosis).Reference Lattanzi, Logullo, Di Bella, Silvestrini and Provinciali 12 , Reference Oh and Levy 13 Routine blood tests and other investigations may be useful to rule out MS mimics (Table 2).Reference Brownlee, Hardy, Fazekas and Miller 14
Table 2 Routine blood tests to rule out multiple sclerosis (MS) mimics
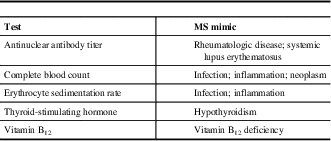
Adapted from Saguil et al.Reference Saguil, Kane and Farnell 11
Diagnosis
The clinical hallmark that characterizes MS is multiple neurological events separated in space and time.Reference McDonald, Compston and Edan 15 It is important to keep in mind that MS is a clinical diagnosis, requiring symptoms or signs typically associated with demyelinating lesions, although MRI of the brain and spine is now routinely employed to make the diagnosis. 16 , Reference Traboulsee, Letourneau-Guillon and Freedman 17 The “dissemination in space” criterion for MS is met by objective evidence of lesions in two or more areas of the brain and/or spinal cord on the clinical exam (polysymptomatic) or based on lesions in suggestive areas of the neuroaxis on an MRI. For the RRMS “dissemination in time” criterion, careful history-taking is essential to identify two discrete episodes of neurological worsening separated by at least 30 days,Reference McDonald, Compston and Edan 15 and to exclude other causes, such as infection or fever. Relapses may be reported by the examination. The “dissemination in time” criterion can also be met by the presence of new lesions on an MRI scan with reference to a baseline scan, irrespective of the timing of the baseline scan, or the simultaneous presence of gadolinium-enhancing and non-enhancing lesions on the same scan (as long as the gadolinium-enhancing lesion is not symptomatic). In PPMS, dissemination in time is characterized by a minimum of 1 year of progressive neurological symptoms.Reference Polman, Reingold and Banwell 18
Patients who present with a typical demyelinating event (e.g., one relapse) but who do not meet the clinical or radiological criteria of dissemination in space and time are categorized as having a clinically isolated syndrome (CIS). Clinically isolated syndrome with MRI lesions is highly predictive of the later development of MS. Also noteworthy is radiologically isolated syndrome (RIS), which is the incidental finding of MS-like lesions on MRI;Reference Okuda, Mowry and Beheshtian 19 an estimated one-third of RIS patients will have an initial clinical event over the next 5 years and will accordingly be diagnosed with CIS or MS.Reference Okuda, Siva and Kantarci 20
MRI findings suggestive of MS include periventricular, infratentorial, juxtacortical, and spinal cord lesions in the CNS. A gadolinium contrast agent, if available, is useful to identify new inflammatory lesions resulting from blood-brain barrier permeability.
Role of the PCP in MS Diagnosis
The temporary, intermittent nature of early MS symptoms can result in considerable diagnostic delays. Recent revisions to the diagnostic criteria (the McDonald criteriaReference McDonald, Compston and Edan 15 , Reference Polman, Reingold and Banwell 18 ) have enabled earlier MS diagnosis,Reference Rovira, Swanton and Tintore 21 although it remains to be determined whether this will result in improved long-term outcomes compared with historical cohorts.
Primary care physicians can play an important role in the early detection of demyelinating disease. The annual incidence of MS in Canada ranges from 5 to 11 cases per 100,000,Reference Marrie, Fisk and Stadnyk 3 , Reference Marrie, Yu, Blanchard, Leung and Elliott 22 suggesting that an average family physician will encounter one new MS case every 1-2 years.
In patients with symptoms or signs suggestive of a first relapse of MS, an MRI of the brain with gadolinium should be ordered if possible, and the patient has no known contraindications to receiving a contrast agent. MRI of the spinal cord should be ordered if the MRI of the brain is non-diagnostic, or if symptomatology is referable to this region.Reference Traboulsee, Letourneau-Guillon and Freedman 17 , Reference Traboulsee and Li 23 , Reference Traboulsee, Simon and Stone 24 To expedite the scan, the requisition should describe the patient’s suspicious signs and symptoms (e.g., monocular vision loss with pain) rather than simply stating “suspected MS.” The MRI should generally take place within 3 months of presentation, and more urgently if the suspicion of MS is high. Patients with lesions on MRI suggestive of MS should be referred to an MS specialty center to make the diagnosis. A reasonable expectation is that the MS specialist will assess the patient within 6 months of MRI. However, if the PCP is concerned about aggressive MS (frequent relapses, significant disability early in disease), it may be necessary to request a more urgent appointment. It is prudent not to confirm a diagnosis of MS until the patient has undergone a full neurological examination and work-up.
