INTRODUCTION
Toxoplasma gondii is a coccidian parasite with worldwide distribution which circulates in nature in three infectious stages; the tachyzoite, the bradyzoite (within tissue cysts) and the sporozoite form (inside oocysts). Most infections in nature occur by ingestion of the parasite, either in the bradyzoite form (e.g. by eating raw or undercooked T. gondii-infected meat) or in the sporozoite form (e.g. ingesting food or water contaminated with oocysts) [Reference Moncada and Montoya1]. There are significant differences in parasite strains circulating in Europe vs. North America. Although most of the T. gondii strains found in both areas belong to one of the three main, closely related, clonal lineages (types 1, 2, 3) [Reference Howe and Sibley2, Reference Khan3]; type 2 strains predominate in Western Europe while a more mixed picture has been observed in North America (types 1, 2, 3 and the recently defined type 12 strains) [Reference Khan3].
Knowledge whether acute T. gondii infections have a seasonal pattern is important, especially for high-risk individuals, to cautiously avoid possible risk factors in certain seasons. There are no data on the seasonal variation of acute toxoplasmosis in the United States and only limited data are available in countries outside the United States [Reference Logar4–Reference Meenken7]. A recent large French study, with about 1500 acutely infected pregnant women from rural areas, documented a seasonal variation of T. gondii infections; with the highest number of cases observed from August to September and from the end of October to early January [Reference Morin8]. The seasonal patterns in the United States may be different compared to European countries for several reasons, including differences in implicated T. gondii strains.
The most appropriate approach for the identification of such seasonal patterns of acute T. gondii infections differs between countries. In countries with routine screenings of pregnant women during gestation for T. gondii, the most appropriate approach would be the study of acute T. gondii infections during gestation [Reference Logar4–Reference Morin8]. However, in countries like the United States, where such screening is not routinely recommended, the most appropriate approach is the study of acute toxoplasmic lymphadenopathy (ATL), which is the most common manifestation of acute toxoplasmosis [Reference Montoya, Huffman and Remington9]. ATL can be a surrogate of acute toxoplasmosis in patients in whom the date of onset of lymphadenopathy matches the window of acute infection predicted by serological tests performed at a reference laboratory [Reference Montoya, Huffman and Remington9–Reference Montoya11].
METHODS
In this retrospective cohort study, we describe the seasonal variation of ATL in the United States. We perused the electronic database of the Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL) over a 15-year period (1997–2011) to identify cases with ATL who fulfilled the following three strict inclusion criteria: (a) serological evidence of recently acquired acute T. gondii infection ⩽4 months from the sample collection date; (b) provided date of lymphadenopathy onset (month/day/year or month/year) by the referring physician and (c) date of lymphadenopathy onset ⩽4 months from the sample collection date. We used these very strict criteria to ascertain that we included patients with acute lymphadenopathy whose aetiology was most likely due to T. gondii infection.
The serological tests performed at PAMF-TSL for the diagnosis of acute T. gondii infection included the following: Sabin–Feldman Dye test (T. gondii IgG), T. gondii IgM and IgA ELISA, differential-agglutination test (AC/HS) and T. gondii IgG avidity test. The serological criteria we used for the diagnosis of acute T. gondii infection acquired ⩽4 months from sample collection date were as follows: (a) IgG Dye test ⩾1024, IgM ELISA ⩾5 and acute AC/HS pattern (if performed); (b) IgG Dye test ⩾1024, 3⩽IgM ELISA ⩽5, acute AC/HS pattern (if performed) and either low avidity ⩽10 (if performed) or IgA ELISA ⩾5 (if performed), and (c) IgG Dye test ⩽512, IgM ELISA ⩾5, acute AC/HS pattern (if performed) and either low avidity ⩽10 (if performed) or IgA ELISA ⩾5 (if performed). These criteria have been routinely used in the everyday clinical practice at PAMF-TSL for the interpretation of serological test results and estimation of the most likely time an acute T. gondii infection occurred. We further excluded those cases where the estimated time of T. gondii infection and the reported date of lymphadenopthy onset were not consistent; suggesting an aetiology for the lymphadenopathy other than T. gondii. The interpretation of serological test results was done independently by two investigators (D.C-I., J.G.M.) who were blind to the reported date of lymphadenopathy onset and characterized whether the T. gondii infections could have occurred ⩽4 months or not from the sample collection date. Any discrepancies were resolved by consensus.