If the PCP foresees a significant delay with the MRI, an urgent referral to a community neurologist may be appropriate to expedite the scan. In addition, if an MS clinic appointment will be delayed >6 months, the MRI reveals white-matter lesions of uncertain etiology, or the patient is unable to travel to a provincial MS clinic, an urgent referral to a community neurologist may be appropriate, who can counsel the patient and initiate a DMT (if necessary) while awaiting an MS clinic appointment.
Referral documentation provided to the neurologist should include relevant findings from the patient’s history and physical examination (including recent changes in health status), patient-reported symptoms (type, frequency, severity), laboratory results, MRI images, and the MRI report, if obtained. A medication history, including recent treatments for suspected MS-related symptoms, is also needed. It is useful to ensure that a patient’s vaccination schedule is up-to-date as MS medications can alter the immune response, which may be associated with an increased risk of infections and an impaired ability to mount an immune response. Of particular importance are live attenuated vaccines (e.g., measles, mumps, chickenpox), as the safety of administering these vaccines is unknown once a DMT has been initiated.
Role of the PCP in Managing Patients Diagnosed with RRMS
There are three principal roles for PCPs in the management of patients diagnosed with MS: health maintenance and promotion, MS symptom management, and DMT safety monitoring. Of particular importance are general health maintenance and promotion. In patients with a chronic illness such as MS, clinicians tend to view health status through the prism of that condition, which may result in neglecting routine preventative measures, and misattributing symptoms to their MS.
Health Maintenance and Promotion
Patients should be counseled about maintaining a healthy lifestyle (smoking cessation, regular exercise, diet), and will require the usual screening measures (routine blood work, vascular risk factor screening, Pap smear, colonoscopy, and so on). Recommendations on a variety of topics related to MS are available on a number of reputable websites (www.mssociety.ca, www.msbrainhealth.org) for both patients and healthcare practitioners.Reference Motl 25
Individuals with MS are at risk of obesity, partly owing to MS fatigue and mobility difficulties. A recent survey reported that a high proportion of individuals with MS were overweight (22.5%) or obese (19.4%), with obesity associated with increased risks of diabetes (odds ratio [OR] 4.8) and hypertension (OR 4.5),Reference Marck, Neate, Taylor, Weiland and Jelinek 26 as is seen in the non-MS population. Obesity may partly account for the increasing incidence of diabetes, hypertension, hyperlipidemia, and ischemic heart disease that has been reported among Canadians with MS.Reference Marrie, Fisk and Tremlett 27 Counseling individuals about smoking cessation is especially relevant: active and passive smoking have been reported to be risk factors for the development of MS,Reference Zhang, Wang and Li 28 and continuing to smoke may accelerate disease progression.Reference McKay, Jahanfar, Duggan, Tkachuk and Tremlett 6 Comorbid illness in MS patients is associated with an increased risk of hospitalizations and mortality, and a significantly poorer quality of life.Reference Berrigan, Fisk and Patten 29 - Reference Marrie, Elliott, Marriott, Cossoy, Tennakoon and Yu 31
Vitamin D supplementation is now recognized as an important aspect of health maintenance in MS, as there is emerging evidence that having an adequate vitamin D level has beneficial effects in MS patients. Many MS patients are vitamin D deficient, and doses up to 4000 IU daily are generally recommended. As part of their routine care, PCPs should thus recommend vitamin D supplementation in newly diagnosed patients, or in those who are not yet on supplementation, and can monitor levels to ensure adequate supplementation. 16 , 32
Primary care physicians can also provide counseling about pregnancy planning. Multiple sclerosis does not appear to affect a woman’s fertility.Reference Roux, Courtillot, Debs, Touraine, Lubetzki and Papeix 33 There is a genetic susceptibility to MS in offspring. The absolute risk of MS in the offspring of a patient with MS is approximately 2%-4%.Reference O’Gorman, Lin, Stankovich and Broadley 34 Pregnancy does not have a negative impact on the long-term prognosis of MS.Reference Coyle 35 For the vast majority of patients, having a known diagnosis of MS does not affect decisions regarding labor, delivery method, use of epidural anesthetic, or use of routine medications during labor and delivery, and in the postpartum period. Thus, patients should make this decision based on the obstetrician’s recommendations. However, in cases in which patients have severe disability, special consideration may need to be given around labor and delivery to minimize harm to the mother and fetus, and the obstetrician and neurologist should both be involved in this decision.Reference Coyle 36
Disease-modifying treatments are not recommended for use in patients who are pregnant, trying to conceive, or breastfeeding.Reference Coyle 35 The effects of DMTs on pregnancy and lactation have not been well studied, but there is evidence that some of these agents have the potential to cause fetal harm. If a patient with MS is contemplating pregnancy, the PCP should recommend that she discuss this in detail with her MS specialist for the most current recommendations on DMT use peri-pregnancy. As most DMTs should not be used during the conception period, it is recommended that patients try to adequately plan pregnancy, and utilize methods to conceive without an excessive delay to minimize time off DMT. If conception does not occur within 6-12 months, fertility counseling should be sought to minimize time off DMT, and risk of relapse.
Generally, all DMT-treated female MS patients should be counseled to practice effective contraception. Although most DMTs are not known to affect oral contraceptives, they may not be optimal contraceptives as some DMTs (teriflunomide) may affect these agents’ pharmacokinetics. 37 Long-acting reversible contraception (e.g., subdermal rod or an intrauterine device) may be preferable.Reference Coyle 35 It is important to advise patients that, in most cases, it is not sufficient to stop their DMT when pregnancy occurs. Some DMTs have long half-lives and drug levels may be detectable for months or years after discontinuation. Women who wish to become pregnant should be counseled to continue using their DMT and practise effective contraception until they have consulted with their neurologist. The clinic will schedule discontinuation of the patient’s DMT to enable an adequate washout period before pregnancy. In patients who are taking teriflunomide (Aubagio), an accelerated elimination procedure is available, and should be performed for patients planning to conceive, or in the case of unexpected pregnancy.
Health maintenance includes the need for PCPs to manage other medical conditions as comorbidities are common in the MS population. The prevalence of irritable bowel syndrome and chronic lung disease is reported to be more than 10%.Reference Marrie, Reider and Stuve 38 Respiratory dysfunction resulting from infection is the most common reason for admission to neuro-intensive care and is associated with a fourfold increased risk of mortality compared with non-MS patients.Reference Karamyan, Dunser and Wiebe 39 There is also some evidence of an increased risk of other autoimmune diseases, notably psoriasis, thyroid disease, and uveitis.Reference Marrie, Reider and Cohen 40
Of particular importance in primary care is the diagnosis and management of depression and anxiety, both of which occur in >20% of patients.Reference Marrie, Reingold and Cohen 41 Indeed, one study found that patients and healthcare professionals view family practitioners as the healthcare provider best suited to play a central role in meeting MS patients’ mental healthcare needs.Reference Methley, Campbell, Cheraghi-Sohi and Chew-Graham 42 Psychological and pharmacological therapies have been shown to be effective in MS patients with depression.Reference Fiest, Walker and Bernstein 43 Patients can also be counseled on the benefits of exercise, mindfulness, yoga, and other stress management techniques that may enable them to cope better with chronic illness.
MS Symptom Management (Table 3)
Second, PCPs can play an important role in managing MS-related symptoms (Table 3). Common problems that PCPs may encounter in MS patients include fatigue, pain syndromes, bladder dysfunction, sexual dysfunction, spasticity, and sleep disorder. For symptoms such as fatigue, pain, and sleep disorders that are also commonly seen in the general population, PCPs should perform standard work-ups for these symptoms. If there is another identifiable cause of the symptom, it should generally be managed as it would be in the non-MS population.