Statistical analysis
Statistical analyses were performed in Stata/SE 12 (StataCorp LP, USA). We tested the uniformity of distribution of ATL cases per month, across the 12 calendar months using the Rayleigh and Kuiper tests for uniformity using circular statistics in Stata (circsumm method) [12]. Weighting was applied to the Rayleigh test, using as analytical weight (aweight) the total number of T. gondii IgG tests performed at PAMF-TSL per month. Calendar months during which ATL cases were detected were represented in degrees from 0o to 360o. A circular plot was generated in R software [13].
We also calculated the cumulative monthly incidence rate (IR) and 95% confidence intervals of ATL cases (IR = total number of cases per month/total number of T. gondii IgG tests performed at PAMF-TSL per month, summarized across the 15-year study period). We used χ 2 tests to test the uniformity of distribution of the cumulative monthly incidence rates across the 12 calendar months.
RESULTS
In the PAMF-TSL database over the 15-year study period, 688 patients had a lymphadenopathy diagnosis; 291 of those had a reported date of onset of lymphadenopathy and 114 of those also had serological evidence of acute T. gondii infection acquired ⩽4 months from sample collection date. Two patients (two relatives) who reported living mostly in Brazil were further excluded. In total, 112 ATL cases from the United States fulfilling our strict inclusion criteria were included for further analyses. The mean cumulative number of cases per month across the 15-year study period was nine (range 4–18) (Table 1). The characteristics of those cases and their serological test results are shown in Supplementary Tables S1 and S2.
Table 1. Cumulative monthly incidence rate of acute toxoplasmic lymphadenopathy, summarized across the 15-year study period (1997–2011)

ATL, Acute toxoplasmic lymphadenopathy; IR, incidence rate; CI, confidence interval. *Per 1000 T. gondii IgG tests.
The distribution of the cumulative number of ATL cases was statistically significant different across the 12 calendar months (Fig. 1). We rejected the null hypothesis of uniformity of distribution of ATL cases per month across the 12 calendar months (weighted Rayleigh test P = 0·006, Kuiper test P < 0·001).
The cumulative monthly incidence rates were also statistically significantly different across the 12 calendar months (P = 0·025, χ 2 test) (Table 1). The highest peak in the cumulative monthly incidence rate was observed in December (2·76 cases/1000 T. gondii IgG tests) and the second highest peak was observed in September (2·61 cases/1000 T. gondii IgG tests). In addition, comparison of the cumulative incidence rate in December vs. the cumulative incidence rate in the remaining months showed a statistically significant difference (P = 0·004, χ 2 test).
DISCUSSION
This is the largest cohort study to date of the seasonal patterns of acute toxoplasmosis in the United States. We documented that the distribution of ATL cases was not uniform across the 12 calendar months and that there was a seasonal variation with the highest peak in December and a second highest peak in September. The results were similar when we performed weighted analyses, weighting the number of ATL cases per month by the total number of T. gondii IgG tests performed per month in the PAMF-TSL laboratory. Results were also similar when we analysed the monthly incidence rates.
As more than half of individuals with documented acute T. gondii infection do not report clinical symptoms suggestive of acute toxoplasmosis and/or conventional risk factors for acute T. gondii infections [Reference Boyer14], our study findings suggest that the pre-test probability for the diagnosis of acute Toxoplasma infections in the United States should be highest around those months.
Other studies have also identified similar peaks around those months. The most recent study of seasonal variation of acute toxoplasmosis in France, using similar analytical methods and analysing 1444 pregnant women from rural areas with acute toxoplasmosis showed that the highest peaks of cases were detected from August to September and from the end of October to early January [Reference Morin8]. Two additional studies, one from Austria [Reference Sagel, Mikolajczyk and Kramer6] and one from Slovenia [Reference Logar4], have also highlighted increased numbers of acute T. gondii infections during the winter season [Reference Logar4] and during the winter months, respectively [Reference Sagel, Mikolajczyk and Kramer6].
The observed seasonal variation of ATL could be due to seasonal variation in exposure to certain risk factors associated with T. gondii infections. Physicians in the United States should be aware of this seasonal pattern of acute toxoplasmosis and promptly advise individuals in certain high-risk groups (such as pregnant women and immunocompromised patients) to avoid risk factors associated with T. gondii infections especially around December and September.