Table 3 Multiple sclerosis (MS) symptom management in primary care
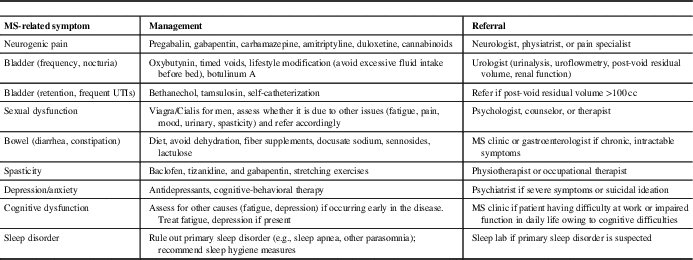
One of the most common MS-related symptoms is fatigue, which is reported to be severe and disabling in up to 70% of patients.Reference Khan and Smith 44 A multimodal approach is needed to manage factors that may contribute to MS fatigue, such as excluding other medical conditions (e.g., thyroid dysfunction, depression, sleep apnea), pain, muscle spasms or nocturia that can disrupt sleep, or medication-related side-effects, which can include both fatigue or insomnia (e.g., psychostimulants, fampridine, some DMTs, anti-spasmodics).Reference Hadjimichael, Vollmer and Oleen-Burkey 45
An estimated 29%-86% of MS patients experience disabling pain symptoms.Reference Tur 46 Neuropathic pain, such as Lhermitte’s sign (electrical sensation down the spine on neck flexion), pain in the extremities, and trigeminal neuralgia are common. Treatment options include antiepileptic agents (e.g., pregabalin, gabapentin, carbamazepine) or antidepressants (amitriptyline, duloxetine); cannabinoids may also be used,Reference Solaro and Messmer Uccelli 47 , Reference Turcotte, Doupe and Torabi 48 although evidence for their efficacy is limited.
Bladder dysfunction in MS is most commonly due to detrusor overactivity with symptoms such as urinary frequency, urgency, and nocturia.Reference Phe, Chartier-Kastler and Panicker 49 Patients may be referred to a urologist for a full work-up, including urinalysis, uroflowmetry, measurement of post-void residual (PVR) volume, and assessment of renal function. First-line treatment is with an anti-muscarinic agent; botulinum toxin A injections may also be useful. In the case of anti-muscarinic agents, PCPs should evaluate PVR as these drugs can worsen urinary retention. Alternatively, patients with MS may have difficulties emptying the bladder, which can result in urinary retention and frequent urinary tract infections (UTIs). Cholinergic agents (e.g., bethanechol) and α-1-agonists (e.g., tamsulosin) can be used in some cases, and self-catheterization may eventually be necessary. In MS patients with symptoms of urinary retention (incomplete emptying, excessive straining to empty bladder, frequent UTIs), PCPs should perform PVR, if possible, and consider a referral to a urologist if the PVR is >100 cc.
Sexual dysfunction (e.g., diminished libido, erectile dysfunction, anorgasmia) is common in MS but is often under recognized and inadequately managed. A multimodal approach is generally needed to manage MS symptoms that may affect sexual functioning (e.g., fatigue, incontinence, spasticity), and address psychosocial issues (e.g., depression, cognitive impairment).Reference Lew-Starowicz and Gianotten 50 Primary care physicians can play an important role in initiating a discussion of sexual functioning, and to refer patients to specialist medical services, psychologists, and therapists, as needed.
Spasticity symptoms range from mild muscle stiffness to sudden or sustained muscle contractions, and may be associated with back pain, arthralgia, and myalgia (most commonly of the legs). Patients should be counseled to engage in daily stretching or other exercises. First-line medications include baclofen, tizanidine, and gabapentin.Reference Otero-Romero, Sastre-Garriga and Comi 51 If no improvement is seen, diazepam or dantrolene may provide some benefit. There is some evidence supporting the use of cannabinoids for spasticity.Reference Whiting, Wolff and Deshpande 52 Botox injections can be considered for focal spasticity and/or refractory cases. A referral to a physical/occupational therapist or physiatrist should be considered.
Sleep disorders often arise secondary to MS symptoms, such as MS fatigue, neuropathic pain, nocturia, spasticity or limb movement disorders, or as a symptom of depression and anxiety. Patients will benefit from symptom management and counseling about sleep hygiene measures, such as avoidance of caffeinated beverages, restricting the consumption of liquids in the evening, and avoidance of stimulating activities (exercise, computer/tablet use) around bedtime. If a primary sleep disorder (obstructive sleep apnea or another parasomnia) is suspected, a referral for a sleep study should be made.
It should be noted that when prescribing symptomatic medications in DMT-treated patients, there are few pharmacological interactions that are noteworthy. Pharmacists are a valuable community resource and can provide information on potential drug-drug interactions.
Finally, PCPs can play a role in monitoring for relapses in patients with RRMS. Sub-acute onset of new neurological symptoms or worsening of pre-existing neurological symptoms that last for at least 24 hours and typically progress over many days warrant further investigation. In this situation, PCPs can first ensure that patients do not have concomitant infections that can exacerbate neurological symptoms and cause “pseudo-relapses,” and order appropriate tests as needed. When an infection has been ruled out, PCPs may be able to facilitate a visit to the neurologist’s office, or local emergency room for patients to be assessed and treated with steroids, if warranted. In some settings, the PCP may even be comfortable prescribing high-dose oral steroids (prednisone 1250 mg daily for 3-5 days), 53 , Reference Lattanzi, Cagnetti, Danni, Provinciali and Silvestrini 54 but patients should be warned of side effects of short-term, high-dose steroids, which can include insomnia, hypertension, gastrointestinal symptoms, hyperglycemia, and avascular necrosis.
Safety and Efficacy Monitoring in DMT-Treated MS Patients
Disease-modifying treatments are associated with a number of acute and chronic adverse effects that require ongoing monitoring. Monitoring requirements differ for each DMT, but necessary investigations can include periodic blood tests (e.g., liver enzymes, complete blood count, lymphocyte count, thyroid function), urine tests, ophthalmic examinations, and MRIs. The prescribing neurologist generally performs safety monitoring, with additional assistance from the drug manufacturers’ patient support programs. On occasion, neurologists may ask PCPs to include specific tests or procedures in the next primary-care visit and to forward the information to the MS clinic.
If a routine test obtained in primary care raises a concern that the result is out of keeping with what is expected with the specific DMT the patient is prescribed, the PCP should contact the consulting neurologist for advice and to schedule an appointment at the MS clinic. Although a comprehensive list of side effects of all DMTs is beyond the scope of this manuscript, common and noteworthy side effects and suggested management are listed in Table 3. Progressive multifocal leukoencephalopathy (PML) is a rare but serious potential adverse event that can occur with a number of DMTs, but has been seen most commonly with natalizumab. Progressive multifocal leukoencephalopathy is an opportunistic infection of the brain and can present in a myriad of ways, which can include weakness, visual or sensory disturbance, confusion, imbalance, and incoordination. Symptom onset can be subtle, but is generally progressive over days to weeks, and can often be difficult to distinguish from an MS-related relapse. If the concern for PML is present, PCPs should immediately send patients to an urgent care facility and notify the treating neurologist, who can arrange for appropriate investigations and management, which can include lumbar puncture, MRI, and discontinuing the culprit medication.
In addition to playing a role in safety monitoring of DMTs, PCPs may also facilitate patient care by monitoring for breakthrough MS disease activity. Although treatment algorithms can differ among MS neurologists, and continue to change as a result of the rapidly evolving therapeutic landscape, patients are generally monitored using both clinical and MRI measures. When a patient who is either untreated or who has been on a particular DMT for an adequate amount of time demonstrates evidence of breakthrough clinical relapses or significant MRI disease activity (usually more than 3-5 new lesions per year), the treating neurologist will typically initiate or change DMT.Reference Freedman, Selchen and Arnold 55 - Reference Río, Rovira and Tintoré 59 The PCP can play an important role in this setting by recommending that the patient be seen more urgently by his/her treatment neurologist. Furthermore, PCPs can inquire about medication compliance, and encourage adherence or recommend that patients be assessed by his/her neurologist more urgently to consider other options if medication adherence is an issue. Medication persistence is one of the most important factors that determine a DMT’s efficacy, and PCPs can play an important role in this regard.Reference Lattanzi, Danni and Taffi 60
Toward a Shared-Care Model of MS
The treatment landscape of MS is dynamic, and continues to increase in complexity as a number of novel treatments are expected to be approved for use in the near future.
Multiple sclerosis is a complex, disabling illness, which like other chronic diseases requires ongoing multidisciplinary care to meet the evolving needs of patients throughout the clinical course.