Information about risk factors was not systematically collected and could not be analysed for this project; but possible speculated risk factors (among others) that could explain the peak we observed in December could be consumption during the holiday season of traditional dishes made with relatively raw meat. Additional, speculated risk factors for the peak we observed in September could be higher consumption of fresh vegetables and fruits at the end of summer.
Some limitations should be acknowledged for our study. The total number of lymphadenopathy cases per month across the whole 15-year period was relatively small due to the very strict inclusion criteria we applied. Despite that, we were able to document that the distribution of acute toxoplasmosis was not uniform across the 12 calendar months. In the French study [Reference Morin8], although a much larger number of acute T. gondii infections was analysed, their unit of analysis was the days during the year [the ratio of analysed cases per day was 4:1 (1440/365)], while in our study we analysed the data per calendar months [the ratio of analysed cases per month was 9:1 (112/12)], and thus we were still able to detect a statistically significant signal for a differential monthly distribution. Moreover, by applying very strict inclusion criteria we ascertained that the most likely aetiology of these lymphadenopathy cases was acute T. gondii infection. Information on the date of onset of lymphadenopathy was based on the reported date of onset by the referring physician, as it was recorded in a short standardized questionnaire that was routinely requested to be completed by the referring physicians for all samples sent to PAMF-TSL, in order to assist in the more appropriate interpretation of serological test results. It should also be taken into account that lymphadenopathy usually develops ~2–4 weeks after the acute primary T. gondii infection; so the month with the peak of acute primary T. gondii infections might have been a month earlier than the month with the peak of ATL cases. The reported date of onset of lymphadenopathy might also depend on how promptly the patients recognized the presence of the lymphadenopathy. It is possible that patients with more systemic influenza-like illness might have sought medical attention earlier than patients without such symptoms. However, the number of patients who reported associated influenza-like symptoms (22/112, 20%) was not differentially distributed across the 12 calendar moths (data not shown). It is also possible that errors in the recorded dates of lymphadenopathy onset could have affected the estimated incidence rate, especially when the number of cases per month is small; however, such errors are more likely to occur randomly across the 12 calendar months and thus, unlikely to have introduced a systematic bias in our results. As the samples were sent to PAMF-TSL from several different laboratories across the United States, we do not know whether the seasonal pattern we observed might have been the same across all states and whether differences between urban and rural areas do exist. Different climatic conditions and probably different habits from South to North or from East to West could possibly influence seasonal acquisition patterns.
It would be important in future studies to evaluate whether the observed seasonal variation of acute toxoplasmosis could be explained by different T. gondii serotypes implicated and/or by different sources of infection (e.g. sporozoite-associated vs. bradyzoite-associated T. gondii infections) by correlating serotyping data for type II and non-type II infections [Reference Kong15] and data for antibodies to sporozoite-specific antigens. Such complementary information could provide additional insight on the seasonal variation in the exposure risk factors; e.g. exposure to infected meat (bradyzoite-associated T. gondii infections) vs. exposure to cat faeces (sporozoite-associated T. gondii infections).
Despite the above limitations, our study is the largest study to date of the seasonal variation of ATL in the United States. We did document that the distribution of ATL is not uniform across the 12 calendar months and that there is a seasonal variation with the highest peak in December and a second highest peak in September.
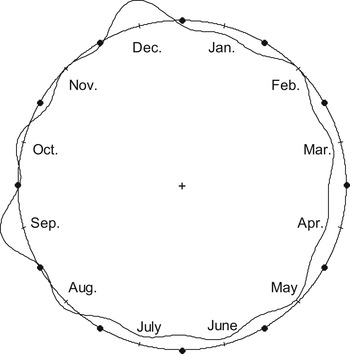
Fig. 1. Distribution of acute toxoplasmic lymphadenopathy cases per month (circular plot; shown is the kernel density line; the round circle corresponds to the mean distribution per month).
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814002945.
ACKNOWLEDGEMENTS
The authors thank Dr John S. Tamaresis, PhD, MS (biostatistician) from the Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, for his kind assistance with the circular statistics analyses and Dr Wei Wang, PhD (information management analyst, biostatistician) from the Palo Alto Medical Foundation Research Institute, Palo Alto, CA, for his kind assistance in generating the circular plot graph in R software.
DECLARATION OF INTEREST
None.