Although the diagnosis and treatment of MS are the responsibility of a specialized MS center or community neurologist, there is an urgent need for PCPs to participate actively in the care of MS patients. Primary care physicians play an essential role in providing continuity of care, educating patients about community resources (Appendix 2), and coordinating the provision of allied healthcare services.
In the shared-care model proposed above, there are a number of specific areas in which PCPs may contribute significantly in the care of people living with MS. Primary care physicians are an important first-contact in the early detection of symptoms suggestive of MS. Following the diagnosis of MS and possible initiation of a DMT, PCPs can provide support with ongoing health maintenance, manage comorbidities and MS-related symptoms, and can also assist in safety monitoring with specific DMTs. Primary care physicians are also vital in providing patients with ongoing advice and encouragement, and are central to the coordination of allied healthcare services, such as physiatry, physiotherapy, occupational therapy, nutritional counseling, psychiatry, and psychological services.
In the modern era of MS treatment, what is becoming evident is that it is only through the combined efforts of PCPs, neurologists, and allied healthcare teams that the evolving and complex needs of patients will be met during the life-long course of MS. Ultimately, the goal of this team approach is to improve the lives of people living with MS.
Acknowledgments
All authors have reviewed the final version of this manuscript and have agreed with the conclusions. Authors received funding from Sanofi Genzyme for participation at a roundtable meeting to discuss the development of the contents of this paper. The sponsor provided no editorial input during the development of the manuscript. The authors acknowledge the editorial assistance of Steven Manners of Communications Lansdowne, whose help was made possible through funding from Sanofi Genzyme.
DISCLOSURES
JO reports grants from Sanofi Genzyme, during the study period, grants from MS Society of Canada, grants from National MS Society, grants from Biogen Idec, grants from Sanofi Genzyme, personal fees from EMD Serono, personal fees from Sanofi Genzyme, personal fees from Biogen-Idec, personal fees from Teva, personal fees from Novartis, and personal fees from Roche, outside the submitted work.
M-SG-B reports grants from Sanofi Genzyme, during the study period.
CL reports personal fees from Teva, personal fees from Novartis, personal fees from Biogen, personal fees from Sanofi Genzyme, and from Merck Serono, outside the submitted work.
FL reports grants from Sanofi Genzyme, during the study period.
SM reports grants from Sanofi Genzyme, during the study period, fellowship funding from Multiple Sclerosis International Federation, grants from Consortium of MS Centers, grants from Western Pacific endMS Regional Research and Training Centre, grants from European Committee for the Treatment and Research in Multiple Sclerosis, personal fees from Sanofi Genzyme, personal fees from Roche, personal fees from Novartis, personal fees from Biogen, and personal fees from EMD Serono, outside the submitted work.
SAM reports grants from Sanofi Genzyme, during the study period, personal fees from Biogen, personal fees from Sanofi Genzyme, personal fees from EMD Serono, personal fees from Novartis, personal fees from Roche outside the submitted work. SAM also worked as the site principal investigator in an industry-sponsored clinical trial with Novartis, Sanofi Genzyme, and Roche, outside the submitted work.
LP-L reports grants from Sanofi Genzyme, during the study period, personal fees from Biogen, personal fees from EMD Serono, and personal fees from Sanofi Genzyme, outside the submitted work.
CR-H reports grants from Sanofi Genzyme, during the study period, personal fees from EMD Serono, personal fees from Novartis, personal fees from Biogen, personal fees from Sanofi Genzyme, and personal fees from Roche, outside the submitted work.
CR reports grants from Sanofi Genzyme, during the study period, personal fees from Bayer Healthcare, personal fees from Biogen Idec, personal fees from Roche, personal fees from EMD Serono, personal fees from MedDay, personal fees from Novartis, personal fees from Sanofi-Aventis, and personal fees from Teva, outside the submitted work.
A-MT reports grants from Sanofi Genzyme, during the study period, personal fees from Biogen-Idec, personal fees from EMD Serono, personal fees from Roche, personal fees from Novartis, personal fees from Sanofi Genzyme, and personal fees from Teva, outside the submitted work.
PSG reports grants from Sanofi Genzyme, during the study period, grants from Biogen, grants from Teva, grants from Alexion, grants from Bayer HealthCare, grants from Biogen, grants from Elan, grants from EMD Serono, grants from GlaxoSmithKline, grants from MedDay, grants from Novartis, grants from Ono, grants from Roche, grants from Sanofi-Aventis, grants from Teva, personal fees from NeuroRx, personal fees from Allergan, personal fees from Bayer HealthCare, personal fees from Biogen, personal fees from EMD Serono, personal fees from Sanofi Genzyme, personal fees from Mrez, personal fees from Novartis, personal fees from Roche, and from Teva, outside the submitted work.
MG has nothing to disclose.
Appendix 1
MS clinics in Canada
British Columbia
∙ Burnaby Hospital
Burnaby, BC
∙ UBC Hospital
Vancouver, BC
∙ Kelowna General Hospital
Kelowna, BC
∙ Royal Jubilee Hospital
Victoria, BC
∙ Prince George Regional Hospital
Prince George, BC
Alberta
∙ Foothills Hospital
Calgary, AB
∙ Red Deer Regional Hospital
Red Deer, AB
∙ Edmonton Clinic
Edmonton AB
Saskatchewan
∙ Saskatoon City Hospital
Saskatoon, SK
Manitoba
∙ Health Sciences Centre
Winnipeg, MB
Ontario
∙ St. Michael’s Hospital
Toronto, ON
∙ The Hospital for Sick Children
Toronto, ON
∙ Sunnybrook Health Sciences Centre
Toronto, ON
∙ Hamilton Health Sciences
Hamilton, ON
∙ Thunder Bay MS Clinic
Thunder Bay, ON
∙ Kingston General Hospital
Kingston, ON
∙ London Health Sciences Centre
London, ON
∙ Ottawa Hospital General Campus
Ottawa, ON
∙ Children’s Hospital of Eastern Ontario
Ottawa, ON
Quebec
∙ Montreal Neurological Institute
Montreal, QC
∙ Hôpital Notre-Dame—CHUM
Montréal, QC
∙ Clinique de SP de Hôpital Maisonneuve-Rosemont
Montréal, QC
∙ Hôpital Sacré-Cœur
Montréal, QC
∙ Montreal Children’s Hospital, Neuropediatric clinic, Glen site
Montréal QC
∙ Clinique de SP (Nord de l'Île de Montréal/Laval)
Saint-Laurent, QC
∙ Neuro Rive-Sud MS Clinic
Greenfield Park, QC
∙ Clinique Neuro-Outaouais
Gatineau, QC
∙ Centre hospitalier régional de Trois-Rivières
Trois-Rivières, QC
∙ Clinique de maladies neuromusculaires et de sclérose en plaques IRDPQ
Quebec City, QC
∙ Centre de Rèadaptation en Déficience Physique
Charny, QC
∙ CHUS—Site Fleurimont
Fleurimont, QC
∙ Clinique Accès-Santé
Rouyn-Noranda, QC
∙ Clinique de SP du CSSS de Rivière-du-Loup
Rivière-du-Loup, QC
∙ Clinique de SP du Centre hospitalier régional de Lanaudière (CHRDL)
Saint-Charles Borromée, QC
∙ Clinique SEP CSSS du Sud de Lanaudière (Centre hospitalier Pierre-Le-Gardeur)
Terrebonne (secteur Lachenaie), QC
∙ Clinique neurologique de l’hôpital régional de St-Jérôme
St-Jérôme, QC
∙ Hôpital St-Jean-sur-Richelieu
St-Jean-sur Richelieu, QC
∙ Clinique neuro spécialisée de Hôpital Chicoutimi
Chicoutimi, QC
New Brunswick
∙ Saint John Regional Hospital
Saint John, NB
∙ The Moncton Hospital
Moncton, NB
Nova Scotia
∙ Dalhousie MS Research Unit
Halifax, NS
∙ Cape Breton Regional Hospital
Sydney, NS
Newfoundland-Labrador
∙ OPD Neurology Clinic
St. John’s NL
Appendix 2
Available resources for MS patients in Canada
∙ MS Society of Canada: https://mssociety.ca/
Includes information on local patient support groups
∙ US National MS Society
∙ US National Library of Medicine: www.nlm.nih.gov/medlineplus/multiplesclerosis.html





